Abstract
The role of autoimmunity targeting epithelial antigens in asthma has been suggested, in particular in non-atopic and severe asthma. Periplakin, a desmosomal component, is involved in epithelial cohesion and intracellular signaling. We detected anti-periplakin IgG antibodies in 47/260 (18 %) patients with asthma, with no association with severity or atopy. In addition, anti-periplakin IgE antibodies were detected in 12 of 138 tested patients (8.7 %) and were more frequently observed in patients with than without nasal polyposis. This study identifies a new autoimmune epithelial target in asthma. Whether periplakin autoimmunity (both IgG and IgE auto-antibodies) is involved in asthma pathogenesis remains to be studied during the disease course of these patients.
Keywords: Airway epithelium, Asthma, Autoimmunity, Desmosome, Nasal polyposis
To the Editor,
Autoimmunity targeting the bronchial epithelium has been consistently observed in patients with asthma, and various auto-antigens have been identified, including alpha enolase, [1] cytokeratine 8 or 9, [2] epidermal growth factor receptor (EGFR), activin A type 1 receptor, collagen V or α-catenin [3]. Autoantibodies (Abs) are observed in 30 % to 40 % of non-atopic patients and 0 to 10 % of atopic ones [3]. Autoimmunity may result from the exposure of self-antigens due to airway epithelial damage induced by respiratory viruses or allergens. However, the demonstration that epithelial-targeted autoimmunity contributes to asthma pathogenesis and severity is still tenuous and poorly documented: in non-atopic patients, asthma severity has been found associated with anti-collagen V Abs [3] and with the presence of IgG Abs, with a cytotoxic effect on airway epithelial cells [4].
Periplakin (PPL), a component of desmosomes, is expressed in proximal and distal airway epithelia [5]. PPL acts as a cytolinker between intermediate filament scaffolding and the desmosomal plaque and is involved in epithelial cohesion, intracellular signal transduction and antigen presentation [5]. We previously described PPL as a target for autoimmunity in idiopathic pulmonary fibrosis and that anti-PPL IgG Abs reduced alveolar cell migration in vitro [6]. Here, we hypothesized that PPL may be a target for autoimmunity in asthma and that anti-PPL IgG frequency might be different in severe compared with mild-to-moderate patients and/or in atopic compared with non-atopic patients.
We prospectively evaluated the prevalence of anti-PPL IgG Abs in the French asthma cohort COBRA (Cohorte Obstruction Bronchique et Asthme). All patients gave written informed consent for follow-up in the COBRA cohort according to the procedure validated by the local Ethics Committee (Comité de Protection des Personnes d’Ile de France 1), which approved the study.
We analysed serum obtained at enrolment in 260 patients (188 with severe and 72 mild to moderate asthma according to GINA guidelines); 40 healthy donors were used as controls. Western blot assay was used to test the presence of anti-PPL IgG Abs as previously described [6]. In 138 patients and 18 healthy donors, we also tested for the presence of anti-PPL IgE Abs. Results were considered positive when a 195-kDa band was detected with both a human placental extract and the recombinant PPL as antigenic targets. Western blot results from 6 selected patients are in Fig. 1.
Fig. 1.
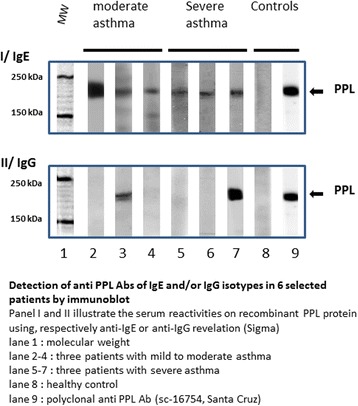
Western blot analysis of anti-periplakin (PPL) antibodies (Abs) of IgE isotype (I) and IgG isotype (II) in 6 patients. Lane 1, molecular weight; lanes 2–4, 3 patients with mild to moderate asthma; lanes 5–7, 3 patients with severe asthma; lane 8, healthy control; lane 9, polyclonal anti-PPL Ab (sc-16754, Santa Cruz Biotechnology)
Statistical analysis involved use of SAS 9.2 (SAS Inst. Inc., Cary, NC). Continuous variables were compared by Student t test or Wilcoxon rank-sum test and categorical variables by chi-square test or Fisher exact test, as appropriate. We tested for a differential association between anti-PPL IgG and severity or atopy with an interaction term in a multivariate logistic regression analysis explaining asthma severity. We did not adjust for multiple comparisons as it is not required in exploratory analysis [7].
Anti-PPL IgG Abs were detected in 47/260 patients (18 %) and in no control subjects. The characteristics of anti-PPL IgG–positive and –negative patients are in Table 1.
Table 1.
Characteristics of anti-periplakin IgG and IgE –positive and –negative patients
| Total (n = 260) | IgG PPL + (n = 47) | IgG PPL– (n = 213) | P value | Total (n = 138) |
IgE PPL + (n = 12) | IgE PPL– (n = 126) | P value | |
|---|---|---|---|---|---|---|---|---|
| Male | 80 (30.8 %) | 20 (42.6 %) | 60 (28.2 %) | 0.053 | 44 (31.9 %) | 6 (50.0 %) | 38 (30.2 %) | 0.19 |
| Age (yr) | 48.5 (14.2) | 47.5 (13.4) | 48.7 (14.4) | 0.59 | 49.5 (15.1) | 47.1 (16.3) | 49.7 (15.0) | 0.57 |
| Asthma severity | 0.89 | 0.67 | ||||||
| Mild | 35 (13.4 %) | 6 (12.7 %) | 29 (13.6 %) | 16 (11.6 %) | 2 (16.6 %) | 14 (11.1 %) | ||
| Moderate | 37 (14.2 %) | 8 (17.0 %) | 29 (13.6 %) | 16 (11.6 %) | 1 (8.3 %) | 15 (11.9 %) | ||
| Severe | 188 (72.3 %) | 33 (70.2 %) | 155(72.8 %) | 106(76.8 %) | 9 (75.0 %) | 97 (77.0 %) | ||
| Atopy | 162 (69.8 %) | 34 (77.3 %) | 128(68.1 %) | 0.23 | 95 (72.0 %) | 10 (83.3 %) | 85 (70.8 %) | 0.5 |
| BMI | 26.9 (5.6) | 26.9 (5.7) | 27.0 (5.6) | 0.91 | 27.5 (5.3) | 25.4 (3.0) | 27.7 (5.4) | 0.14 |
| Asthma duration (yr) | 23.2 [9.1–35.9] | 28.4 [14.5–37.7] | 21.1 [8.0–35.7] | 0.28 | 21.5 [8.2–36.4] | 13.6 [8.0–31.7] | 23.0 [8.8–36.4] | 0.31 |
| FEV1 (% predicted) | 81.0 (23.4) | 84.1 (20.8) | 80.4 (23.9) | 0.32 | 78.7 (24.8) | 87.8 (28.2) | 77.8 (24.4) | 0.18 |
| FEV1/FVC (%) | 68.6 (14.4) | 68.3 (15.6) | 68.7 (14.2) | 0.88 | 66.5 (15.3) | 65.3 (15.0) | 66.7 (15.4) | 0.77 |
| Total IgE (kUI/l) | 424.0 (643.5) | 446.6 (826.7) | 418.6 (593.9) | 0.45 | 425.0 (665.5) | 318.2 (377.9) | 436.1 (688.8) | 0.82 |
| Blood eosinophils (/mm3) | 305.4 (278.8) | 322.3 (185.1) | 301.4 (297.1) | 0.06 | 316.5 (251.7) | 378.9 (248.0) | 310.7 (252.2) | 0.32 |
| Nasal polyposis | 51 (19.9 %) | 13 (27.7 %) | 38 (18.2 %) | 0.14 | 27 (19.6 %) | 6 (50.0 %) | 21 (16.7 %) | 0.01 |
| Smoker or former smoker | 100 (38.6 %) | 22 (46.8 %) | 78 (36.8 %) | 0.26 | 56 (38.6 %) | 4 (33.3 %) | 52 (41.2 %) | 0.8 |
| Occupational asthma | 32 (12.4 %) | 9 (19.1 %) | 23 (10.9 %) | 0.12 | 18 (13.1 %) | 1 (8.3 %) | 17 (13.6 %) | 1 |
| GERD | 103(39.6 %) | 19 (40.4 %) | 84 (39.4 %) | 0.9 | 58 (42.0 %) | 4 (33.3 %) | 54 (42.9 %) | 0.52 |
| Asthma control | ||||||||
| well-controlled | 78 (30.2 %) | 17 (36.2 %) | 61 (28.9 %) | 0.61 | 38 (27.7 %) | 4 (33.3 %) | 34 (27.2 %) | 0.92 |
| partly controlled | 76 (29.5 %) | 13 (27.7 %) | 63 (29.9 %) | 36 (26.3 %) | 3 (25.0 %) | 33 (26.4 %) | ||
| uncontrolled | 104(40.3 %) | 17 (36.2 %) | 87 (41.2 %) | 63 (46.0 %) | 5 (41.7 %) | 58 (46.4 %) | ||
| JUNIPER score | 10.2 (7.3) | 9.1 (8.1) | 10.5 (7.0) | 0.29 | 10.4 (7.3) | 6.1 (8.4) | 10.8 (7.1) | 0.08 |
| At least 1 exacerbation in the previous year | 147 (58.8 %) | 22 (48.9 %) | 125 (61.0 %) | 0.13 | 81 (60.4 %) | 8 (72.7 %) | 73 (59.3 %) | 0.52 |
| No. of oral steroid bursts in the previous year | 4.0 [2.0–8.0] | 3.0 [2.0–4.5] | 4.0 [2.0–8.0] | 0.39 | 4.0 [2.0–8.0] | 3.5 [2.0–4.0] | 5.0 [2.0–8.0] | 0.38 |
| Inhaled steroids | 223 (85.8 %) | 42 (89.4 %) | 181 (85.0 %) | 0.43 | 123 (89.1 %) | 10 (83.3 %) | 113 (89.7 %) | 0.62 |
| Daily dose of inhaled steroids (μg/d) | 1781.1 (1154.1) | 1463.5 (1113.6) | 1854.8 (1153.8) | 0.01 | 1801.8 (1092.8) | 1700.0 (1032.8) | 1810.8 (1101.9) | 0.86 |
| Daily oral steroids | 47 (18.1 %) | 7 (14.9 %) | 40 (18.9 %) | 0.52 | 25 (18.2 %) | 2 (16.7 %) | 23 (18.4 %) | 1 |
| Omalizumab | 15 (5.8 %) | 2 (4.3 %) | 13 (6.2 %) | 1 | 8 (5.8 %) | 0 (0.0 %) | 8 (6.3 %) | 0.36 |
Data are mean (SD), n (%) or median (interquartile range)
PPL periplakin, GERD gastroesophageal reflux disease, FEV forced expiratory volume in 1 s, FCV forced vital capacity, BMI body mass index Atopy was defined by a positive skin prick test and/or specific IgE level > 0.15 kU/l for at least one aeroallergen
The proportion of patients with anti-PPL IgG was similar in mild-to-moderate asthma (19.4 % [95 % CI 10.3–28.6 %]) and in severe asthma (17.6 % [95 % CI 12.6–23 %]) (p = 0.72). However, the association between anti-PPL IgG positivity and asthma severity differed by atopy (pinteraction = 0.04): anti-PPL IgG–positive non-atopic patients tended to have less severe asthma, although not significantly (odds ratio [OR] 0.26 [95 % CI 0.07–1.05], p = 0.059), while anti-PPL IgG-positivity was not associated with asthma severity in atopic patients (OR 1.42 [95 % CI 0.59–3.42], p = 0.42).
Moreover, anti-PPL IgG–positive and –negative patients only differ for the mean daily inhaled corticosteroids dose, which was lower in the positive group. Non-significant trends to a greater proportion of males, frequency of occupational asthma and nasal polyposis were observed in the anti-PPL IgG–positive group, together with a trend to a lower number of asthma exacerbations and a longer median duration of asthma.
Anti-PPL IgE Abs were detected in none of the healthy donors and in 12/138 patients (8.7 %) (Table 1); 7 were negative for anti-PPL IgG Abs. Anti-PPL IgE–positive and –negative patients did not differ in clinical characteristics, particularly atopy, except for the proportion of nasal polyposis, which was higher for anti-PPL IgE–positive than –negative patients (50 % vs 16.7 %, p < 0.01).
This study identifies a new epithelial target for autoimmunity in asthma. Regardless of atopic status and degree of asthma severity, about 20 % of our asthmatic patients exhibited circulating auto-Abs (IgG and/or IgE) targeting PPL. Anti-PPL IgG-positive patients received lower doses of inhaled corticosteroids, suggesting a less active disease, which is in line with a trend for a lower proportion of patients presenting an asthma exacerbation, but could also suggest a potential modulation of the anti-PPL Abs production by corticosteroids.
We consider anti-PPL Abs a marker of airway epithelium damage. Because the presence of anti-PPL IgG or IgE Abs was tested only once during the asthma time course, we cannot determine how anti-PPL autoimmunity developed during the disease course nor how it could relate to earlier periods of uncontrolled disease, beyond the 1-year period preceding inclusion. A close prospective follow-up of anti-PPL Ab–negative patients could help assess the history of anti-PPL autoimmunity, as well as the role of asthma exacerbations and inhaled/oral steroids in the development of anti-PPL Abs. A longer follow-up could also help determine whether anti-PPL–positive patients may have a different prognosis than –negative patients, for example, show a more pronounced obstructive pattern or not. The titration of Abs could also give additional information because anti-collagen V Ab levels have been found to be increased in severe asthma [3].
Our results emphasize the potential role of auto-reactive IgE (alone or associated with IgG) in asthma. Of note, anti-PPL IgE Abs were more frequent in patients with than without nasal polyposis. This is an intriguing observation because nasal polyposis is characterized by altered upper airway epithelium (loss of epithelial barrier function and loss of desmosomes) [8], as is observed in asthma. Autoreactive IgE have been described in auto immune diseases, where they can act in synergy with IgG auto-Abs to potentiate the activation of inflammatory components [9]. Most importantly, IgE auto-Abs have been also reported in atopic dermatitis [10] and in chronic urticaria [11], and are considered as potential targets for omalizumab treatment in this setting [11].
The present data do not allow for determining whether anti-PPL autoimmunity is really involved in the pathogenesis of asthma. However, since we previously showed that anti-PPL Abs inhibit epithelial repair in vitro [6], we suggest anti-PPL Abs may contribute to a vicious cycle leading to chronic airway epithelial injury, a central feature of asthma.
To conclude, in this exploratory analysis, we observed autoimmunity features (IgG and/or IgE Abs) directed against PPL, an important airway epithelial protein, in 20 % of a large cohort of patients with asthma. These results add to the controversial hypothesis about the role of autoimmunity in the pathogenesis of asthma and autoimmunity as a target for asthma treatment but also emphasize the potential role of IgE auto-Abs in asthma and nasal polyposis.
Acknowledgements
We would like to acknowledge Dr Joëlle Benessiano, and all staff members of the Bichat Hospital Biological Resource Centre (Paris) for their help in centralising and managing biological data collection, and the COBRA investigators. We thank Laura Smales (BioMedEditing, Toronto, Canada) for English editing.
Funding
This study was supported by a grant from the French Ministry of Health (PHRC Régional 2010, AOR10114) and managed by the Assistance Publique-Hôpitaux de Paris.
Availability of data and materials
Many data were recorded during follow up in the COBRA cohort. We do not wish to share these data, that can be used for further studies.
Authors’ contributions
CT and SGM were in charge of the conception, design and supervision of the study, of data interpretation and of writing the manuscript. SGM and SLKS realized and interpreted all immunoblots. MA and MP were involved in patient inclusion and in management of the COBRA collection. BC and SCM participated in study design, data interpretation and writing of the manuscript. CE and CR participated in study design, performed statistical analyses and were involved in data interpretation. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
NA.
Ethics approval and consent to participate
This study is part of the national COBRA cohort. All patients gave written informed consent for follow-up in the COBRA cohort according to the procedure validated by the local Ethics Committee (Comité de Protection des Personnes d’Ile de France 1), which approved the study.
Abbreviations
- BMI
Body mass index
- FCV
Forced vital capacity
- FEV
Forced expiratory volume in 1 s
- GERD
Gastroesophageal reflux disease
- PPL
Periplakin
Contributor Information
Camille Taillé, Phone: 331 40256800, Email: camille.taille@aphp.fr.
Sabine Grootenboer-Mignot, Email: sabine.grootenboer-mignot@aphp.fr.
Candice Estellat, Email: candice.estellat@aphp.fr.
Carine Roy, Email: carine.roy@aphp.fr.
Sophie Ly Ka So, Email: sophie.lykaso@gmail.com.
Marina Pretolani, Email: marina.pretolani@inserm.fr.
Michel Aubier, Email: michel.aubier@aphp.fr.
Bruno Crestani, Email: bruno.crestani@aphp.fr.
Sylvie Chollet-Martin, Email: sylvie.chollet-martin@u-psud.fr.
References
- 1.Nahm DH, Lee KH, Shin JY, Ye YM, Kang Y, Park HS. Identification of alpha-enolase as an autoantigen associated with severe asthma. J Allergy Clin Immunol. 2006;118(2):376–81. doi: 10.1016/j.jaci.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Nahm DH, Lee YE, Yim EJ, Park HS, Yim H, Kang Y, et al. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002;165(11):1536–9. doi: 10.1164/rccm.200201-009OC. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Subramanian V, Christie C, Castro M, Mohanakumar T. Immune responses to self-antigens in asthma patients: clinical and immunopathological implications. Hum Immunol. 2012;73(5):511–6. doi: 10.1016/j.humimm.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon B, Lee HA, Choi GS, Ye YM, Nahm DH, Park HS. Increased IgG antibody-induced cytotoxicity against airway epithelial cells in patients with nonallergic asthma. J Clin Immunol. 2009;29(4):517–23. doi: 10.1007/s10875-009-9276-x. [DOI] [PubMed] [Google Scholar]
- 5.Bouameur JE, Favre B, Borradori L. Plakins, a versatile family of cytolinkers: roles in skin integrity and in human diseases. J Invest Dermatol. 2014;134(4):885–94. doi: 10.1038/jid.2013.498. [DOI] [PubMed] [Google Scholar]
- 6.Taille C, Grootenboer-Mignot S, Boursier C, Michel L, Debray MP, Fagart J, et al. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(6):759–66. doi: 10.1164/rccm.201001-0076OC. [DOI] [PubMed] [Google Scholar]
- 7.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–9. doi: 10.1016/S0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 8.Shahana S, Jaunmuktane Z, Asplund MS, Roomans GM. Ultrastructural investigation of epithelial damage in asthmatic and non-asthmatic nasal polyps. Respir Med. 2006;100(11):2018–28. doi: 10.1016/j.rmed.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Henault J, Riggs JM, Karnell JL, Liarski VM, Li J, Shirinian L, et al. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat Immunol. 2016;17(2):196–203. doi: 10.1038/ni.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeller S, Rhyner C, Meyer N, Schmid-Grendelmeier P, Akdis CA, Crameri R. Exploring the repertoire of IgE-binding self-antigens associated with atopic eczema. J Allergy Clin Immunol. 2009;124(2):278–85, 85 e1-7. doi: 10.1016/j.jaci.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135(2):337–42. doi: 10.1016/j.jaci.2014.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Many data were recorded during follow up in the COBRA cohort. We do not wish to share these data, that can be used for further studies.


