Abstract
Background
The APOA5 rs662799 polymorphism has been widely reported regarding its associations with the plasma lipid levels and the occurrence of coronary heart disease (CHD), whereas its relationship with the severity of CHD has not yet been explored.
Methods
Four hundred and seventy-eight angiografically defined subjects (325 CHD patients and 153 CHD-free controls) were enrolled in this study. The rs662799 polymorphism was genotyped, and the fasting lipid data were collected for all participants. The severity of CHD was evaluated for the CHD patients by using Gensini scores.
Results
The variant C allele of the rs662799 polymorphism was associated with lower levels of HDL-C in CHD-free women, and higher levels of TG and TG/HDL-C in women with CHD (P < 0.05 for all). The C allele was associated with higher prevalence of dyslipidemia and higher levels of Gensini scores only in women (P < 0.05 for both), but not in men. Multivariate linear regression analysis showed that the rs662799 polymorphism was independently associated with the Gensini scores in women after adjustment for other potential CHD risk factors (Beta = 0.157, 95 % CI: 0.017–0.298, P = 0.028).
Conclusion
Our data indicate that the rs662799 polymorphism is associated with dyslipidemia and the severity of CHD in Chinese women.
Keywords: APOA5, rs662799, Coronary heart disease, Lipid, Severity
Background
Coronary heart disease (CHD) is a major cause of death in developed countries, and in some developing countries like China [1]. CHD is recognized as a multifactorial disease, and dyslipidemia is closely associated with the occurrence and development of CHD. It has been demonstrated that dyslipidemia could account for around 50 % of the population-attributable risk for CHD [2]. Dyslipidemia is the elevation of plasma cholesterol, triglycerides (TG), or both, or a reduction of high-density lipoprotein cholesterol (HDL-C) that contributes to the development of atherosclerosis. Of the dyslipidemia components, low-density lipoprotein cholesterol (LDL-C) can directly deposit on the walls of blood vessels to form atherosclerotic plaques and plasma triglycerides exert their influence on CHD by increasing the coagulation of blood [3]. Plasma cholesterol and TG levels are known to be affected by many factors, among which genetic polymorphisms in the apolipoprotein genes were considered as the key factors [4].
The APOA1/C3/A4/A5 gene cluster, located on chromosome 11q23, has been shown to be among the most well characterized regions of the human genome with reference to their dynamic associations with plasma lipid levels [5]. APOA5 is a member of the APOA1/C3/A4/A5 gene cluster and has been recognized as a key regulator of TG metabolism [6]. This gene is exclusively expressed in liver, and its product, apolipoprotein AV (apoAV), is combined with chylomicron, very low-density lipoprotein (VLDL), high-density lipoprotein (HDL), but not with low-density lipoprotein (LDL). APOA5-knockout mice exhibited enhanced TG concentrations and conversely, APOA5-overexpressing mice had lower TG levels than those control mice [7]. In humans, a mutation in APOA5, which generates a truncated apoAV, has been associated with severe hypertriglyceridemia [8] and hyperchylomicronemia [9]. The underlying mechanisms by which apoAV reduces plasma TG and cholesterol levels are not well-known so far. However, experimental evidence supports the view that this apolipoprotein may function as an activator of lipoprotein lipase in the clearance of TG from the circulation [10]. Furthermore, Vu-Dac et al. [11] reported that APOA5 is a highly responsive peroxisome proliferator-activated receptor alpha (PPARα)-target gene and fenofibrate, a plasma TG-lowering drug, can increase the APOA5 expression in hepatocytes by stimulating the PPARα pathway.
Of several variants within APOA5, a transition from T to C located upstream of the promoter of this gene results in a rs662799 polymorphism (also known as -1131T>C). The rs662799 polymorphism has been extensively explored regarding its associations with plasma lipid levels and the occurrence of CHD over the past decades. In the literature, some studies demonstrated that this polymorphism is associated with higher levels of TG [12–25], total cholesterol (TC) [17] and LDL-C [18], and lower levels of HDL-C [14–22]. A significant association between this variant and the presence of CHD was also reported in several studies [22–24, 26, 27]. However, whether this polymorphism is associated with the severity of CHD has not been reported before.
Considering the association between the rs662799 polymorphism and dyslipidemia, it is logically plausible to hypothesize a link between the rs662799 polymorphism and the severity of CHD. In this study, a hospital-based study with angiographically defined CHD patients was conducted to systematically investigate the associations of the rs662799 polymorphism with dyslipidemia, the occurrence and severity of CHD. Our study results can provide the opportunity to elucidate the interrelationships among the rs662799 polymorphism, dyslipidemia and CHD.
Results
The clinical and genetic characteristics of the study population
As shown in Table 1, the CHD patients had higher age (P = 0.001) and higher prevalence of dyslipidemia (P = 0.011) and hypertension (P = 0.001) than the CHD-free subjects in men; the CHD patients had higher age (P < 0.001) and higher prevalence of menopause (P = 0.015), dyslipidemia (P = 0.027), hypertension (P = 0.010) and diabetes (P = 0.042) than the CHD-free subjects in women. The CHD patients had higher levels of apoB (P = 0.022), TG/HDL-C (P = 0.036), TC/HDL-C (P = 0.001) and apoB/apoAI (P = 0.002), and lower levels of HDL-C (P = 0.010) and apoAI (P = 0.002) than the CHD-free subjects in men; the CHD patients had higher levels of TG (P = 0.016), LDL-C (P = 0.017), apoB (P = 0.020), TG/HDL-C (P = 0.015), TC/HDL-C (P = 0.001), LDL-C/HDL-C (P < 0.001) and apoB/apoAI (P = 0.004) than the CHD-free subjects in women. The APOA5 rs662799 genotype or allele frequencies did not differ significantly between the CHD patients and the CHD-free subjects in both men and women.
Table 1.
Clinical and genetic characteristics of the study population
| Men | Women | |||||
|---|---|---|---|---|---|---|
| CHD-free (n = 81) | CHD (n = 205) | P | CHD-free (n = 72) | CHD (n = 120) | P | |
| Demographic characteristics | ||||||
| Age, years | 59.88 ± 12.58 | 64.63 ± 9.51 | 0.001 | 59.14 ± 9.69 | 64.48 ± 8.30 | <0.001 |
| Weight, kg | 65.35 ± 9.43 | 65.58 ± 8.54 | 0.863 | 59.65 ± 9.39 | 59.15 ± 9.09 | 0.902 |
| BMI, kg/m2 | 23.58 ± 3.13 | 24.04 ± 3.03 | 0.311 | 24.26 ± 3.58 | 24.42 ± 3.68 | 0.507 |
| Menopause, n (%) | NA | NA | 62 (86.11) | 115 (95.83) | 0.015 | |
| Dyslipidemia, n (%) | 49 (60.49) | 155 (75.61) | 0.011 | 32 (44.44) | 73 (60.83) | 0.027 |
| Hypertension, n (%) | 29 (35.80) | 117 (57.07) | 0.001 | 27 (37.50) | 68 (56.67) | 0.010 |
| Diabetes, n (%) | 9 (11.11) | 27 (13.17) | 0.636 | 6 (8.33) | 23 (19.17) | 0.042 |
| Lipid levels | ||||||
| TG, mmol/L | 1.27 ± 0.67 | 1.58 ± 1.31 | 0.056 | 1.42 ± 0.68 | 1.75 ± 1.10 | 0.016 |
| TC, mmol/L | 3.87 ± 1.01 | 4.13 ± 1.21 | 0.087 | 4.11 ± 1.03 | 4.40 ± 1.14 | 0.105 |
| LDL-C, mmol/L | 2.54 ± 1.85 | 2.61 ± 0.95 | 0.662 | 2.45 ± 0.77 | 2.77 ± 0.93 | 0.017 |
| HDL-C, mmol/L | 1.03 ± 0. 29 | 0.95 ± 0.23 | 0.010 | 1.11 ± 0.26 | 1.06 ± 0.24 | 0.118 |
| ApoAI, g/L | 1.08 ± 0.16 | 1.01 ± 0.20 | 0.002 | 1.13 ± 0.19 | 1.11 ± 0.18 | 0.394 |
| ApoB, g/L | 0.73 ± 0.24 | 0.81 ± 0.29 | 0.022 | 0.75 ± 0.23 | 0.84 ± 0.27 | 0.020 |
| Lp(a), mg/L | 264.04 ± 320.60 | 310.96 ± 332.45 | 0.140 | 239.74 ± 249.33 | 322.45 ± 340.38 | 0.087 |
| TG/HDL-C | 1.41 ± 1.03 | 1.82 ± 1.55 | 0.036 | 1.38 ± 0.83 | 1.85 ± 1.60 | 0.015 |
| TC/HDL-C | 3.94 ± 1.26 | 4.52 ± 1.35 | 0.001 | 3.75 ± 0.75 | 4.30 ± 1.31 | 0.001 |
| LDL-C/HDL-C | 2.55 ± 1.51 | 2.87 ± 1.10 | 0.052 | 2.24 ± 0.65 | 2.73 ± 1.08 | <0.001 |
| ApoB/apoAI | 0.68 ± 0.25 | 0.85 ± 0.46 | 0.002 | 0.66 ± 0.19 | 0.78 ± 0.28 | 0.004 |
| rs662799 Genotype frequency | ||||||
| TT, n (%) | 43 (53.09) | 103 (50.24) | 0.751 | 42 (58.33) | 63 (52.50) | 0.351 |
| TC, n (%) | 33 (40.74) | 90 (43.90) | 28 (38.89) | 51 (42.50) | ||
| CC, n (%) | 5 (6.17) | 12 (5.85) | 2 (2.78) | 6 (5.00) | ||
| rs662799 Allele frequency | ||||||
| T allele | 0.735 | 0.722 | 0.761 | 0.778 | 0.738 | 0.376 |
| C allele | 0.265 | 0.278 | 0.222 | 0.263 | ||
CHD coronary heart disease, BMI body mass index, NA not available, TG triglycerides, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, apoAI apolipoprotein AI, apoB apolipoprotein B
Comparisons of the clinical and biochemical variables among the APOA5 rs662799 genotypes
The demographic variables and lipid levels among the genotypes of the rs662799 polymorphism are shown in Table 2 for the CHD-free subjects and in Table 3 for the CHD patients. HDL-C (P = 0.045) levels decreased orderly with the number of C alleles in CHD-free women. TG (P = 0.009) and TG/HDL-C (P = 0.006) levels increased orderly with the number of C alleles in women with CHD. There were no significant differences in other variables among the genotypes in both men and women.
Table 2.
Non-lipid variables and lipid levels of the CHD-free subjects by the APOA5 rs662799 genotypes
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| TT genotype (n = 43) | CT genotype (n = 33) | CC genotype (n = 5) | P | TT genotype (n = 42) | CT genotype (n = 28) | CC genotype (n = 2) | P | |
| Non-lipid variables | ||||||||
| Age, years | 59.44 ± 12.99 | 59.88 ± 12.95 | 63.60 ± 5.89 | 0.787 | 59.00 ± 10.86 | 59.29 ± 7.72 | 64.50 ± 7.77 | 0.737 |
| Weight, kg | 64.00 ± 8.87 | 66.53 ± 9.73 | 69.00 ± 12.08 | 0.366 | 59.13 ± 10.76 | 59.46 ± 8.92 | 52.50 ± 3.53 | 0.635 |
| BMI, kg/m2 | 23.30 ± 3.11 | 23.68 ± 2.96 | 25.28 ± 4.67 | 0.483 | 23.93 ± 4.08 | 24.48 ± 3.26 | 20.88 ± 0.85 | 0.404 |
| Menopause, n (%) | NA | NA | NA | 36 (85.71) | 24 (85.71) | 2 (100.00 %) | 0.784 | |
| Hypertension, n (%) | 14 (32.56) | 13 (39.39) | 2 (40.00) | 0.545 | 12 (28.57) | 14 (50.00) | 1 (50.00) | 0.079 |
| Diabetes, n (%) | 4 (9.30) | 5 (15.15) | 0 (0.00) | 0.898 | 2 (4.76) | 4 (14.29) | 0 (0.00) | 0.305 |
| Lipid variables | ||||||||
| TG, mmol/L | 1.20 ± 0.68 | 1.24 ± 0.50 | 2.07 ± 1.06 | 0.060 | 1.33 ± 0.65 | 1.58 ± 0.74 | 1.15 ± 0.23 | 0.288 |
| TC, mmol/L | 3.91 ± 1.08 | 3.77 ± 0.93 | 4.27 ± 0.68 | 0.554 | 4.30 ± 1.01 | 3.89 ± 1.05 | 3.51 ± 0.09 | 0.174 |
| LDL-C, mmol/L | 2.73 ± 2.44 | 2.26 ± 0.75 | 2.73 ± 0.63 | 0.550 | 2.59 ± 0.76 | 2.26 ± 0.77 | 2.10 ± 0.00 | 0.161 |
| HDL-C, mmol/L | 1.08 ± 0.35 | 0.98 ± 0.20 | 0.92 ± 0.25 | 0.264 | 1.18 ± 0.25 | 1.02 ± 0.24 | 0.92 ± 0.01 | 0.045 |
| ApoAI, g/L | 1.10 ± 0.15 | 1.06 ± 0.17 | 1.08 ± 0.17 | 0.705 | 1.16 ± 0.16 | 1.09 ± 0.22 | 0.99 ± 0.16 | 0.152 |
| ApoB, g/L | 0.71 ± 0.25 | 0.73 ± 0.24 | 0.85 ± 0.22 | 0.456 | 0.77 ± 0.25 | 0.73 ± 0.21 | 0.66 ± 0.10 | 0.680 |
| Lp(a), mg/L | 251.09 ± 323.39 | 280.68 ± 327.62 | 272.26 ± 321.52 | 0.926 | 236.26 ± 248.07 | 260.84 ± 271.67 | 211.95 ± 17.32 | 0.911 |
| TG/HDL-C | 1.29 ± 0.97 | 1.38 ± 0.78 | 2.62 ± 2.11 | 0.066 | 1.22 ± 0.78 | 1.63 ± 0.87 | 1.25 ± 0.25 | 0.131 |
| TC/HDL-C | 3.86 ± 1.43 | 3.91 ± 0.96 | 4.85 ± 1.24 | 0.247 | 3.69 ± 0.73 | 3.85 ± 0.82 | 3.83 ± 0.07 | 0.693 |
| LDL-C/HDL-C | 2.64 ± 1.92 | 2.36 ± 0.81 | 3.11 ± 0.92 | 0.506 | 2.23 ± 0.63 | 2.24 ± 0.69 | 2.29 ± 0.02 | 0.992 |
| ApoB/apoAI | 0.66 ± 0.27 | 0.69 ± 0.22 | 0.81 ± 0.22 | 0.424 | 0.66 ± 0.19 | 0.67 ± 0.17 | 0.69 ± 0.21 | 0.951 |
CHD coronary heart disease, BMI body mass index, NA not available, TG triglycerides, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, apoAI apolipoprotein AI, apoB apolipoprotein B
Table 3.
Non-lipid variables and lipid levels of the CHD patients by the APOA5 rs662799 genotypes
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| TT genotype (n = 103) | CT genotype (n = 90) | CC genotype (n = 12) | P | TT genotype (n = 63) | CT genotype (n = 51) | CC genotype (n = 6) | P | |
| Non-lipid variables | ||||||||
| Age, years | 64.97 ± 9.34 | 64.17 ± 10.00 | 65.17 ± 7.60 | 0.827 | 65.17 ± 7.49 | 63.82 ± 9.20 | 62.40 ± 9.29 | 0.589 |
| Weight, kg | 65.71 ± 8.27 | 65.57 ± 9.13 | 64.00 ± 7.05 | 0.822 | 59.07 ± 8.14 | 58.59 ± 10.35 | 66.17 ± 5.63 | 0.156 |
| BMI, kg/m2 | 24.17 ± 3.02 | 23.86 ± 3.10 | 23.81 ± 2.74 | 0.762 | 24.15 ± 3.06 | 24.55 ± 4.48 | 26.59 ± 1.83 | 0.298 |
| Menopause, n (%) | NA | NA | NA | 62 (98.41) | 48 (94.12) | 5 (83.33) | 0.068 | |
| Hypertension, n (%) | 57 (55.34) | 55 (61.11) | 5 (41.47) | 0.988 | 42 (66.7) | 23 (45.10) | 3 (50.00) | 0.065 |
| Diabetes, n (%) | 16 (15.53) | 10 (11.11) | 1 (8.33) | 0.303 | 13 (20.6) | 9 (17.65) | 1 (16.67) | 0.674 |
| Lipid variables | ||||||||
| TG, mmol/L | 1.36 ± 0.74 | 1.76 ± 1.73 | 1.83 ± 0.91 | 0.073 | 1.51 ± 0.86 | 1.96 ± 1.27 | 3.01 ± 1.55 | 0.009 |
| TC, mmol/L | 4.08 ± 1.18 | 4.24 ± 1.29 | 3.82 ± 0.84 | 0.448 | 4.31 ± 1.23 | 4.48 ± 1.03 | 4.44 ± 1.22 | 0.722 |
| LDL-C, mmol/L | 2.57 ± 0.91 | 2.71 ± 1.03 | 2.30 ± 0.61 | 0.307 | 2.70 ± 1.03 | 2.82 ± 0.79 | 2.94 ± 1.04 | 0.749 |
| HDL-C, mmol/L | 0.96 ± 0.20 | 0.95 ± 0.23 | 0.82 ± 0.24 | 0.124 | 1.09 ± 0.24 | 1.03 ± 0.24 | 0.91 ± 0.24 | 0.149 |
| ApoAI, g/L | 1.00 ± 0.16 | 1.02 ± 0.20 | 1.00 ± 0.15 | 0.861 | 1.13 ± 0.16 | 1.08 ± 0.20 | 1.11 ± 0.18 | 0.234 |
| ApoB, g/L | 0.80 ± 0.30 | 0.83 ± 0.30 | 0.77 ± 0.19 | 0.618 | 0.80 ± 0.27 | 0.88 ± 0.26 | 0.83 ± 0.33 | 0.255 |
| Lp(a), mg/L | 328.47 ± 362.37 | 271.98 ± 285.15 | 412.95 ± 371.18 | 0.271 | 369.27 ± 374.08 | 258.82 ± 274.92 | 441.90 ± 437.05 | 0.164 |
| TG/HDL-C | 1.53 ± 1.04 | 1.98 ± 1.81 | 2.60 ± 1.78 | 0.057 | 1.52 ± 1.00 | 2.14 ± 2.02 | 3.83 ± 2.73 | 0.006 |
| TC/HDL-C | 4.40 ± 1.41 | 4.59 ± 1.30 | 4.86 ± 1.17 | 0.411 | 4.07 ± 1.27 | 4.53 ± 1.33 | 4.99 ± 1.19 | 0.080 |
| LDL-C/HDL-C | 2.80 ± 1.16 | 2.95 ± 1.09 | 2.90 ± 0.68 | 0.665 | 2.57 ± 1.08 | 2.88 ± 1.07 | 3.26 ± 0.86 | 0.150 |
| ApoB/apoAI | 0.85 ± 0.45 | 0.87 ± 0.51 | 0.78 ± 0.19 | 0.808 | 0.72 ± 0.26 | 0.84 ± 0.29 | 0.86 ± 0.29 | 0.057 |
CHD coronary heart disease, BMI body mass index, NA not available, TG triglycerides, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, apoAI apolipoprotein AI, apoB apolipoprotein B
The frequencies of the APOA5 rs662799 genotypes and alleles in the non-dyslipidemic and dyslipdemic subjects
The distribution of the rs662799 genotypes and alleles was significantly different between the non-dyslipidemic subjects and dyslipidemic subjects in women (Table 4). The frequencies of TC (P = 0.022) and CC (P = 0.050) genotypes were higher in the dyslipidemic women than in the non-dyslipidemic women. The C allele frequency was higher in the dyslipidemic women than in the non-dyslipidemic women (P < 0.001). There were no significant differences in the distribution of genotypes or alleles between the non-dyslipidemic subjects and the dyslipidemic subjects in men.
Table 4.
The distribution of the APOA5 rs662799 genotypes and alleles in the non-dyslipidemic and dyslipdemic subjects
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Non-dyslipidemic (n = 82) | Dyslipidemic (n = 204) | P | Non-dyslipidemic (n = 87) | Dyslipidemic (n = 105) | P | |
| Genotype frequency | ||||||
| TT, n (%) | 45 (54.88) | 101 (49.51) | 0.280 | 59 (67.82) | 46 (43.81) | <0.001 |
| TC, n (%) | 34 (41.46) | 89 (43.63) | 28 (32.18) | 51 (48.57) | ||
| CC, n (%) | 3 (3.66) | 14 (6.86) | 0 (0.00) | 8 (7.62) | ||
| Allele frequency | ||||||
| T allele | 0.756 | 0.713 | 0.299 | 0.839 | 0.681 | <0.001 |
| C allele | 0.244 | 0.287 | 0.161 | 0.319 | ||
The Gensini score levels of the CHD patients according to the APOA5 rs662799 genotypes
As shown in Fig. 1, the Gensini score levels increased orderly with the number of C alleles in women patients (P = 0.045). In men patients, the Gensini score levels were similar among the genotypes.
Fig. 1.
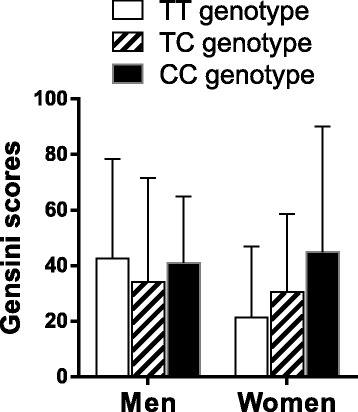
The Gensini score levels of the CHD patients according to the APOA5 rs662799 genotypes. The Gensini score levels were significantly different among the women with different rs662799 genotypes (P < 0.05)
Linear regression analysis of the APOA5 rs662799 polymorphism and other variables with the Gensini scores in the CHD patients
The results of the linear regression analysis of the potential CHD risk factors and the Gensini scores are shown in Table 5. In men, univariate linear regression analyses were conducted and 9 viaribles [hypertension, TC, LDL-C, apoAI, apoB, Lp(a), TC/HDL-C, LDL-C/HDL-C and apoB/apoAI] were found to be associated with the Gensini scores (P < 0.1 for all). Then the 9 variables were taken into the stepwise multivariate linear regression analysis and Lp(a) (P = 0.035) and apoB/apoAI (P < 0.001) were found to be independently associated with the Gensini scores after controlling for the confounding variables including hypertension, TC, LDL-C, apoAI, apoB, TC/HDL-C and LDL-C/HDL-C. In women, 9 viaribles [APOA5 rs662799 polymorphism, TC, LDL-C, apoAI, apoB, Lp(a), TC/HDL-C, LDL-C/HDL-C and apoB/apoAI] were found to be associated with the Gensini scores in univariate linear regression analyses (P < 0.1 for all). Then the 9 variables were taken into the stepwise multivariate linear regression analysis and APOA5 rs662799 polymorphism (P = 0.028), Lp(a) (P = 0.009) and apoB/apoAI (P = 0.005) were found to be independently associated with the Gensini scores after controlling for the confounding variables including hypertension, TC, LDL-C, apoAI, apoB, TC/HDL-C and LDL-C/HDL-C.
Table 5.
Linear regression analysis of the Gensini scores (dependent variable) and the potential CHD risk factors (independent variables) in CHD patients
| Variables | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate linear regression | P | Multivariate linear regression | P | Univariate linear regression | P | Multivariate linear regression | P | |
| Beta (95 % CI) | Beta (95 % CI) | Beta (95 % CI) | Beta (95 % CI) | |||||
| APOA5 rs662799 | −0.053 (−0.164–0.057) | 0.342 | 0.187 (0.041–0.334) | 0.013 | 0.157 (0.017–0.298) | 0.028 | ||
| Age | 0.001 (−0.006–0.008) | 0.777 | 0.008 (−0.003–0.018) | 0.157 | ||||
| Weight | 0.003 (−0.005–0.011) | 0.530 | −0.004 (−0.015–0.006) | 0.391 | ||||
| BMI | 0.018 (−0.004–0.041) | 0.113 | −0.007 (−0.003–0.019) | 0.591 | ||||
| Menopause | NA | NA | −0.031 (−0.474–0.411) | 0.889 | ||||
| Hypertension | 0.063 (0.013–0.112) | 0.013 | 0.001 (−0.065–0.066) | 0.981 | ||||
| Diabetes | 0.106 (−0.091–0.303) | 0.289 | 0.021 (−0.205–0.247) | 0.854 | ||||
| TG | −0.016 (−0.067–0.036) | 0.556 | 0.031 (−0.047–0.109) | 0.427 | ||||
| TC | 0.059 (0.004–0.113) | 0.035 | 0.074 (−0.003–0.151) | 0.061 | ||||
| LDL-C | 0.096 (0.027–0.165) | 0.007 | 0.095 (0.001–0.189) | 0.048 | ||||
| HDL-C | −0.113 (−0.401–0.176) | 0.442 | −0.221 (−0.586–0.143) | 0.232 | ||||
| ApoAI | −0.384 (−0.717– −0.051) | 0.024 | −0.841 (−1.330– −0.352) | 0.001 | ||||
| ApoB | 0.372 (0.147–0.597) | 0.001 | 0.430 (0.105–0.755) | 0.010 | ||||
| Lp(a) | 0.192 (0.038–0.346) | 0.015 | 0.162 (0.012–0.312) | 0.035 | 0.360 (0.152–0.568) | 0.001 | 0.278 (0.071–0.486) | 0.009 |
| TG/HDL-C | −0.001 (−0.046–0.043) | 0.957 | 0.012 (−0.041–0.065) | 0.647 | ||||
| TC/HDL-C | 0.073 (0.025–0.121) | 0.003 | 0.079 (0.012–0.146) | 0.021 | ||||
| LDL-C/HDL-C | 0.101 (0.042–0.160) | 0.001 | 0.094 (0.013–0.175) | 0.024 | ||||
| ApoB/apoAI | 0.277 (0.137–0.416) | <0.001 | 0.261 (0.124–0.398) | <0.001 | 0.628 (0.332–0.923) | <0.001 | 0.446 (0.137–0.756) | 0.005 |
CHD coronary heart disease, BMI body mass index, NA not available, 95 % CI 95 % confidence interval, TG triglycerides, TC total cholesterol, ApoAI apolipoprotein AI, ApoB apolipoprotein B, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, Lp(a) lipoprotein (a)
Discussion
The present study demonstrated that the rs662799 polymorphism was independently associated with the severity of CHD in Chinese women patients. To our knowledge, this is the first demonstration of a significant association between the rs662799 polymorphism and the severity of CHD, although the associations of the variant with abnormal lipid profiles and the occurrence of CHD have been reported in Chinese [22, 26, 27], Hungarian [23] and Japanese [24].
The significant association between the rs662799 polymorphism and the severity of CHD was only observed in women patients, but not in men patients. The gender-specific effects of the rs662799 polymorphism on the severity of CHD are not well understood. One reason could be that the rs662799 polymorphism was associated with dyslipidemia only in women (Table 4). The women carrying one or more C alleles of the rs662799 polymorphism had higher prevalence of dyslipidemia than those without this allele. It is logical to suppose that the C allele carrying women experienced faster deposition of lipids in the walls of coronary arteries and had severer coronary stenosis than those without this allele. Another raised question could be that why the rs662799 polymorphism was associated with higher prevalence of dyslipidemia only in women, but not in men in our study population. This can be partly explained by the different lifestyles between Chinese men and women. It has been reported that the rs662799 polymorphism had interaction with lifestyles on the lipid levels in Asians [28]. The gender-specific effects of the rs662799 polymorphism on lipid levels were also reported in other populations. In Turkish adults, Komurcu-Bayrak et al. [14]. demonstrated that the C carriers had higher fasting TG levels in both genders, but lower HDL-C levels only in women than the non-carriers. In our study population, the CHD-free subjects were categorized into three subgroups [subjects with normal coronary arteries, subjects with coronary atherosclerosis and subjects with minimal coronary stenosis (stenosis < 50 %)] according to the extent of coronary atherosclerosis. Interestingly, there was a weak trend in the association between the rs662799 polymorphism and the extent of coronary atherosclerosis in CHD-free women, but not in men (data not shown).
Our data did not support a significant association between the rs662799 polymorphism and the occurrence of CHD, although an independent and significant association was observed between the rs662799 polymorphism and the severity of CHD in women. This result is beyond our understanding since a series of case–control studies demonstrated a significant association between the rs662799 polymorphism and the occurrence of CHD [22–24, 26, 27]. One of the reasons could be that the control group in our study was not made up of healthy individuals, but the subjects who underwent angiography for suspected CHD. In the control group, 54.90 % of them had normal coronary arteries, 33.99 % of them had coronary atherosclerosis and 11.11 % of them had minimal coronary stenosis (stenosis < 50 %). Some other studies [21, 29] which demonstrated negative association between the rs662799 polymorphism and the occurrence of CHD also enrolled the subjects with coronary atherosclerosis or minimal coronary stenosis as controls. On the other hand, all the studies [22–24, 26, 27] which concluded a positive association between the rs662799 polymorphism and the occurrence of CHD used healthy individuals as controls.
The C allele frequency of the rs662799 polymorphism was 0.26 in the present study population, which is consistent with the ranges reported for other Asians, including Chinese (0.29–0.39) [16, 17, 22, 26], Korean (0.31) [15] and Japanese (0.33–0.35) [24], but is higher than those reported for Caucasians (0.06–0.11) [13, 20, 21, 23]. The rs662799 polymorphism might have some effects on the expression pattern of APOA5. The circulating levels of apoAV protein were affected by the rs662799 polymorphism in several studies, but the results were inconsistent [30, 31]. Kang et al. [30] demonstrated that the C allele carriers had higher levels of apoAV and TG than the non-carriers in type 2 diabetes patients, whereas Jang et al. [31] reported that the C allele carriers had lower levels of apoAV but higher levels of TG in healthy subjects and CHD patients. ApoAV is combined with chylomicron, VLDL, HDL and considered as a key regulator of TG metabolism. The plasma levels of apoAV were reported to be negatively associated with the TG levels [7–9]. Therefore, the association between the rs662799 polymorphism and the severity of CHD could be mediated by apoAV and hypertriglyceridemia.
Our findings should be considered in the context of several potential limitations. Firstly, the small sample size in each group may have limited the power to detect a significant relationship. Secondly, the control group was not made up of healthy individuals, but rather the subjects who underwent angiography with suspected CHD at our hospital, which may have led to a selection bias. However, it was difficult to enroll healthy subjects who underwent coronary angiography from general population. Thirdly, the patients included in this study were exclusively Chinese Han people, and therefore our findings may not apply to other ethnic origins.
Conclusions
The rs662799 polymorphism is significantly associated with dyslipidemia and the severity of CHD in Chinese women, but further investigations with large sample size and multi-ethnicities are required to validate the findings from the present study.
Methods
Study participants
This study was designed as a hospital-based study. A total of 478 consecutive and unrelated adult subjects who underwent coronary angiography for suspected CHD at the Department of Cardiology, the Affiliated Hospital of North Sichuan Medical College (Nanchong, China) were enrolled in the study between April 2014 and July 2015. Of these subjects, 325 subjects were diagnosed with CHD, while the rest 153 subjects were free of CHD and considered as the control group. Subjects taking lipid lowering drugs or the drugs that might affect the glucose or lipid metabolism were excluded from the study. In order to enlarge the sample size, those who took the drugs which were thought not to affect plasma lipid levels were still enrolled in this study. Subjects with renal or hepatic dysfunction, active inflammatory disease, significant valvular disease, myocarditis, and malignant disease were also excluded from the study. All the subjects were Chinese Han people. The Han people are the largest ethnic group in East Asia, constituting approximately 92 % of the population of Mainland China, 98 % of the population of Taiwan, and 74 % of the population of Singapore. The tenets of the Declaration of Helsinki were adhered to in all the procedures reported in the article. The study protocol was reviewed and approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College. Signed informed consent was provided by all the participants or their guardians prior to their participation in the study.
Definitions
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III), dyslipidemia was defined as the presence of one or more of the following conditions: TG ≥ 2.26 mmol/L, TC ≥ 6.22 mmol/L, LDL-C ≥ 4.14 mmol/L and HDL-C < 1.04 mmol/L [32]. Smoking was defined as regular cigarette smoking. Hypertension was defined as the measurement of systolic/diastolic blood pressure higher than 140/90 mmHg or active use of antihypertensive drugs. Diabetes mellitus was defined as the fasting glucose levels above 126 mg/dL or active use of antidiabetic drugs or insulin. Body mass index (BMI) was calculated by dividing weight by height squared (kg/m2).
Biochemical measurement
Fasting blood samples were taken on the first morning of the in-hospital day when no lipid-lowering drugs were used. Samples were immediately shipped to the Department of Clinical Laboratory of the Affiliated Hospital of North Sichuan Medical College for measurement of plasma lipids. TG, TC, LDL-C and HDL-C were measured directly by enzymatic methods. ApoB, apoAI and Lp(a) were measured by immunoturbidimetric assays. All the measurements were carried out using an automatic clinical chemistry analyzer (Beckman Coulter AU5800, USA). The lipoprotein ratios were also calculated.
Diagnosis of CHD and evaluation of the severity of CHD
CHD was diagnosed in the patients who had angiographic evidence of stenosis greater than 50 % in at least one major coronary artery. Those with normal coronary arteries, coronary atherosclerosis, or minimal stenosis (less than 50 %) in any of the major coronary arteries were considered as CHD-free control subjects. Two experienced cardiologists who were unaware of the clinical history and laboratory results of the subjects performed the coronary angiography. Standard coronary angiography with at least four views of the left coronary system and two views of the right coronary artery was performed using the Judkins technique by Allura Xper FD20 (Philips Medical Systems Nederland B.V. Netherlands). The Gensini scoring system [33] was used to assess the severity of CHD. In this system, the lumen narrowing of coronary arteries is graded as 1 point for 1–25 % narrowing, 2 points for 26–50 % narrowing, 4 points for 51–75 % narrowing, 8 points for 76–90 % narrowing, 16 points for 91–99 % narrowing, and 32 points for complete occlusion. Each score is then multiplied by a factor that takes into account the importance of the lesion’s position in the coronary arterial tree, for example, 5 points for the left main coronary artery, 2.5 points for the proximal left anterior descending branch (LAD) or proximal left circumflex branch (LCX), 1.5 points for the middle LAD or middle LCX, and 1 point for the distal LAD, distal LCX or right coronary arteries. The Gensini score was calculated as the sum of the scores for all coronary arteries.
Statistical analysis
Continuous data were presented as mean ± standard deviation (SD) unless otherwise stated. Continuous variables were tested for normality, and log transformation was conducted for those which did not conform to a normal distribution. The differences between the CHD patients and the CHD-free subjects, the dyslipidemic subjects and the non-dyslipidemic subjects, or among the subjects with different genotypes were calculated by Chi-square test for categorical variables, and one-way ANOVA analysis for continuous variables. The Gensini score levels among the patients with different genotypes were compared by one-way ANOVA analysis. The P values were adjusted by using the Bonferroni correction in multiple comparisons of the variables among the rs662799 genotypes. The associations between the rs662799 polymorphism and the other potential CHD risk factors and the Gensini scores were analyzed by univariate and stepwise multivariate linear regression analyses; the variables with a P value less than 0.1 in univariate analysis were taken into the multivariate analysis. The results of regression analysis were presented as Beta with 95 % confidence interval (95 % CI). All P values were two-tailed and the differences were considered as significant if P ≤ 0.05. All statistical analyses were done by using 13.0 version SPSS (Chicago, IL, USA).
Acknowledgements
This research was supported by the grants from the Basic and Applied Research Project of Sichuan Province, China (2013JY0072) and the Key Cultivation Project of North Sichuan Medical College, Sichuan Province, China (CBY12-A-ZP06).
Authors’ contributions
YYS, YMW and ZL conceived of the study, participated in the design, and drafted the manuscript. ZL and XMZ conducted the coronary angiography. YYS and JS performed the statistical analysis. JXZ, YMW and YY collected the data. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Abbreviations
- ApoAI
Apolipoprotein AI
- ApoB100
Apolipoprotein B100
- BMI
Body mass index
- CHD
Coronary heart disease
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- Lp(a)
Lipoprotein (a)
- TC
Total cholesterol
- TG
Triglycerides
References
- 1.Bi Y, Jiang Y, He J, Xu Y, Wang L, Xu M, et al. Status of cardiovascular health in Chinese adults. J Am Coll Cardiol. 2015;65:1013–25. doi: 10.1016/j.jacc.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Simpson HC, Mann JI, Meade TW, Chakrabarti R, Stirling Y, Woolf L. Hypertriglyceridaemia and hypercoagulability. Lancet. 1983;1:786–90. doi: 10.1016/S0140-6736(83)91849-4. [DOI] [PubMed] [Google Scholar]
- 4.Lange LA, Willer CJ, Rich SS. Recent developments in genome and exome-wide analyses of plasma lipids. Curr Opin Lipidol. 2015;26:96–102. doi: 10.1097/MOL.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 5.Gombojav B, Lee SJ, Kho M, Song YM, Lee K, Sung J. Multiple susceptibility loci at chromosome 11q23.3 are associated with plasma triglyceride in East Asians. J Lipid Res. 2016;57:318–24. doi: 10.1194/jlr.P063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forte TM, Ryan RO. Apolipoprotein A5: extracellular and intracellular roles in triglyceride metabolism. Curr Drug Targets. 2015;16:1274–80. doi: 10.2174/1389450116666150531161138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–73. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 8.Priore Oliva C, Pisciotta L, Li Volti G, Sambataro MP, Cantafora A, Bellocchio A, et al. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2005;25:411–7. doi: 10.1161/01.ATV.0000153087.36428.dd. [DOI] [PubMed] [Google Scholar]
- 9.Marçais C, Verges B, Charrière S, Pruneta V, Merlin M, Billon S, et al. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J Clin Invest. 2005;115:2862–9. doi: 10.1172/JCI24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaap FG, Rensen PC, Voshol PJ, Vrins C, van der Vliet HN, Chamuleau RA, et al. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J Biol Chem. 2004;279:27941–7. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 11.Vu-Dac N, Gervois P, Jakel H, Nowak M, Bauge E, Dehondt H, et al. Apolipoprotein A5, a crucial determinant of plasma triglyceride levels, is highly responsive to peroxisome proliferator-activated receptor alpha activators. J Biol Chem. 2003;278:17982–5. doi: 10.1074/jbc.M212191200. [DOI] [PubMed] [Google Scholar]
- 12.Halalkhor S, Jalali F, Tilaki KH, Shojaei S. Association of two common polymorphisms of apolipoprotein A5 gene with metabolic syndrome indicators in a North Iranian population, a cross-sectional study. J Diabetes Metab Disord. 2014;13:48. doi: 10.1186/2251-6581-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundl I, Guardiola M, Khoschsorur G, Solà R, Vallvé JC, Godàs G, et al. Increased concentrations of circulating vitamin E in carriers of the apolipoprotein A5 gene - 1131T>C variant and associations with plasma lipids and lipid peroxidation. J Lipid Res. 2007;48:2506–13. doi: 10.1194/jlr.M700285-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Komurcu-Bayrak E, Onat A, Poda M, Humphries SE, Palmen J, Guclu F, et al. Gender-modulated impact of apolipoprotein A5 gene (APOA5) -1131T>C and c.56C>G polymorphisms on lipids, dyslipidemia and metabolic syndrome in Turkish adults. Clin Chem Lab Med. 2008;46:778–84. doi: 10.1515/CCLM.2008.161. [DOI] [PubMed] [Google Scholar]
- 15.Song KH, Yu SG, Cha S, Kim JY. Association of the Apolipoprotein A5 Gene -1131T > C Polymorphism with Serum Lipids in Korean Subjects: Impact of Sasang Constitution. Evid Based Complement Alternat Med. 2012;2012:598394. doi: 10.1155/2012/598394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu LA, Ko YL, Chang CJ, Hu CF, Wu S, Teng MS, et al. Genetic variations of apolipoprotein A5 gene is associated with the risk of coronary artery disease among Chinese in Taiwan. Atherosclerosis. 2006;185:143–9. doi: 10.1016/j.atherosclerosis.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Li GP, Wang JY, Yan SK, Chen BS, Xue H, Wu G. Genetic effect of two polymorphisms in the apolipoprotein A5 gene and apolipoprotein C3 gene on serum lipids and lipoproteins levels in a Chinese population. Clin Genet. 2004;65:470–6. doi: 10.1111/j.1399-0004.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Matsuo H, Warita S, Watanabe S, Kato K, Oguri M, et al. Prediction of genetic risk for dyslipidemia. Genomics. 2007;90:551–8. doi: 10.1016/j.ygeno.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Aouizerat BE, Kulkarni M, Heilbron D, Drown D, Raskin S, Pullinger CR, et al. Genetic analysis of a polymorphism in the human apoA-V gene: effect on plasma lipids. J Lipid Res. 2003;44:1167–73. doi: 10.1194/jlr.M200480-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Charriere S, Bernard S, Aqallal M, Merlin M, Billon S, Perrot L, et al. Association of APOA5 -1131T > C and S19W gene polymorphisms with both mild hypertriglyceridemia and hyperchylomicronemia in type 2 diabetic patients. Clin Chim Acta. 2008;394:99–103. doi: 10.1016/j.cca.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW, Ayyobi AF, Frohlich JJ, Hill JS. APOA5 gene polymorphism modulates levels of triglyceride, HDL cholesterol and FERHDL but is not a risk factor for coronary artery disease. Atherosclerosis. 2004;176:165–72. doi: 10.1016/j.atherosclerosis.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Zhang S, Lin J, Li H, Huang A, Xiao C, et al. Association between DNA variant sites in the apolipoprotein A5 gene and coronary heart disease in Chinese. Metabolism. 2005;54:568–72. doi: 10.1016/j.metabol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Szalai C, Keszei M, Duba J, Prohászka Z, Kozma GT, Császár A, et al. Polymorphism in the promoter region of the apolipoprotein A5 gene is associated with an increased susceptibility for coronary artery disease. Atherosclerosis. 2004;173:109–14. doi: 10.1016/j.atherosclerosis.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi F, Isono M, Katsuya T, Yokota M, Yamamoto K, Nabika T, et al. Association of genetic variants influencing lipid levels with coronary artery disease in Japanese individuals. PLoS One. 2012;7:e46385. doi: 10.1371/journal.pone.0046385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinelli N, Trabetti E, Bassi A, Girelli D, Friso S, Pizzolo F, et al. The -1131 T>C and S19W APOA5 gene polymorphisms are associated with high levels of triglycerides and apolipoprotein C-III, but not with coronary artery disease: an angiographic study. Atherosclerosis. 2007;191:409–17. doi: 10.1016/j.atherosclerosis.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Ye H, Zhou A, Hong Q, Tang L, Xu X, Xin Y, et al. Positive Association between APOA5 rs662799 Polymorphism and Coronary Heart Disease: A Case–control Study and Meta-Analysis. PLoS One. 2015;10:e0135683. doi: 10.1371/journal.pone.0135683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen B, Zhao W, Zheng Y, Chen X, Xu W, Li S. An Association Study Identifies Two Single Nucleotide Polymorphisms on Chromosome 11q23.3 as a Risk Locus for Acute Myocardial Infarction in the Chinese Han Population. Clin Lab. 2015;61:1609–16. doi: 10.7754/clin.lab.2015.150331. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki M, Mutombo PB, Iwamoto M, Nogi A, Hashimoto M, Nabika T, et al. The interaction of Apolipoprotein A5 gene promoter region T-1131C polymorphism (rs12286037) and lifestyle modification on plasma triglyceride levels in Japanese. Nutr Res Pract. 2015;9:379–84. doi: 10.4162/nrp.2015.9.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Xu L, Huang RS, Huang Y, Le Y, Jiang D, et al. Apolipoprotein A5 gene variants and the risk of coronary heart disease: a case–control study and meta-analysis. Mol Med Rep. 2013;8:1175–82. doi: 10.3892/mmr.2013.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang R, Kim M, Chae JS, Lee SH, Lee JH. Consumption of whole grains and legumes modulates the genetic effect of the APOA5 -1131C variant on changes in triglyceride and apolipoprotein A-V concentrations in patients with impaired fasting glucose or newly diagnosed type 2 diabetes. Trials. 2014;15:100. doi: 10.1186/1745-6215-15-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang Y, Paik JK, Hyun YJ, Chae JS, Kim JY, Choi JR, et al. The apolipoprotein A5–1131T > C promoter polymorphism in Koreans: association with plasma APOA5 and serum triglyceride concentrations, LDL particle size and coronary artery disease. Clin Chim Acta. 2009;402:83–7. doi: 10.1016/j.cca.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Cholesterol Education Program (NCEP) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 33.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/S0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]


