Abstract
Introduction:
Transplant renal artery stenosis (TRAS) is a well-known vascular complication of renal transplantation. The aim of this analysis was to assess the short and midterm outcomes of endovascular therapy to salvage transplant kidney.
Methods:
We retrospectively analyzed our transplant database from 2000 to 2015. Percutaneous transluminal angioplasty/stenting was done in 24 patients (22 men and two women) with significant TRAS. The mean age was 59 ± 12 years. The parameters analyzed were: Technical success, pre- and post-treatment serum creatinine and number of antihypertensive drugs before and after treatment and vessel patency on Doppler ultrasonography at 3 and 6 months.
Results:
Overall incidence of TRAS in this study was 5.06%. Incidence of TRAS following live donor transplantation was 4.68% while that in deceased donors was 11.5%. Technical success was 100%. There were no periprocedural deaths. Renal function was improved from 2.32 ± 0.5 mg/dL to 1.72 ± 0.3 mg/dL (P < 0.001) and number of antihypertensive medications after the procedure was reduced from 2.9 ± 0.7 to 2 ± 0.6 (P < 0.001) at 6 months follow-up. One patient developed restenosis within 5 months (4.2%). Clinical success at 6 months follow-up was 79.2%.
Conclusions:
Endovascular treatment of TRAS has high technical success with minimal complications. It also provides satisfactory clinical success with improvement in overall transplant renal function and renovascular hypertension in early follow-up.
Key words: Angioplasty and stenting, endovascular treatment, renovascular hypertension, transplant renal artery stenosis
INTRODUCTION
Transplant renal artery stenosis (TRAS) is a reversible cause of graft dysfunction which is manifest by refractory hypertension or rising serum creatinine.[1] It is a rare vascular complication with incidence varying between 1% and 23%.[2,3,4,5] It usually becomes apparent between 3 months and 2 years after renal transplantation, but it can present at any time.[6] The stenosis can be preanastomotic because of the recipient's atherosclerotic arterial disease, at the anastomotic site due to surgical trauma along with postoperative fibrosis, or postanastomotic whose etiology is not well defined, though mechanical and immunological factors are implicated as possible causes.[7]
The gold standard for diagnosis and treatment of TRAS is percutaneous transluminal angiography and angioplasty (PTA).[8,9] We retrospectively reviewed our series of patients who had undergone PTA, with or without stenting, for suspected TRAS and report the early outcomes of the intervention.
METHODS
Between 2000 and 2015, 474 patients underwent renal transplantation (live donor – 448; deceased donor – 26) at our institution. A retrospective analysis of our transplant database was performed for this study. All patients who underwent intervention for a suspected TRAS on the basis of sudden increase in blood pressure from baseline, refractory hypertension, or raised serum creatinine were included in this report. The patients were screened with Doppler ultrasonography (USG) and those with peak systolic velocities (PSVs) >200 cm/s were further subjected to magnetic resonance angiography (MRA) to confirm the diagnosis.
We collected data concerning the patient's characteristics (age, gender, comorbidities), organ donors characteristics, type of anastomosis, clinical presentation, time to presentation, number and location of stenotic lesions relative to anastomosis, type of procedure (angioplasty with or without stenting), early and late outcomes, and serum creatinine levels, blood pressure value, and number of antihypertensive drugs before and after the procedure.
Informed consent was obtained from all patients before the intervention. Diagnostic renal arteriography was performed through a contralateral femoral arterial approach. Under fluoroscopy, 7 Fr crossover sheath was placed over a 0.035-inch hydrophilic guide wire. Nonselective iliac arteriography was performed to look for preanastomotic lesion (pseudo-TRAS). Size, extent, and level of stenosis were noted. Patients with significant TRAS (>70% reduction in diameter) underwent PTA with or without stenting. A guide wire was advanced across the stenosis and angioplasty was performed using 2–4 mm × 20 mm low-profile balloon. Subsequently, stenting was performed using balloon-expandable stents [Figure 1]. A check angiogram was performed to look for stent position and residual stenosis. Technical success was defined as residual stenosis <30% without dissection or extravasation after revascularization.
Figure 1.
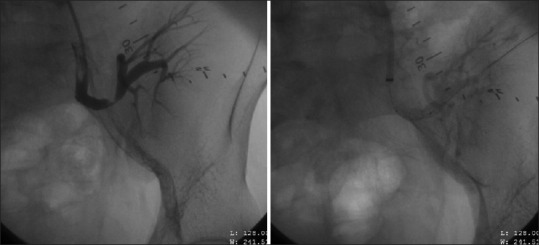
Selective left internal iliac angiogram confirms stenosis at anastomotic site. Second image demonstrates patent renal artery after percutaneous transluminal angioplasty and stenting
Before discharge, a Doppler USG was performed, and serum creatinine levels were documented. Follow-up protocol consisted of serial evaluation of serum creatinine, measurement of blood pressure and Doppler USG at 1 month, 3 months, and 6 months and at any time if recurrence was suspected. All patients were followed for minimum of 6 months. Restenosis was considered significant if PSVs were >200 cm/s on Doppler USG and/or >50% residual stenosis on MRA.
The primary end point of this study was stenosis-free primary transplant renal artery patency, and secondary end points were freedom from reintervention, graft survival, blood pressure, and renal function evolution. Clinical success at 6 months follow-up was defined as (i) more than a 15% reduction in serum creatinine level and/or (ii) reduction in a number of antihypertensive medications.
The serum creatinine values before and after treatment of TRAS with PTA were compared by using a paired two-tailed Student's t-test. The number of blood pressure medications was compared with the use of the Wilcoxon signed ranks test. Statistical Package for the Social Sciences software (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.) was used for analysis. A P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Twenty-four (5.06%) patients (22 male, 2 female; mean age 59 ± 12 years) were diagnosed to have TRAS. Their demographics and clinical details are summarized in Table 1. The mean donor age was 44 ± 8 years. 87.5% (21/24) patients had undergone live donor renal transplantation and 12.5% (3/24) patients had received their renal grafts from deceased donors.
Table 1.
Transplant demographics and clinical details
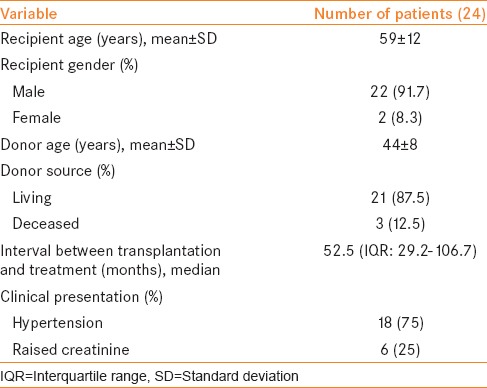
Eighteen patients presented with sudden increase in blood pressure from baseline or refractory hypertension while six patients had raised serum creatinine. Doppler USG demonstrated increased PSV of 315 ± 40 cm/s. Median time from renal transplantation to presentation was 52.5 months (interquartile range [IQR] 29.2–106.7 months). All patients had single renal artery anastomosed in end-to-end fashion to internal iliac artery.
Angiography and procedure details are compiled in Table 2. Nineteen patients had stenosis at the anastomotic site for which angioplasty and stenting were performed in all patients except in one patient where only angioplasty was performed, and stenting was not possible due to complexity of the lesion (kink at anastomotic site). This patient had received a deceased donor graft, and he developed restenosis within 5 months and was managed conservatively. Two patients had isolated stenosis proximal to the anastomotic site for which angioplasty and stenting was performed.
Table 2.
Stenosis characteristics and procedure details

Three patients had stenosis at both anastomotic site and proximal to the anastomosis. In two patients, angioplasty was performed at the proximal site and a stent was placed at the anastomotic site. In one, calcified atheromatous debris was dislodged and migrated distally which was aspirated immediately. The third patient underwent stenting at both the anastomotic and preanastomotic sites. Procedure-related complications, i.e., dislodged atheromatous debris were seen in one patient (4.17%). Technical success rate was 100% and patency rate at 6 months follow-up was 95.8% as one patient developed restenosis after 5 months.
The outcomes of the procedure are shown in Table 3. The mean serum creatinine decreased from preprocedure value of 2.32 ± 0.5 mg/dL to 1.63 ± 0.4 mg/dL (P < 0.001) at 3 months and 1.72 ± 0.3 mg/dL (P < 0.001) at 6 months [Figure 2]. A reduction in serum creatinine of >15% was seen in 23 patients (95.8%) after 3 months and 20 patients (83.3%) after 6 months. Before the procedure, the patients required a mean of 2.9 ± 0.7 antihypertensive agents which decreased to 1.8 ± 0.6 agents (P < 0.001) at 3 months and 2 ± 0.6 agents (P < 0.001) at 6 months [Figure 3]. A decrease in number of anti-hypertensive agents was noted in 23 patients (95.8%) at 3 months and 20 (83.3%) patients at 6 months. Mean PSV decreased from 315 ± 40 cm/s to 164 ± 11 cm/s (P < 0.001) at 3 months and 181 ± 32 cm/s (P < 0.001) at 6 months. All 24 patients (100%) had improvement in PSV (<200 cm/s) at 3 months which decreased to 18 patients (75%) at 6 months.
Table 3.
Outcomes of the procedure
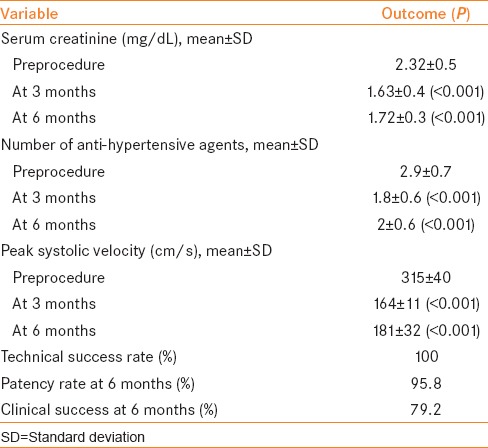
Figure 2.
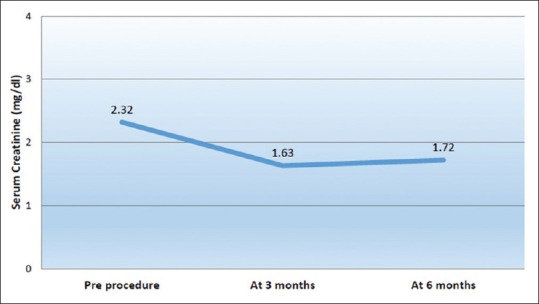
Evolution of serum creatinine
Figure 3.
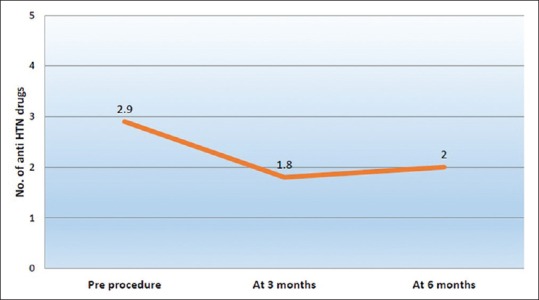
Mean number of antihypertensive drugs
Clinical success at 6 months follow-up was achieved in 79.2% patients (19/24). There were no procedure-related deaths, and there was no graft loss at 6 months follow-up.
DISCUSSION
The incidence of TRAS in this study was 5.06%. Depending on the definition and diagnostic techniques used, various published series report incidence ranging between 1% and 23%.[2,3,4,5] Several risk factors for TRAS have been reported including older recipient age, retrieval damage to renal artery, intimal damage at the time of perfusion, atheroma at the site of anastomosis,[10] faulty suture technique, disparity in diameters of donor and recipient arteries in end-to-end anastomosis,[11] and prolonged cold ischemia time, acute rejection,[12] and cytomegalovirus infection.[13] Some authors have reported higher incidence of TRAS in cadaveric donors (13.2–17.7%) than living donor renal transplant.[3,14,15] In this study, the incidence of TRAS in deceased donors was 11.5%.
Most patients present with accelerated hypertension with or without biochemical evidence of allograft dysfunction occurring between 3 months and 2 years after renal transplantation, but it can present at any time.[6] Median time to the presentation in this cohort was 52.5 months (IQR 29.2–106.7 months).
The Doppler USG criteria for diagnosis are PSV higher than 200 cm/s, resistive index <0.5, and velocity gradient between stenotic and prestenotic segment of more than 2:1.[16,17] We used Doppler USG (PSV > 200 cm/s) for initial diagnosis and for follow-up after endovascular intervention. In all patients diagnosis of TRAS was confirmed by MRA and PTA during the intervention (>70% luminal narrowing).
Two therapeutic approaches have been described for treatment of TRAS: Revision open surgery which is considered as rescue therapy and is reserved for patients with unsuccessful angioplasty or with complicated stenosis.[18] The success rate ranges from 63% to 92%, and the recurrence rate is around 12%. Surgery has high complication rates such as ureteral injury (14%), graft loss (15–20%), reoperation (13%), and mortality (5%).[6,19] PTA with stenting is now recognized as the treatment of choice. Technical success rate of endovascular treatment is reported between 89% and 100%[20,21,22,23,24] while clinical success being around 65.5–94%.[20,22,24] In our series, technical success was 100%, and clinical success was 79.2% at 6 months follow-up.
PTA alone carries higher risk of restenosis with an incidence ranging from 16% to 62%.[25] However, restenosis following PTA and stenting are low, and it varies from 5.5% to 20%.[26,27] In our series, restenosis rate was 4.2% at 6 months follow-up. In this patient, only angioplasty was performed, and stenting was not possible due to complexity of the lesion (kink at the anastomotic site) and later he was managed conservatively. Procedure related complication was seen in one patient (4.2%). In this patient, calcified atheromatous debris was dislodged and migrated distally after stenting. It was recognized immediately, and atheroma was aspirated. Check angiogram confirmed the patency of lumen.
We acknowledge certain limitations in this study. This was a retrospective study and could have a selection bias as some patients may have been inadvertently missed. There is the possibility of delayed failure of the procedure, and since the follow-up is only of 6 months, these events may not have been captured. Finally, the sample size is limited.
CONCLUSIONS
Endovascular treatment of TRAS has high technical success with minimal complications. It also provides satisfactory clinical success with improvement in overall transplant renal function and renovascular hypertension in early follow-up.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15:134–41. doi: 10.1097/01.asn.0000099379.61001.f8. [DOI] [PubMed] [Google Scholar]
- 2.Fervenza FC, Lafayette RA, Alfrey EJ, Petersen J. Renal artery stenosis in kidney transplants. Am J Kidney Dis. 1998;31:142–8. doi: 10.1053/ajkd.1998.v31.pm9428466. [DOI] [PubMed] [Google Scholar]
- 3.Lacombe M. Arterial stenosis complicating renal allotransplantation in man: A study of 38 cases. Ann Surg. 1975;181:283–8. doi: 10.1097/00000658-197503000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan HW, Ho YW, Chan CM, Yiu TF, Tong MK, Wong PH. Treatment of anastomotic ostial allograft and renal artery stenosis with the Palmaz stent. Transplantation. 1995;59:436–9. [PubMed] [Google Scholar]
- 5.Greenstein SM, Verstandig A, McLean GK, Dafoe DC, Burke DR, Meranze SG, et al. Percutaneous transluminal angioplasty. The procedure of choice in the hypertensive renal allograft recipient with renal artery stenosis. Transplantation. 1987;43:29–32. [PubMed] [Google Scholar]
- 6.Roberts JP, Ascher NL, Fryd DS, Hunter DW, Dunn DL, Payne WD, et al. Transplant renal artery stenosis. Transplantation. 1989;48:580–3. [PubMed] [Google Scholar]
- 7.Rengel M, Gomes-Da-Silva G, Incháustegui L, Lampreave JL, Robledo R, Echenagusia A, et al. Renal artery stenosis after kidney transplantation: Diagnostic and therapeutic approach. Kidney Int Suppl. 1998;68:S99–106. doi: 10.1038/sj.ki.4490573. [DOI] [PubMed] [Google Scholar]
- 8.Spinosa DJ, Isaacs RB, Matsumoto AH, Angle JF, Hagspiel KD, Leung DA. Angiographic evaluation and treatment of transplant renal artery stenosis. Curr Opin Urol. 2001;11:197–205. doi: 10.1097/00042307-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Koukoulaki M, Brountzos E, Loukopoulos I, Pomoni M, Antypa E, Vougas V, et al. Successful endovascular treatment of transplant intrarenal artery stenosis in renal transplant recipients: Two case reports. World J Transplant. 2015;5:68–72. doi: 10.5500/wjt.v5.i2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland RS, Spees EK, Jones JW, Fink DW. Renal artery stenosis after renal transplantation: The impact of the hypogastric artery anastomosis. J Urol. 1993;149:980–5. doi: 10.1016/s0022-5347(17)36273-0. [DOI] [PubMed] [Google Scholar]
- 11.Rijksen JF, Koolen MI, Walaszewski JE, Terpstra JL, Vink M. Vascular complications in 400 consecutive renal allotransplants. J Cardiovasc Surg (Torino) 1982;23:91–8. [PubMed] [Google Scholar]
- 12.Wong W, Fynn SP, Higgins RM, Walters H, Evans S, Deane C, et al. Transplant renal artery stenosis in 77 patients – Does it have an immunological cause? Transplantation. 1996;61:215–9. doi: 10.1097/00007890-199601270-00009. [DOI] [PubMed] [Google Scholar]
- 13.Pouria S, State OI, Wong W, Hendry BM. CMV infection is associated with transplant renal artery stenosis. QJM. 1998;91:185–9. doi: 10.1093/qjmed/91.3.185. [DOI] [PubMed] [Google Scholar]
- 14.Sankari BR, Geisinger M, Zelch M, Brouhard B, Cunningham R, Novick AC. Post-transplant renal artery stenosis: Impact of therapy on long-term kidney function and blood pressure control. J Urol. 1996;155:1860–4. doi: 10.1016/s0022-5347(01)66030-0. [DOI] [PubMed] [Google Scholar]
- 15.Halimi JM, Al-Najjar A, Buchler M, Birmelé B, Tranquart F, Alison D, et al. Transplant renal artery stenosis: Potential role of ischemia/reperfusion injury and long-term outcome following angioplasty. J Urol. 1999;161:28–32. doi: 10.1016/s0022-5347(01)62051-2. [DOI] [PubMed] [Google Scholar]
- 16.Snider JF, Hunter DW, Moradian GP, Castaneda-Zuniga WR, Letourneau JG. Transplant renal artery stenosis: Evaluation with duplex sonography. Radiology. 1989;172(3 Pt 2):1027–30. doi: 10.1148/172.3.1027. [DOI] [PubMed] [Google Scholar]
- 17.de Morais RH, Muglia VF, Mamere AE, Garcia Pisi T, Saber LT, Muglia VA, et al. Duplex Doppler sonography of transplant renal artery stenosis. J Clin Ultrasound. 2003;31:135–41. doi: 10.1002/jcu.10147. [DOI] [PubMed] [Google Scholar]
- 18.Merkus JW, Huysmans FT, Hoitsma AJ, Buskens FG, Skotnicki SH, Koene RA. Renal allograft artery stenosis: Results of medical treatment and intervention. A retrospective analysis. Transpl Int. 1993;6:111–5. doi: 10.1007/BF00336655. [DOI] [PubMed] [Google Scholar]
- 19.Benoit G, Moukarzel M, Hiesse C, Verdelli G, Charpentier B, Fries D. Transplant renal artery stenosis: Experience and comparative results between surgery and angioplasty. Transpl Int. 1990;3:137–40. doi: 10.1007/BF00355459. [DOI] [PubMed] [Google Scholar]
- 20.Peregrin JH, Stríbrná J, Lácha J, Skibová J. Long-term follow-up of renal transplant patients with renal artery stenosis treated by percutaneous angioplasty. Eur J Radiol. 2008;66:512–8. doi: 10.1016/j.ejrad.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Patel NH, Jindal RM, Wilkin T, Rose S, Johnson MS, Shah H, et al. Renal arterial stenosis in renal allografts: Retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001;219:663–7. doi: 10.1148/radiology.219.3.r01jn30663. [DOI] [PubMed] [Google Scholar]
- 22.Pappas P, Zavos G, Kaza S, Leonardou P, Theodoropoulou E, Bokos J, et al. Angioplasty and stenting of arterial stenosis affecting renal transplant function. Transplant Proc. 2008;40:1391–6. doi: 10.1016/j.transproceed.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Ghazanfar A, Tavakoli A, Augustine T, Pararajasingam R, Riad H, Chalmers N. Management of transplant renal artery stenosis and its impact on long-term allograft survival: A single-centre experience. Nephrol Dial Transplant. 2011;26:336–43. doi: 10.1093/ndt/gfq393. [DOI] [PubMed] [Google Scholar]
- 24.Beecroft JR, Rajan DK, Clark TW, Robinette M, Stavropoulos SW. Transplant renal artery stenosis: Outcome after percutaneous intervention. J Vasc Interv Radiol. 2004;15:1407–13. doi: 10.1097/01.RVI.0000141338.62574.F4. [DOI] [PubMed] [Google Scholar]
- 25.Audard V, Matignon M, Hemery F, Snanoudj R, Desgranges P, Anglade MC, et al. Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant. 2006;6:95–9. doi: 10.1111/j.1600-6143.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 26.Su CH, Lian JD, Chang HR, Wu SW, Chen SC, Tsai CF, et al. Long-term outcomes of patients treated with primary stenting for transplant renal artery stenosis: A 10-year case cohort study. World J Surg. 2012;36:222–8. doi: 10.1007/s00268-011-1312-3. [DOI] [PubMed] [Google Scholar]
- 27.Voiculescu A, Schmitz M, Hollenbeck M, Braasch S, Luther B, Sandmann W, et al. Management of arterial stenosis affecting kidney graft perfusion: A single-centre study in 53 patients. Am J Transplant. 2005;5:1731–8. doi: 10.1111/j.1600-6143.2005.00927.x. [DOI] [PubMed] [Google Scholar]


