Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
PF4/heparin ultra-large complexes activate complement and bind preferentially to B cells via CR2 (CD21).
Complement-fixed PF4/heparin complexes can be detected on circulating B cells in patients receiving heparin therapy.
Abstract
Heparin-induced thrombocytopenia is a prothrombotic disorder caused by antibodies to platelet factor 4 (PF4)/heparin complexes. The mechanism that incites such prevalent anti-PF4/heparin antibody production in more than 50% of patients exposed to heparin in some clinical settings is poorly understood. To investigate early events associated with antigen exposure, we first examined the interaction of PF4/heparin complexes with cells circulating in whole blood. In healthy donors, PF4/heparin complexes bind preferentially to B cells (>90% of B cells bind to PF4/heparin in vitro) relative to neutrophils, monocytes, or T cells. Binding of PF4 to B cells is heparin dependent, and PF4/heparin complexes are found on circulating B cells from some, but not all, patients receiving heparin. Given the high proportion of B cells that bind PF4/heparin, we investigated complement as a mechanism for noncognate antigen recognition. Complement is activated by PF4/heparin complexes, co-localizes with antigen on B cells from healthy donors, and is present on antigen-positive B cells in patients receiving heparin. Binding of PF4/heparin complexes to B cells is mediated through the interaction between complement and complement receptor 2 (CR2 [CD21]). To the best of our knowledge, these are the first studies to demonstrate complement activation by PF4/heparin complexes, opsonization of PF4/heparin to B cells via CD21, and the presence of complement activation fragments on circulating B cells in some patients receiving heparin. Given the critical contribution of complement to humoral immunity, our observations provide new mechanistic insights into the immunogenicity of PF4/heparin complexes.
Introduction
Immune responses to heparin are common and include the well-recognized complication of heparin-induced thrombocytopenia (HIT), a prothrombotic disorder caused by antibodies to complexes of platelet factor 4 (PF4) and heparin. It is not known how heparin and PF4, which individually are host constituents, become recognized as non-self when combined in vivo. Previous studies have shown that ultra-large complexes (ULCs) formed through electrostatic interactions of PF4 and heparin elicit T-cell–dependent immune responses in vivo. In murine models, PF4/heparin ULCs formed at certain molar ratios of PF4:heparin initiate antibody production, whereas PF4 itself or ULCs formed with excess heparin are rarely associated with antibody formation.1
How the immune system responds to a subset of PF4/heparin complexes is uncertain. Multivalent antigens can trigger adaptive, T-cell–mediated immunity by activating generalized host-defense mechanisms through cell-surface pattern recognition receptors and/or humoral pattern recognition molecules.2 To date, only 2 studies have examined the role of pattern recognition receptors in the pathogenesis of HIT. In mice, anti-PF4/heparin antibody formation does not require TLR/MyD88 signaling, because MyD88-deficient and wild-type mice have comparable rates of seroconversion.3 In the other study, TLR4, which signals by both MyD88-dependent and MyD88-independent mechanisms, has been implicated in HIT immune activation through effects on interleukin-8 production.4
Far less is known about the contribution of humoral-based molecules, such as complement, in the initiation of the PF4/heparin immune response. The complement system is a tightly regulated innate host defense mechanism that is rapidly activated by molecular patterns found on invading pathogens. In addition to its primary role in clearance and destruction of microorganisms, complement also subserves essential functions in adaptive immunity.5 Transient depletion of complement6 or targeted disruption of genes that encode complement proteins7 impair T-cell–dependent antibody responses. This costimulatory effect is primarily transacted by complement receptors (CRs) expressed on B cells.5 Indeed, binding of complement-coated antigen to B-cell CR2 (CD21) enhances the immunogenicity of some antigens 1000 to 10 000-fold.8
We undertook these studies to examine the role of complement in the immune response to PF4/heparin complexes. The studies we discuss in this article show marked preferential binding of PF4/heparin ULCs to B cells compared with other leukocytes in whole blood, heparin-dependent binding of PF4/heparin complexes to B cells in vitro and in vivo, complement activation by PF4/heparin complexes, and binding of complement activation fragments onto circulating B cells in patients receiving heparin. We also identify a critical role for CD21 in binding PF4/heparin complexes to B cells. Together, these findings identify a previously unrecognized pathway that likely contributes to the immunogenicity of PF4/heparin complexes.
Methods
Materials and cell lines
Recombinant human PF4 was purified as previously described.9 Studies were performed by using unfractionated heparin (UFH; Elkins-Sinn Inc.), low molecular weight heparin (LMWH; Sanofi-Aventis Pharmaceuticals), and fondaparinux (GlaxoSmithKline). Lymphoblastic cell lines Ramos, Raji, and P3HR-1 and the acute lymphocytic leukemia cell line, Reh, were purchased through a license agreement from the Duke Cell Culture Facility. Unless specified, chemicals and tissue culture reagents for preparing buffers were purchased from Sigma.
Blood samples
Blood from healthy donors or patients receiving heparin therapy was collected into sodium citrate or acid-citrate dextrose-A (1:7 volume) with written consent following an institutional review board–approved protocol (Duke Institutional Review Board #Pro00012901). Human patients were enrolled in the study in accordance with the Declaration of Helsinki. Where indicated, studies were performed in whole blood or in 100% plasma from healthy donors.
Studies of PF4/ heparin and protamine/heparin complex binding and flow cytometry
For a detailed description/schema describing whole blood studies of healthy donor or patient samples, see the supplemental Data, available on the Blood Web site. In brief, whole blood was incubated with buffer or with antigens (PF4 or protamine [PRT] with or without UFH, LMWH, or fondaparinux) at 37°C for 1 hour or as indicated. Unless specified, PF4 and heparin concentrations were 25 µg/mL and 0.25 U/mL, respectively, and PRT and heparin concentrations were 100 µg/mL and 10 U/mL, respectively. To block CD21, blood was incubated with 10 µg/mL sheep anti-human CD21 (R&D Systems) or isotype control antibody at room temperature for 20 minutes and then incubated with PF4 with or without heparin as described above. Red blood cells (RBCs) were then lysed with a 10-fold excess of ammonium chloride-potassium lysing buffer for 5 minutes at room temperature. Patient samples were processed for RBC lysis without the addition of PF4 or heparin. After RBC lysis, cells were centrifuged and washed in 2% v/v fetal bovine serum in phosphate-buffered saline. To block Fc receptors, cells were first incubated with intravenous immunoglobulin (Grifols) at 4°C for 10 minutes, followed by incubation with fluorescently labeled or isotype control antibodies for 30 minutes at 4°C for surface staining. One or more biotin-labeled or fluorescently labeled antibodies were used in the studies discussed below: KKO (anti-hPF4/heparin10), biotinylated anti-C3c (Quidel), biotinylated anti-C4c (My BioSource), anti-CD19 (B cells), anti-CD3 (T cells), anti-CD66b (neutrophils), anti-CD14 (monocytes), and anti-CD41 (platelets). Details regarding fluorescent markers and sources of antibodies are provided in supplemental Data. For studies that used lymphoblastoid cell lines, cells (1 × 106/mL) were incubated in 100% plasma or 100% plasma supplemented with antigen and analyzed by flow cytometry. Cells were analyzed by using a BD FACS Canto Flow cytometer (BD Biosciences). Signals from a minimum of 10 000 cells were acquired from each sample. Analyses were performed by using FCS Express software, Version 4.07.0011 (De Novo Software).
Image analysis
Cell specificity of PF4/heparin binding was confirmed with ImageStream under conditions similar to those described for flow cytometry. Events were acquired by the Amnis ImageStreamX Mark II Imaging Flow Cytometer (EMD Millipore) using ×60 magnification and the manufacturer’s software (Inspire). Data from a minimum of 20 000 cells were acquired for each experiment and analyzed with IDEAS Application v6.2 (EMD Millipore).
Studies of complement activation
Cells and plasma were separated from whole blood by centrifugation and reconstituted according to the experimental conditions outlined below. To inactivate complement, plasma was heat-inactivated at 56°C for 30 minutes. Pelleted cells were washed twice with serum-free RPMI and re-mixed with separated normal plasma or heat-inactivated plasma (at a 1:1.5 ratio) followed by incubation with buffer or 10 mM EDTA for 10 minutes at room temperature to block complement. Re-mixed plasma and cells were then incubated in the presence or absence of antigen (PF4 with or without heparin) for 1 hour at 37°C or at 0°C followed by staining with antibody. To determine the sequence of complement activation and PF4/heparin binding to B cells, plasma was first incubated with 10 mM EDTA with or without PF4/heparin for 30 minutes, followed by the addition of blood cells for 30 minutes. Alternatively, antigen was added to plasma at 37°C for 30 minutes followed by addition of 10 mM EDTA and blood cells for 30 minutes.
Statistics
Data are expressed as mean ± standard deviation. Significance was calculated by using Student t test. Statistical analyses were performed by using GraphPad Prism (Graph Pad Software, Version 5.02).
Results
PF4/heparin complexes bind preferentially to peripheral blood B cells
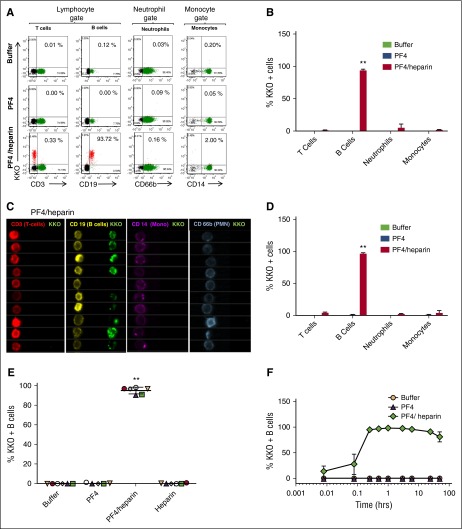
On the basis of recent findings showing monocyte uptake of PF4/heparin complexes,11 we asked if other antigen-presenting cells, such as B cells, interact with PF4/heparin ULCs in whole blood. To accomplish this, we added unlabeled PF4/heparin complexes to whole blood and measured binding of PF4/heparin complexes to various leukocyte populations by using fluorescently labeled KKO, a murine monoclonal antibody to hPF4/heparin complexes10 and cell-specific markers. PF4/heparin complexes bound to >90% of B cells as measured by KKO binding but to <1% of T cells, ∼2% of monocytes, and <1% of neutrophils (Figure 1A, bottom panel) compared to the absence of binding of PF4 alone or buffer (Figure 1B). To confirm cell specificity, we used an alternate imaging modality, ImageStream, an imaging flow cytometer that renders cell-surface images of fluorescently bound antibodies.12 Imaging analysis confirmed similar binding patterns of PF4/heparin complexes (Figure 1C). Similar to findings in Figure 1A-B, ImageStream analysis confirmed KKO binding to B cells in the presence of PF4/heparin complexes but not PF4 alone or buffer (Figure 1D; supplemental Figure 1A). Binding of PF4/heparin could not be attributed to heterocellular complexes with platelet-bound PF4, because platelets were not found in association with B cells (supplemental Figure 1B) nor did they bind PF4/heparin complexes in whole blood directly (data not shown). A similar pattern of PF4/heparin binding was seen with B cells from every healthy donor studied (Figure 1E). To ensure that B-cell binding was indeed due to PF4/heparin and not unique to the binding characteristics of the monoclonal antibody KKO, we performed a series of experiments involving directly labeled antigen (PF4/heparin-fluorescein isothiocyanate [FITC]), HIT immunoglobulin G (IgG), and a monoclonal antibody to PF4 (RTO10). Similar results were seen whether B-cell binding was measured with PF4/heparin-FITC complexes (supplemental Figure 2A) or with HIT antibodies (supplemental Figure 2B). PF4 alone, as indicated by RTO binding, does not bind to monocytes, neutrophils, or platelets at the concentrations we studied (data not shown).
Figure 1.
PF4/heparin complexes bind preferentially to B cells in the peripheral blood. Whole blood from healthy donors was incubated with buffer or antigen (PF4 with or without heparin) followed by staining for cell-specific markers and KKO. Mean ± standard deviation (SD) of 3 independent experiments is shown. (A) Flow cytometric analyses of peripheral blood leukocytes. Leukocyte subpopulations were defined by forward scatter and side scatter characteristics (lymphocytes, neutrophils, and monocytes). Gated subpopulations were identified by cell-surface markers for T lymphocytes (CD3), B lymphocytes (CD19), neutrophils (CD66b), and monocytes (CD14) on the x-axis and for KKO staining on the y-axis for each antigen. The percentage of KKO-positive cells for each cell lineage appears in the upper right corner of each graph. (B) Graph of cell lineage–specific staining of flow data from (A). Binding of KKO to cell lineages incubated with antigen is shown. (C) ImageStream analysis of PF4/heparin binding to B cells. Cell-surface binding of PF4/heparin complexes is shown by imaging flow cytometry using fluorescently labeled cell-surface markers (CD3-APC, CD19-PE, CD14-APC, and CD66b-APC) and KKO-AF488 (green). For clarity, colors were reassigned to show T cells in red, B cells in yellow, monocytes in purple, and neutrophils in blue. (D) Graph of cell lineage–specific staining of ImageStream data from (C). (E) PF4/heparin complexes bind preferentially to B cells from multiple healthy donors. CD19+ cells were gated for KKO binding for various antigens. The graphs show percentage of KKO-positive B cells (mean ± SD) in various conditions from 6 healthy donors. Each colored symbol represents % KKO + B cells in an individual donor. (F) Kinetics of PF4/heparin binding to B cells. Whole blood was incubated with antigen for defined time points and stained with KKO/CD19. For each condition, time is shown on the x-axis and binding of KKO shown on the y-axis. **P < .005 compared with other data in graph.
To examine the kinetics of PF4/heparin binding to B cells, PF4/heparin complexes were incubated with whole blood for 30 seconds to 48 hours. Antigen binding to B cells was detected within 30 seconds of incubation, reached saturation within 30 minutes, and remained stable over the ensuing 24 hours (Figure 1F) without internalization (data not shown).
PRT/heparin complexes show similar selective binding to B cells
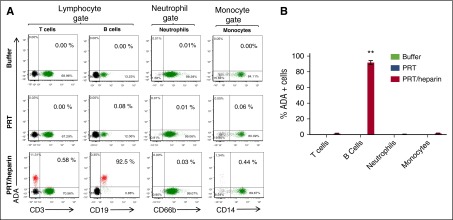
To examine whether binding of PF4/heparin complexes to B cells was generalizable to other protein/heparin ULCs, we performed similar experiments using PRT/heparin ULCs and ADA (G.M.L., Manali Joglekar, S.E.K., Rui Qi, L.R., and G.M.A., manuscript in preparation), which is a monoclonal antibody to PRT/heparin, or PRT/heparin-FITC. Similar to PF4/heparin complexes, PRT/heparin ULCs bound preferentially to B cells in the peripheral blood (Figure 2A-B), which was assessed by using ADA or PRT/heparin-FITC (data not shown). B-cell binding of PRT, like PF4, was also heparin dependent (data not shown). These studies show that binding of protein/heparin ULCs to B cells is a general property of heparin-containing complexes and is not antigen specific.
Figure 2.
PRT/heparin complexes show similar selective binding to B cells. Whole blood from a representative healthy donor was incubated with buffer or antigen (PRT with or without heparin) followed by staining for cell-specific markers and ADA, a monoclonal antibody to PRT/heparin complexes. Mean ± SD of 3 independent experiments is shown. (A) Flow cytometric analyses of peripheral blood leukocytes incubated with buffer, PRT, or PRT/heparin. Flow cytometry was performed on whole blood. Leukocyte subpopulations were defined by forward and side scatter characteristics (lymphocytes, neutrophils, and monocytes). Gated subpopulations were identified by cell-surface markers for T lymphocytes (CD3), B lymphocytes (CD19), neutrophils (CD66b), and monocytes (CD14) on the x-axis and for ADA staining on the y-axis for each antigen. The percentage of ADA-positive cells for each cell lineage appears in the upper right corner of each graph. (B) Graph of cell lineage–specific staining of flow data shown in (A). Binding of ADA to cell lineages incubated with antigen is shown. **P < .005 compared with other data in graph.
B-cell binding of PF4/heparin complexes is heparin dependent
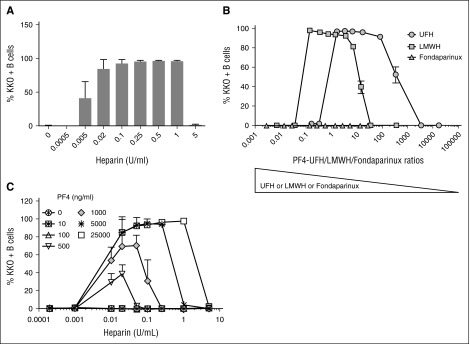
Formation of PF4/heparin ULCs shows heparin dependence.1,13-15 To determine whether B-cell binding of PF4/heparin complexes is also heparin dependent, we incubated whole blood with a fixed concentration of PF4 and varying concentrations of heparin. As shown in Figure 3A, binding of PF4/heparin to B cells showed marked dependence on heparin concentration. No binding was detected when PF4 was added alone or in the presence of low concentrations of heparin (<0.005 U/mL), but as the concentration of heparin was increased (0.005 to 1 U/mL), binding of KKO (PF4/heparin complexes) was markedly enhanced. Consistent with prior studies showing disruption of ULCs at high heparin concentrations,1,13-15 cell-surface binding of KKO was lost at heparin concentrations ≥5 U/mL. We next examined the impact of polysaccharide length on PF4 binding to B cells in the presence of UFH, LMWH, or the synthetic pentasaccharide fondaparinux. At a fixed concentration of PF4 (25 µg/mL), optimal binding of PF4 complexes containing UFH occurred at PF4:heparin molar ratios (PHRs) ranging from 1.5 to 100 (Figure 3B). By contrast, at this same PF4 concentration, binding of complexes containing LMWH occurred over a lower range of PHRs (0.15-7). This indicates that B-cell binding of PF4/LMWH complexes requires higher concentrations of LMWH than UFH, confirming previous observations on the effects of UFH and LMWH on formation of ULCs.14 Also consistent with prior observations,14 fondaparinux did not form complexes with PF4 that could be recognized by KKO.
Figure 3.
PF4/heparin complexes bind B cells in a heparin concentration–dependent manner. Whole blood was incubated with various concentrations of PF4 in combination with UFH, LMWH, or fondaparinux as indicated. Cells were stained with CD19 and KKO and the percentage of B cells binding with KKO under each condition is shown on the y-axis. (A) PF4/heparin binding to B cells is heparin dependent. Whole blood was incubated with a fixed amount of PF4 (25 µg/mL) and various concentrations of UFH (0-5 U/mL). Shown are the mean ± SD for percentage of KKO-positive B cells as a function of UFH concentration from 4 independent experiments. (B) B cell binding of complexes formed with PF4 and UFH, LMWH, or fondaparinux. Whole blood was incubated with a fixed amount of PF4 (25 µg/mL) and various concentrations of UFH, LMWH, or fondaparinux. Shown are the mean ± SD for percentage of KKO-positive B cells as a function of PF4 and UFH, LMWH, and fondaparinux molar ratios from 3 independent experiments. (C) B cell binding of PF4/heparin ULCs increases with PF4/heparin concentrations. Whole blood was incubated with increasing amounts of PF4 (0-25 000 ng/mL) and increasing UFH concentrations (0-5 U/mL). KKO binding for a given PF4 concentration is shown as a function of heparin concentration. Shown are the mean ± SD for percentage of KKO-positive B cells from 3 independent experiments.
We have previously shown that if the PHR is held constant, corresponding changes in the absolute amounts of PF4 and heparin alter the size of ULCs. For example, increasing PF4 (µg/mL):heparin (U/mL) concentrations from 100:2.5 to 200:5 while maintaining the molar ratio at 2.6:1 doubles the size of the ULCs.1 To determine whether B-cell binding of complexes is affected by ULC size, we varied the concentrations of PF4 (0-25 µg/mL) and heparin (0-5 U/mL). As shown in Figure 3C and supplemental Figure 3, maximal binding of KKO to B cells was linearly related to increased concentrations of PF4 and heparin. Binding to B cells occurred over a broad range of concentrations of PF4 (starting at 500 ng/mL) and heparin (starting at 0.01 U/mL). Binding of PF4/heparin complexes to B cells reached a maximum (>90% B cells) at PF4 concentrations ≥5 µg/mL. Together, these studies are consistent with the known biology of PF4/heparin interactions and demonstrate that cell-surface binding to B cells correlates with size and formation of ULCs in solution.
In situ generation of PF4/heparin complexes in whole blood
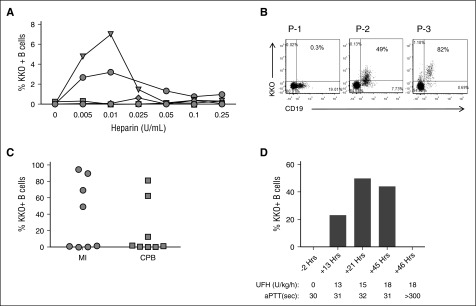
Low levels of PF4 are found on the platelet surface16,17 and in plasma as a consequence of physiologic clearance or intravascular activation.18,19 On the basis of this knowledge, we asked whether heparin could displace endogenous PF4 from the platelet surface to form PF4/heparin complexes that bind to B cells. As shown in Figure 4A, addition of UFH (0.005-0.25 U/mL) to whole blood displaces PF4 from other binding sites that combine to form complexes that bind B cells, although this was not seen with all donors.
Figure 4.
B cells bind PF4/heparin complexes ex vivo and in vivo. (A) Heparin displaces variable amounts of PF4 leading to B-cell binding of PF4/heparin complexes in some healthy subjects. Blood from 6 healthy donors was incubated with various concentrations of UFH as indicated. Binding of PF4/heparin complexes to B cells as assessed by binding of KKO is shown. (B) Detection of PF4/heparin complex bound circulating B cells in 3 patients receiving heparin. Blood was stained for CD19 and KKO. Dot plots show variation in binding of KKO to lymphocyte-gated populations. (C) Variations in KKO binding of PF4/heparin complexes to B cells in vivo in patients receiving UFH for medical indications (MI; n = 8) or for cardiopulmonary bypass surgery (CPB; n = 8). Each symbol represents B cell binding in an individual patient. The y-axis indicates maximal percentage of KKO-bound B cells. (D) In vivo B-cell binding is heparin dependent. A patient undergoing UFH therapy for treatment of cerebral venous thrombosis was monitored for percentage of KKO-positive B cells over 48 hours of UFH therapy. Time from start of heparin therapy, UFH dose, and activated partial thromboplastin time (aPTT) are shown.
Patients treated with UFH have detectable PF4/heparin-positive B cells
On the basis of the finding that endogenous PF4 can be displaced by heparin to form PF4/heparin complexes capable of binding to B cells, we next investigated binding of KKO to circulating B cells in patients treated with UFH for medical or surgical (cardiac) indications. For these studies, whole blood from patients treated with heparin was analyzed in the absence of exogenous PF4 or heparin. Figure 4B shows dot plots of data from 3 representative patients receiving heparin. Binding of KKO to patient B cells, indicative of endogenous PF4/heparin complexes, ranged from 0.3% to 82%. Overall, B cells from 6 (37%) of 16 patients studied bound KKO (Figure 4C). As with healthy donors (Figure 1A), in vivo binding of PF4/heparin was essentially confined to the B-cell population (supplemental Figure 4). We noted that binding of PF4 to B cells in vivo was also heparin induced. As noted in Figure 4D, PF4/heparin-positive B cells were not detected before UFH therapy (–2 hours). However, when UFH was given at a dose of 13 to 18 U/kg per hour, there was a steady rise in the percentage of PF4/heparin-positive B cells over time (13-45 hours). At supratherapeutic levels of heparin, as indicated by activated partial thromboplastin time >300 seconds, there was complete loss of antigen binding. Together, these findings corroborate in vitro observations (Figures 1 and 3) that show significant, heparin-dependent B-cell binding of PF4/heparin complexes in some patients receiving heparin therapy.
Complement mediates binding of PF4/heparin complexes to B cells
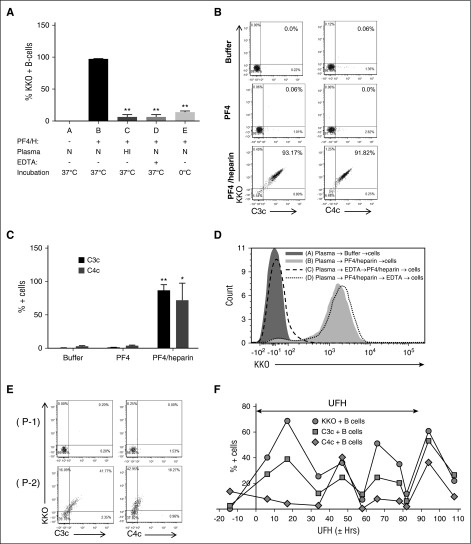
The observation that a high proportion of circulating B cells in healthy donors are capable of binding PF4/heparin (or PRT/heparin) complexes suggests that binding is not mediated by antigen-specific receptors. Because the complement system facilitates antigen-independent binding to B cells,8,20 we investigated the role of complement in binding of PF4/heparin complexes to B cells. Whole blood was incubated with PF4/heparin under several experimental conditions known to inhibit complement activation (heat inactivated sera/ice/EDTA) that do not interfere with formation of PF4/heparin complexes (supplemental Figure 5). KKO did not bind to B cells incubated in normal plasma with buffer (Figure 5A, column A), but showed robust binding at 37°C in the presence of PF4/heparin (Figure 5A, column B). PF4/heparin binding to B cells was markedly impaired under conditions associated with complement inhibition or inactivation (Figure 5A: column C, heat inactivated plasma; column D, 10 mM EDTA; column E, ice).
Figure 5.
Complement mediates binding of PF4/heparin complexes to B cells. (A) Effect of complement inhibition on PF4/heparin binding to B cells. Plasma and blood cells were separated from blood by centrifugation, subjected to conditions associated with complement inactivation (heat inactivation and treatment with 10 mM EDTA or ice), and exposed to antigen or buffer. Conditions A to E correspond to various incubation conditions. Mean ± SD from 3 independent experiments. **P < .005 compared with condition B. N, normal; HI, heat inactivated. (B) and (C) PF4/heparin binding to B cells correlates with C3c/C4c deposition. Percentage of double-positive cells, which represent KKO and C3c or C4c binding, appears in the right upper quadrant of dot plots (B) and as quantified in (C) from 3 independent experiments. *P < .05, and **P < .005 compared with buffer condition. (D) Complement fixation occurs primarily in the solution phase and not on the B-cell surface. Plasma and blood cells were separated, and sequence of B-cell binding was determined as described in “Methods.” Overlay histogram for KKO staining on the CD19-gated B cells is shown by the sequence of incubations as indicated in the key. (E) Binding of PF4/heparin complexes and complement to B cells from heparinized patients. Blood from 2 patients (P-1 and P-2) was stained concurrently for CD19, KKO, C3c, or C4c. Dot plots show binding of KKO and C3c/C4c binding on CD19+ gated cells. (F) Binding of PF4/heparin, C3c, and C4c to B cells over time in a patient treated with heparin. Shown is the percentage of KKO-positive/C3c-positive/C4c-positive B cells in the circulation of a heparinized patient during the course of heparin therapy. The x-axis shows time from start of heparin therapy, the y-axis shows % KKO + B cells, and the 2-headed arrow in the figure indicates duration of UFH therapy.
To determine whether PF4/heparin binding to B cells was associated with complement fixation, we incubated whole blood with PF4/heparin complexes and examined binding of complement fragments C3c/C4c to B cells by flow cytometry. We noted significant amounts of complement fragments C3c and C4c deposited on B cells pre-incubated with PF4/heparin (Figure 5B, bottom panel, and 5C), but not with buffer or PF4 alone (Figure 5B, top 2 panels, and 5C). Binding showed a rocket shape demonstrating a strong correlation between PF4/heparin antigen and complement deposition on B cells. We next examined the sequence of events involved in antigen and complement binding to B cells. Cells incubated in plasma with buffer did not bind PF4/heparin (Figure 5D, condition A), whereas PF4/heparin complexes added to plasma without EDTA showed robust binding to B cells (Figure 5D, positive control, condition B). When plasma was incubated with EDTA before the addition of PF4/heparin (Figure 5D, condition C), no antigen bound to B cells. However, if plasma was first incubated with PF4/heparin complexes and then EDTA was added (Figure 5D, condition D), B-cell binding was preserved.
To demonstrate the clinical relevance of these findings in patients treated with UFH, we examined complement deposition on PF4/heparin antigen-positive B cells in heparinized patients. As shown in Figure 5E, B cells from 2 patients receiving heparin were stained for PF4/heparin antigen and C3c or C4c. B cells from patient 1 (top panel) showed no binding of PF4/heparin or C3/C4. Conversely, patient 2 had double-positive B cells, indicating both PF4/heparin and C3/C4 binding. Figure 5F shows serial studies of KKO and C3/C4 on peripheral B cells from a patient over the course of heparin therapy. KKO-positive cells were detected throughout UFH therapy, indicating persistent antigen binding. There was also a strong correlation between bound antigen and C3/C4 binding. These studies provide experimental evidence of in vitro and in vivo complement activation by PF4/heparin complexes and demonstrate that complement activation occurs in the fluid phase and that complement fixation is required for binding of complexes to B cells.
Complement-fixed PF4/heparin complexes bind to CR2 (CD21) on B cells
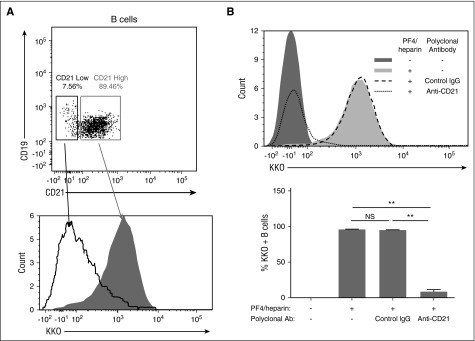
We next investigated the involvement of various cell-surface receptors in mediating binding of complement-coated PF4/heparin to B cells. We found no specific role for cell-surface glycosaminoglycans, IgG-Fc receptors, or cell-surface IgM (supplemental Figure 6). Rather, investigations of PF4/heparin binding to various lymphoblastic cell lines showed strong correlation of PF4/heparin binding with CD21 expression (Table 1; supplemental Figure 7), but not other surface receptors such as major histocompatibility class II molecules and B-cell receptors (Table 1; supplemental Figure 7). A small proportion of B cells in healthy donors express low levels of CD21. Overlay histograms show that B cells expressing low levels of CD21 (Figure 6A, lower panel, open curve with solid line) express low levels of PF4/heparin compared with B cells expressing high levels of CD21 (Figure 6A, lower panel, shaded peak). To examine the contribution of CD21 in mediating binding of complement-fixed PF4/heparin complexes more directly, we blocked CD21 expression on B cells by using a polyclonal CD21 blocking antibody. As shown in Figure 6B, PF4/heparin complexes bound to B cells in the presence of buffer or control IgG, but did not bind to B cells pre-incubated with polyclonal anti-CD21. Together, these results establish that complement mediates binding of PF4/heparin complexes to human B lymphocytes via CD21.
Table 1.
Examination of PF4/heparin binding and expression of receptors on B cell lines
| Cell lines | IgM | MHC II | CD21 | KKO (PF4/heparin) |
|---|---|---|---|---|
| P3HR-1 | + + | + + + | – | – |
| Reh | – | ++ | – | – |
| Ramos | + + + | + + | + | + |
| Bjab | + + + + | + | + + | + + |
| Raji | + + | + + + + | + + + + | + + + + |
Relative receptor expression or KKO binding is indicated by “−” or “+” (1-4) signs.
+, Expression or binding; −, no or very poor expression or binding; MHC II, major histocompatibility class II.
Figure 6.
Complement-containing PF4/heparin complexes bind to CR2 (CD21) on B cells. (A) Binding of PF4/heparin to peripheral blood B cells correlates with CD21 expression. Whole blood was incubated with PF4/heparin and stained with labeled antibodies to CD19, CD21, and KKO. Top panel: gating of B cells based on CD21 expression; bottom panel: overlay histogram of KKO staining as a function of CD21 expression as low- (open curve with solid line) or high- (shaded area) expressing B cells. (B) CD21 mediates binding of complement-coated PF4/heparin complexes to B cells. Blood was incubated with anti-CD21 or control IgG before the addition of PF4/heparin. Top panel: a representative overlay histogram is shown for KKO staining of B cells with anti-CD21 or control IgG. Bottom panel: summary of results (mean ± SD) from 3 experiments for PF4/heparin binding to B cells after expression of CD21 was blocked with a polyclonal anti-CD21 antibody. **P < .005.
Discussion
We undertook these studies to examine the proximate events leading to sensitization of the immune system to PF4/heparin. Our findings provide a new construct for understanding the immune pathogenesis of HIT by defining a major role for complement activation and complement-mediated binding of PF4/heparin complexes to peripheral blood B cells via CR2 (CD21).
Our studies suggest that a major effect of heparin in activating the immune system involves its ability to assemble PF4 or other positively charged proteins into ULCs capable of activating complement. Intravenous heparin displaces PF4 from platelets and endothelium into the circulation in healthy individuals, increasing its intravascular concentration by approximately 10-fold.21-24 Our studies are consistent with the concept that displacement of PF4 by heparin is associated with a redistribution of PF4 from preexisting cellular binding sites to circulating PF4/heparin ULCs that bind to B cells. Preferential binding of PF4/heparin to B cells relative to monocytes9 or neutrophils25 may be attributable to our use of whole blood, which provides a source of complement, rather than use of isolated cell populations as described in previous studies9,25
By using binding of KKO to B cells as a correlate of circulating PF4/heparin complexes, we show that PF4 is displaced by low concentrations of heparin (0.005-0.01 U/mL; Figure 4A) in vitro and, to a greater extent, in patients receiving therapeutic heparin (Figures 4B-D and 5F) to form circulating ULCs capable of activating complement. UFH induces binding of ULCs to B cells at a 10-fold lower concentration (0.01-0.5 µM or 0.02-1 U/mL) than LMWH (0.11-5.5 µM or 0.5-25 µg/mL; Figure 3B), following a pattern that parallels their clinical immunogenicity. Fondaparinux, which has little or no capacity to form ULCs, does not induce binding of KKO (Figure 3B).
PF4/heparin ULCs activate complement in plasma and in whole blood over a range of PF4 concentrations described in health and disease.18,26 The role of complement as an important contributor of antigen binding to B cells is supported by the following observations. First, the finding that >90% of B cells from healthy donors (Figure 1) are capable of binding PF4/heparin complexes suggests that binding is unlikely to be mediated by antigen-specific B cells, which occur at a low frequency in the circulation (<0.05%27). Second, several approaches to inactivate complement (Figure 5A,D) markedly attenuate ULC binding to B cells. Finally, binding of KKO to B cells correlates closely with C3/C4 binding both in vitro and in vivo (Figure 5B,C,F), affirming the relationship between ULC formation, generation, and incorporation of complement fragments, followed by antigen binding to B cells. Binding of PRT/heparin complexes to B cells shows the same requirement, suggesting that complement activation by protein/heparin complexes leading to B-cell expression of antigen may be a general phenomena that extends beyond the immune pathogenesis of HIT (Figure 2).
Our data suggest that complement activation is likely dependent on ULC size and charge, 2 variables known to affect immunogenicity in murine studies.1 The influence of surface charge and topography on complement activation has been shown in several artificial and biologic systems. The effects of surface charge on complement activation have been demonstrated by using phosphatidylcholine:cholesterol liposomes (55:45 mol/mol) modified to exhibit a positive or negative charge.28 At neutrality, liposomes had little effect on complement activation. In these studies, positively charged liposomes triggered activation of the alternative pathway, and negatively charged liposomes activated the classical pathway.28 Another variable affecting complement activation is antigen size, which pathogens exploit for the purpose of immune evasion.29
Complement activated by ULCs becomes incorporated into PF4/heparin complexes and mediates binding of antigen to B cells via the complement receptor, CD21 (Figure 6). Binding of antigen to B-cell CD21 augments immunogenicity in vitro.8 The mechanisms by which CD21 transduces immunity have yet to be fully clarified, but its importance in antigen capture, B-cell activation, and antigen transport are well established. Binding to CD21 might enhance interactions of antigen-specific B cells with cognate antigen directly, or provide an essential second signal for BCR activation. In vitro, co-ligation of CD19/CD21 complex and the BCR by complement-tagged antigens lowers the threshold for B-cell activation30 by enhancing BCR signaling by a variety of mechanisms, including direct signaling,31,32 and indirectly by prolonging dwell times in lipid rafts.33 Ligation of CD19/CD21 complex also augments antigen presentation by the major histocompatibility complex class II molecules, whether the antigen is bound to BCR or taken up by pinocytosis.32
CD21 also facilitates antigen transport by way of antigen transfer from B cells to follicular dendritic cells (FDCs). FDCs are stromal-derived cells that reside in B-cell follicles and present unprocessed antigen in the form of immune complexes or opsonized antigen.34 Unlike conventional dendritic cells, they do not process antigen for subsequent presentation, but instead retain antigen for recognition by antigen-specific B cells. Complement-coated antigen can be transferred from B cells to FDCs for long-term antigen retention35,36
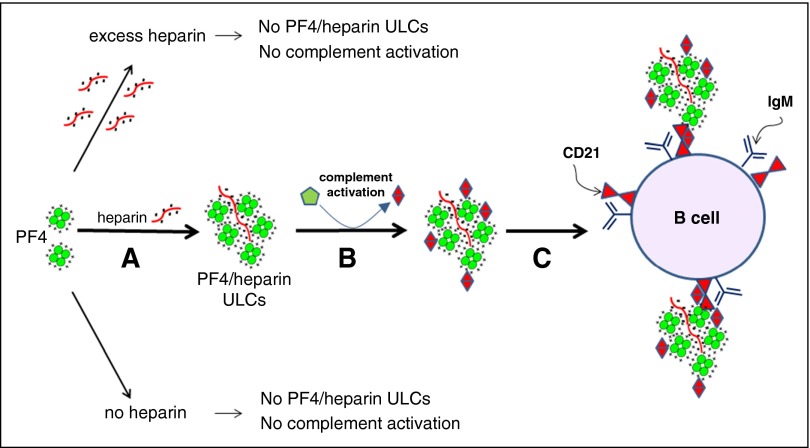
Although our studies do not address the mechanisms by which complement incorporation and CD21 engagement by PF4/heparin complexes leads to a productive immune response, we speculate that these are critical early events in the process. On the basis of our findings, we propose the following model of immune activation leading to HIT. Heparin displaces PF4 from cell surfaces into the circulation, which generates ULCs that vary in size and charge (Figure 7A). By as yet uncharacterized mechanisms, complexes of PF4 and heparin formed at optimal ratios of reactants (ULCs) in solution activate complement, which leads to incorporation of complement activation fragments (C3/C4) into the PF4/heparin complexes (Figure 7B). Complement-coated PF4/heparin complexes then bind to circulating B cells via CD21 (Figure 7C). Binding of antigen to CD21 facilitates either direct activation of B cells and/or antigen transport to secondary lymphoid follicles and antigen transfer culminating in an immune response to the heparin-containing complex. These findings suggest that complement and/or CD21 may serve as potential therapeutic targets for reducing the immune-mediated complications of heparin therapy.
Figure 7.
Activation of complement by PF4/heparin complexes and binding to B-cell CD21. (A) PF4 and heparin interact over a narrow range of molar ratios to generate ULCs. PF4 alone or PF4 with excess of heparin do not make ULCs. (B) ULCs activate complement and bind complement activation products (C3/C4). (C) Complement-coated ULCs bind to a B cell via CR2 (CD21).
Acknowledgments
The authors thank Sandeep Dave for providing the BJAB cell line.
This work was supported by the National Institutes of Health Grant No. P01 HL110860 from the National Heart, Lung, and Blood Institute (G.M.A., L.R., D.B.C., B.S.S., S.E.M., and M.P.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.K., G.K., M.F., and G.M.A. conceived and designed the study; G.M.A., S.K., C.G.H., L.R., D.B.C., and M.P. provided study materials or patients: S.K. and G.M.L. collected and assembled data; G.M.A., S.K., C.G.H., L.R., D.B.C., M.P., B.S.S., G.K., M.F., and S.E.M. analyzed and interpreted data; G.M.A., S.K., D.B.C., M.P., B.S.S., G.K., M.F., and S.E.M. wrote the manuscript; and G.M.A., S.K., G.M.L., C.G.H., L.R., D.B.C., M.P., B.S.S., G.K., M.F., and S.E.M. approved the final version of the manuscript.
Conflict-of-interest disclosure: G.M.A. has an awarded patent for KKO (US Application No. 60/143536); G.M.A., M.P., and D.B.C. have pending intellectual property applications. The remaining authors declare no competing financial interests.
Correspondence: Gowthami M. Arepally, Division of Hematology, Duke University Medical Center, Box 3486, Room 356A, Alex H. Sands Building, Research Dr, Durham, NC 27710; e-mail: arepa001@mc.duke.edu.
References
- 1.Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110(13):4253–4260. doi: 10.1182/blood-2007-08-105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand J Immunol. 2013;78(2):181–193. doi: 10.1111/sji.12070. [DOI] [PubMed] [Google Scholar]
- 3.Suvarna S, Qi R, Hollingsworth JW, Arepally GM. Platelet factor 4-heparin complexes trigger immune responses independently of the MyD88 pathway. Br J Haematol. 2008;142(4):671–673. doi: 10.1111/j.1365-2141.2008.07240.x. [DOI] [PubMed] [Google Scholar]
- 4.Prechel MM, Walenga JM. Complexes of platelet factor 4 and heparin activate Toll-like receptor 4. J Thromb Haemost. 2015;13(4):665–670. doi: 10.1111/jth.12847. [DOI] [PubMed] [Google Scholar]
- 5.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 6.Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140(1):126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer MB, Ma M, Goerg S, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157(2):549–556. [PubMed] [Google Scholar]
- 8.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 9.Rauova L, Hirsch JD, Greene TK, et al. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116(23):5021–5031. doi: 10.1182/blood-2010-03-276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arepally GM, Kamei S, Park KS, et al. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood. 2000;95(5):1533–1540. [PubMed] [Google Scholar]
- 11.Joglekar M, Khandelwal S, Cines DB, Poncz M, Rauova L, Arepally GM. Heparin enhances uptake of platelet factor 4/heparin complexes by monocytes and macrophages. J Thromb Haemost. 2015;13(8):1416–1427. doi: 10.1111/jth.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27(3):653–670. doi: 10.1016/j.cll.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bock PE, Luscombe M, Marshall SE, Pepper DS, Holbrook JJ. The multiple complexes formed by the interaction of platelet factor 4 with heparin. Biochem J. 1980;191(3):769–776. doi: 10.1042/bj1910769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauova L, Poncz M, McKenzie SE, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105(1):131–138. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- 15.Greinacher A, Gopinadhan M, Günther JU, et al. Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler Thromb Vasc Biol. 2006;26(10):2386–2393. doi: 10.1161/01.ATV.0000238350.89477.88. [DOI] [PubMed] [Google Scholar]
- 16.Capitanio AM, Niewiarowski S, Rucinski B, et al. Interaction of platelet factor 4 with human platelets. Biochim Biophys Acta. 1985;839(2):161–173. doi: 10.1016/0304-4165(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 17.George JN, Onofre AR. Human platelet surface binding of endogenous secreted factor VIII-von Willebrand factor and platelet factor 4. Blood. 1982;59(1):194–197. [PubMed] [Google Scholar]
- 18.Files JC, Malpass TW, Yee EK, Ritchie JL, Harker LA. Studies of human plate alpha-granule release in vivo. Blood. 1981;58(3):607–618. [PubMed] [Google Scholar]
- 19.Handin RI, McDonough M, Lesch M. Elevation of platelet factor four in acute myocardial infarction: measurement by radioimmunoassay. J Lab Clin Med. 1978;91(2):340–349. [PubMed] [Google Scholar]
- 20.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 21.Cella G, Vittadello O, Gallucci V, Girolami A. The release of beta-thromboglobulin and platelet factor 4 during extracorporeal circulation for open heart surgery. Eur J Clin Invest. 1981;11(3):165–169. doi: 10.1111/j.1365-2362.1981.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 22.Cella G, Colby SI, Taylor AD, McCracken L, Parisi AF, Sasahara AA. Platelet factor 4 (PF4) and heparin-released platelet factor 4 (HR-PF4) in patients with cardiovascular disorders. Thromb Res. 1983;29(5):499–509. doi: 10.1016/0049-3848(83)90345-6. [DOI] [PubMed] [Google Scholar]
- 23.Cella G, Scattolo N, Stevanato F, Girolami A. The release of platelet factor 4 (PF4) induced by heparin and related glycosaminoglycans (GAGs). Thromb Haemost. 1984;52(1):94–95. [PubMed] [Google Scholar]
- 24.Rucinski B, Knight LC, Niewiarowski S. Clearance of human platelet factor 4 by liver and kidney: its alteration by heparin. Am J Physiol. 1986;251(4 Pt 2):H800–H807. doi: 10.1152/ajpheart.1986.251.4.H800. [DOI] [PubMed] [Google Scholar]
- 25.Petersen F, Brandt E, Lindahl U, Spillmann D. Characterization of a neutrophil cell surface glycosaminoglycan that mediates binding of platelet factor 4. J Biol Chem. 1999;274(18):12376–12382. doi: 10.1074/jbc.274.18.12376. [DOI] [PubMed] [Google Scholar]
- 26.Chesterman CN, McGready JR, Doyle DJ, Morgan FJ. Plasma levels of platelet factor 4 measured by radioimmunoassay. Br J Haematol. 1978;40(3):489–500. doi: 10.1111/j.1365-2141.1978.tb05819.x. [DOI] [PubMed] [Google Scholar]
- 27.Leyendeckers H, Odendahl M, Löhndorf A, et al. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29(4):1406–1417. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146(12):4234–4241. [PubMed] [Google Scholar]
- 29.Dalia AB, Weiser JN. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe. 2011;10(5):486–496. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256(5053):105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 31.Mongini PK, Vilensky MA, Highet PF, Inman JK. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2). J Immunol. 1997;159(8):3782–3791. [PubMed] [Google Scholar]
- 32.Cherukuri A, Cheng PC, Pierce SK. The role of the CD19/CD21 complex in B cell processing and presentation of complement-tagged antigens. J Immunol. 2001;167(1):163–172. doi: 10.4049/jimmunol.167.1.163. [DOI] [PubMed] [Google Scholar]
- 33.Cherukuri A, Cheng PC, Sohn HW, Pierce SK. The CD19/CD21 complex functions to prolong B cell antigen receptor signaling from lipid rafts. Immunity. 2001;14(2):169–179. doi: 10.1016/s1074-7613(01)00098-x. [DOI] [PubMed] [Google Scholar]
- 34.Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35(3):105–113. doi: 10.1016/j.it.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 35.El Shikh ME, Pitzalis C. Follicular dendritic cells in health and disease. Front Immunol. 2012;3:292. doi: 10.3389/fimmu.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Link A, Zabel F, Schnetzler Y, Titz A, Brombacher F, Bachmann MF. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J Immunol. 2012;188(8):3724–3733. doi: 10.4049/jimmunol.1103312. [DOI] [PubMed] [Google Scholar]