Key Points
Human cytokine knock-in mice are improved in vivo models for multilineage engraftment of mobilized PB CD34+ cells.
Humanized mouse models might open new avenues for personalized studies of human pathophysiology of the hematopoietic and immune system.
Abstract
Human CD34+ hematopoietic stem and progenitor cells (HSPCs) can reconstitute a human hemato-lymphoid system when transplanted into immunocompromised mice. Although fetal liver-derived and cord blood–derived CD34+ cells lead to high engraftment levels, engraftment of mobilized, adult donor-derived CD34+ cells has remained poor. We generated so-called MSTRG and MISTRG humanized mice on a Rag2−/−Il2rg−/− background carrying a transgene for human signal regulatory protein α (SIRPα) and human homologs of the cytokine macrophage colony-stimulating factor, thrombopoietin, with or without interleukin-3 and granulocyte-macrophage colony-stimulating factor under murine promoters. Here we transplanted mobilized peripheral blood (PB) CD34+ cells in sublethally irradiated newborn and adult recipients. Human hematopoietic engraftment levels were significantly higher in bone marrow (BM), spleen, and PB in newborn transplanted MSTRG/MISTRG as compared with nonobese diabetic/severe combined immunodeficient Il2rg−/− or human SIRPα-transgenic Rag2−/−Il2rg−/− recipients. Furthermore, newborn transplanted MSTRG/MISTRG mice supported higher engraftment levels of human phenotypically defined HSPCs in BM, T cells in the thymus, and myeloid cells in nonhematopoietic organs such as liver, lung, colon, and skin, approximating the levels in the human system. Similar results were obtained in adult recipient mice. Thus, human cytokine knock-in mice might open new avenues for personalized studies of human pathophysiology of the hematopoietic and immune system in vivo.
Introduction
Modeling the human hemato-lymphoid system in human hematopoietic stem and progenitor cell (HSPC) xenografted mice has advanced our understanding of hematopoiesis and leukemogenesis and allowed testing novel therapeutic approaches.1-4 Although granulocyte colony-stimulating factor (G-CSF)–mobilized CD34+ peripheral blood (PB) cells are clinically widely used for autologous and allogenic hematopoietic stem cell transplantation,5 these cells, in contrast to fetal liver (FL) and cord blood (CB) CD34+ cells, have shown relatively poor engraftment at similar numbers in mice and are thus rarely used.6,7 However, some cell-intrinsic pathways might differ in FL and CB compared with adult HSPCs, and fetal and neonatal cells are inappropriate to study individual patients’ acquired conditions.
We recently generated immunodeficient Rag2−/−Il2rg−/− mice with genes encoding human macrophage colony-stimulating factor (M-CSF),8 combined interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF),9 and thrombopoietin (TPO),10 knocked into the respective mouse loci, either individually or in combination (MISTRG).3,11 Additionally, MISTRG mice carry a transgene encoding human signal regulatory protein α (SIRPα), known to bind human CD47 and thereby inhibiting mouse phagocytes of human cells.12-14 We demonstrated that transplantation of human FLCD34+ cells into newborn MISTRG mice leads to high-level engraftment, multilineage differentiation, and functionality of innate immune cells.11 Also, proof of principle engraftment of CB and PB CD34+ cells into newborn mice has been shown.11
To test utility and value of these novel mouse strains for PB-derived human HSPC research, we compared engraftment, lineage differentiation, and tissue seeding of healthy adult mobilized PB human CD34+ cells and their progeny in various immune-deficient strains, including newborn and adult recipient MISTRG mice.
Study design
Humanized cytokine knock-in (KI) mice were generated as reported previously.11 G-CSF–mobilized adult PB cells and CB cells were collected from healthy donors after obtaining informed consent. The study was approved by the ethics boards of the Canton of Zurich and Yale University Human Investigation Committee, respectively. Human (h) CD34+ cells were purified by density gradient centrifugation followed by positive immunomagnetic selection. Newborn and adult mice were sublethally irradiated. Newborn mice were intrahepatically injected with 300 000 to 500 000 (Zurich cohort) or 100 000 to 140 000 (Yale cohort) hCD34+ cells per mouse.15,16 Adult mice were injected IV with 800 000 to 1 000 000 hCD34+ cells per mouse. Mice were bled at several time points and terminally analyzed 10 to 16 weeks posttransplantation. Experiments were approved by the Veterinäramt des Kantons Zurich and Yale University Institutional Animal Care and Use Committee. For further details, see supplemental Methods, available on the Blood Web site.
Results and discussion
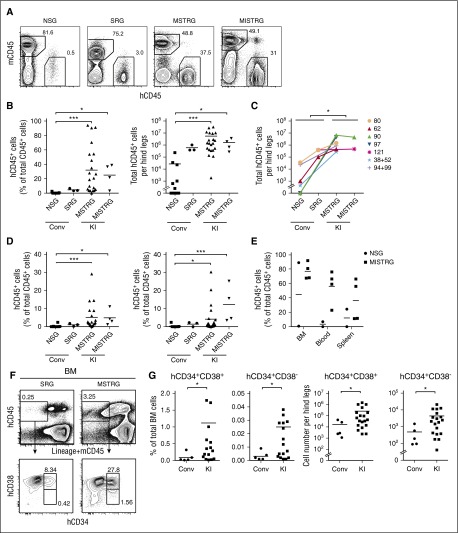
We first examined the engraftment of hCD34+ cells isolated from G-CSF mobilized PB upon intrahepatic injection into sublethally irradiated newborn NOD-scid (nonobese diabetic-severe combined immunodeficiency) Il2rg−/− (NSG), hSIRPα-transgenic Rag2−/−Il2rg−/− (SRG),12 and mouse strains with human cytokine KI genes for hM-CSF combined with hTPO (MSTRG) or the addition of hIL-3/hGM-CSF (MISTRG).11 PB analysis to determine hCD45+ cells at 4, 8, and 14 to 16 weeks revealed an increasing frequency of hCD45+ cells over time among total CD45+ cells in cytokine KI but not in control mice (supplemental Figure 1). When we compared percentage and absolute numbers of engrafted human cells in the bone marrow (BM) of recipient mice, the number was greatly increased in MSTRG and MISTRG in comparison with NSG and SRG mice (Figure 1A-B). Superior engraftment efficiency of cytokine KI mice was reproducible in an independent experimental cohort at Yale, where even lower numbers of PB CD34+ cells were transplanted (supplemental Figure 2A). Importantly, split donor sample transplantation into newborn cytokine KI vs NSG and SRG mice resulted in significantly higher engraftment in the KI group (Figure 1C). Although engraftment was highest in BM, %hCD45+ cells in PB and spleen of cytokine KI mice also exceeded engraftment of control groups (Figure 1D; supplemental Figure 3). Following transplantation of adult PB CD34+ cells, similar to previous studies using FL or CB CD34+ cells, we observed a lower frequency of hCD45+ cells in PB and spleen in comparison with BM in all recipient strains used. Interestingly, this difference was less prominent when transplanting CB CD34+ cells in newborn KI recipients (supplemental Figure 4). The reason for these differences needs to be further explored but might be in part attributable to extramedullary mouse hematopoiesis and/or differences in migration potential of human cells.11 Irrespective of this observation and more importantly, adult recipient MISTRG mice transplanted with mobilized PB CD34+ cells also supported substantial overall hCD45+ and myeloid cell engraftment in BM, PB, spleen, and liver with a trend toward superiority compared with NSG mice (Figure 1E; supplemental Figure 5).
Figure 1.
Human cytokine KI mice support engraftment of human mobilized PB CD34+ cells. (A) Representative fluorescence-activated cell sorter (FACS) analysis of the frequency of mouse and human CD45+ cells in BM in newborn mice 14 weeks posttransplantation. Numbers besides gated areas indicate percentages of cells. (B) Engraftment of hCD45+ cells in BM of newborn mice 10 to 16 weeks posttransplantation. Left panel indicates percentage of hCD45+ cells among total CD45+ cells. Right panel indicates absolute number of hCD45+ cells per hind legs (2 femurs and tibias). Each symbol represents an individual mouse; bars indicate mean values; n = 11 (NSG), n = 3 (SRG), n = 22 (MSTRG), n = 4 (MISTRG). Conv, conventional; KI, human cytokine KI. *P < .05; ***P < .001 (1-way analysis of variance [ANOVA] Kruskal-Wallis test with Dunn’s multiple comparison test). (C) hCD45+ cell engraftment in BM of newborn mice from split, matched individual donors. Each symbol represents mean frequency of hCD45% cells among total CD45+ cells. Mean frequency of human CD45+ cells in Conv vs KI mice was significantly different. *P < .05 (Conv vs KI; paired t test). (D) Engraftment of hCD45+ cells in blood (left) and spleen (right) 10 to 16 weeks posttransplantation. Each symbol represents an individual mouse; bars indicate mean values; n = 11 (NSG), n = 3 (SRG), n = 22 (MSTRG), n = 4 (MISTRG). *P < .05; ***P < .001 (1-way ANOVA Kruskal-Wallis test with Dunn’s multiple comparison test). (E) Engraftment of hCD45+ cells in BM, blood, and spleen in adult mice 12 to 16 weeks after transplantation. Panel shows percentage of hCD45+ cells among total CD45+ cells in each organ. Each symbol represents an individual recipient engrafted with hCD45+ cells; bars indicate mean values; n = 2 (NSG), n = 4 (MISTRG). (F) Representative FACS plots for hCD34+CD38+ and hCD34+CD38− cells in newborn recipients gated on mCD45− Lineage (Lin)− hCD45+ cells. Numbers beside gate indicate percentages of cells. (G) Frequency (left 2 graphs) as well as absolute number (right 2 graphs) of hCD34+CD38+, hCD34+CD38− cells per hind legs. Each symbol represents an individual newborn recipient engrafted with hCD45+ cells; bars indicate mean values; n = 5 (Conv; 2 NSG and 3 SRG), n = 19 (KI; 15 MSTRG and 4 MISTRG). *P < .05 (Mann-Whitney U test).
We next compared immature human HSPC engraftment and maintenance in different newborn recipients. Cytokine KI mice showed significantly increased relative and absolute numbers of human hematopoietic progenitor cells (CD34+CD38+) and hematopoietic stem cells containing CD34+CD38− cells in BM (Figure 1F-G). Thus, we conclude that, in line with prior in vitro and in vivo observations,17-19 human cytokines such as M-CSF, IL-3/GM-CSF, and TPO support maintenance and differentiation of human HSPCs.
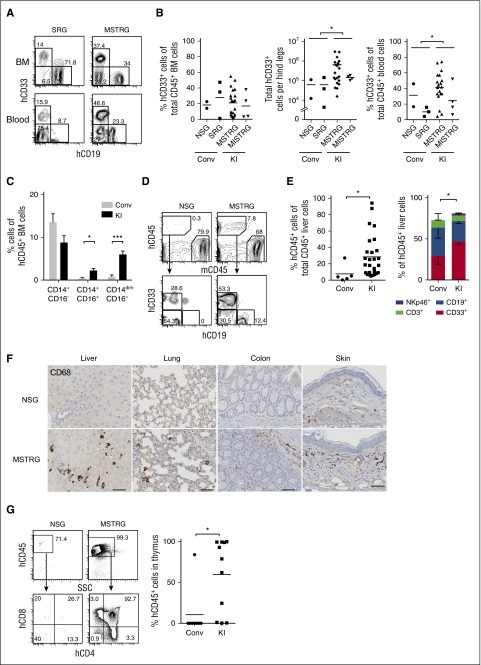
The percentage of human CD33+ myeloid cells in the BM was not significantly different between newborn recipients, whereas absolute numbers were higher in KI than in conventional mice (Figure 2A-B; supplemental Figure 2B), regardless of a comparable BM cellularity (supplemental Figure 6). Importantly, hCD33+ cells were significantly increased in PB as well as nonlymphoid tissues such as liver and lung in the KI group (Figure 2B,D-E; supplemental Figure 7), and human CD68+ myeloid cells were detectable in nonlymphoid tissues such as lung, liver, colon, and skin of KI but not of control mice (Figure 2F). In adult recipients, we observed a trend toward superior myeloid development in different organs (supplemental Figure 5).
Figure 2.
Human cell engraftment in lymphoid and nonlymphoid tissues in newborn recipients. (A) Representative FACS plots for hCD19+ and hCD33+ cells gated on hCD45+ cells in BM (top panels) and PB (bottom panels). (B) Frequency and absolute number of hCD33+ cells in BM (left) and frequency of hCD33+ cells in PB (right). Each plot shows data from an individual recipient engrafted with hCD45+ cells. Numbers beside gates indicate percentages of cells; n = 5 (Conv; 2 NSG and 3 SRG), n = 20 (KI; 16 MSTRG and 4 MISTRG). *P < .05 (Mann-Whitney U test). (C) Frequency of classical (CD14+CD16−) and nonclassical monocytes (CD14+CD16+ or CD14dimCD16+) among lineage (Lin; CD3, CD19, CD20)− hCD45+ cells in BM of engrafted recipient mice; data show mean + standard error of the mean; n = 5 (Conv; 2 NSG and 3 SRG), n = 22 (KI; 18 MSTRG and 4 MISTRG). *P < .05; ***P<.001 (Mann-Whitney U test). (D) Representative FACS plots of recipient mice (in liver 14 weeks posttransplantation) for hCD45+ cells (top panels), CD33+ or CD19+ cells gated on hCD45+ cells (bottom panels). Numbers beside gates indicate percentages of cells shown. (E) Percentage of hCD45+ cells among total CD45+ cells in liver (left graph); each symbol represents an individual recipient engrafted with hCD45+ cells, and bars indicate mean values. CD33+, CD19+, CD3+, and NKp46+ population among hCD45+ cells in the liver of engrafted mice (right); data show mean + standard error of the mean; n = 5 (Conv; 2 NSG and 3 SRG), n = 25 (KI; 21 MSTRG and 4 MISTRG). *P < .05 (Mann-Whitney U test; left) and *P < .05 (for CD33+ cells, 1-way ANOVA Kruskal-Wallis test with Dunn’s multiple comparison test; right). (F) Immunohistological staining of human myeloid cells (hCD68+) in nonlymphoid tissues (liver, lung, colon, and skin) of recipient mice. Bars represent 20 μm. Images are representative of at least 3 mice analyzed per group. (G) Representative FACS plots for hCD45+ cells (top panels) and double-negative, double-positive, CD4+ single-positive, and CD8+ single-positive thymocytes among hCD45+ cells (bottom panels) in the thymus of indicated recipient mice. Number of hCD45+ cells in the thymus is shown (right). Each symbol represents an individual recipient engrafted with hCD45+ cells; bars indicate mean values; n = 4 (Conv; 2 NSG and 2 SRG), n = 9 (KI; 9 MSTRG). *P < .05; ***P < .001 (Mann-Whitney U test).
CD14+CD16− cells are classical monocytes, whereas CD14+CD16+ and CD14dimCD16+ cells are categorized as nonclassical “patrolling” monocytes.20 The number of both CD14+CD16+ and CD14dimCD16+ nonclassical monocytes in BM of the KI group was higher than in the control group (Figure 2C). In addition, CD141+ (BDCA-3+) dendritic cells with cross-presentation activity were significantly increased in the BM of KI mice (supplemental Figure 8).
As previously reported, we observed splenomegaly because of extramedullary mouse hematopoiesis in highly engrafted animals.11 Immunohistochemical staining of BM of an engrafted MISTRG mouse (32% hCD45+ cells) confirmed the presence of human macrophages and showed evidence of hemophagocytosis, underlining human myeloid cell functionality (supplemental Figure 9). Interestingly, and in contrast to MISTRG mice engrafted with 1 × 105 hCD34+ FL cells,11 mice engrafted with up to 5 × 105 mobilized PB CD34+ cells survived clinically healthy until terminal analysis up to 16 weeks. This might be a result of lower human BM engraftment levels, leading to relatively less reduction of mouse hematopoiesis and less phagocytosis of mouse cells by human phagocytes.11
Analysis of human lymphocyte subsets revealed that the frequency of natural killer cells in BM and PB was significantly higher in KI mice (supplemental Figure 10A). Also, the CD4/8 ratio of T cells in the BM of MSTRG and MISTRG mice was higher, whereas naïve and memory CD4+ T-cell subsets and B-cell subsets were similar between KI and control groups (supplemental Figure 10B-C). Of interest, thymus hCD45+ cells and thymocyte development in KI mice were significantly enhanced as compared with control mice (Figure 2G).
Taken together, these data indicate that human cytokine KI mice support the development of mature lineage cells, both myeloid and lymphoid, from adult mobilized PB CD34+ cells in hematopoietic, lymphoid, and nonlymphoid tissues. Even though this study focused on newborn recipients, we are also showing confirmatory proof-of-principle data from adult recipient cytokine KI mice, a setting that might, because of easier experimental handling, facilitate clinical translational research. Indeed, it will now become feasible to use these and other humanized mice for individualized patient sample–derived in vivo research that allows preclinical, likely predictive testing of pathophysiology and novel therapeutic strategies in a personalized manner in infectious and neoplastic diseases11,21
Acknowledgments
This work was supported by a Hanne-Liebermann research fellowship (J.M.E.); the University of Zurich clinical research priority program Human Hemato-Lymphatic Diseases (M.G.M.); the Bill and Melinda Gates Foundation; the National Cancer Institute, National Institutes of Health (grant CA156689) (R.A.F. and M.G.M.); the US Department of Defense (grant CA120128) (S.H.); and the State of Connecticut Department of Public Health (grant RFP 2014-0135) (S.H.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y. Saito, J.M.E., Y. Song, and S.H. designed research, performed experiments, analyzed data, and wrote the manuscript; A. Rafiei, A. Rongvaux, D.K., and M.H. performed experiments; and R.A.F. and M.G.M. directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Hematology, University Hospital and University of Zurich, Raemistr 100, CH-8091 Zurich, Switzerland; e-mail: markus.manz@usz.ch.
References
- 1.Rongvaux A, Takizawa H, Strowig T, et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011;32(7):321–327. doi: 10.1016/j.it.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26(5):537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31–40. doi: 10.1016/j.blre.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André MC, Erbacher A, Gille C, et al. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J Immunol. 2010;185(5):2710–2720. doi: 10.4049/jimmunol.1000583. [DOI] [PubMed] [Google Scholar]
- 7.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 8.Rathinam C, Poueymirou WT, Rojas J, et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118(11):3119–3128. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- 9.Willinger T, Rongvaux A, Takizawa H, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci USA. 2011;108(6):2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rongvaux A, Willinger T, Takizawa H, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci USA. 2011;108(6):2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rongvaux A, Willinger T, Martinek J, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32(4):364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strowig T, Rongvaux A, Rathinam C, et al. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci USA. 2011;108(32):13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takenaka K, Prasolava TK, Wang JCY, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8(12):1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa H, Manz MG. Macrophage tolerance: CD47-SIRP-alpha-mediated signals matter. Nat Immunol. 2007;8(12):1287–1289. doi: 10.1038/ni1207-1287. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Ellegast JM, Manz MG. Generation of humanized mice for analysis of human dendritic cells. Methods Mol Biol. 2016;1423:309–320. doi: 10.1007/978-1-4939-3606-9_22. [DOI] [PubMed] [Google Scholar]
- 16.Traggiai E, Chicha L, Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 17.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Demoulin S, Roncarati P, Delvenne P, Hubert P. Production of large numbers of plasmacytoid dendritic cells with functional activities from CD34(+) hematopoietic progenitor cells: use of interleukin-3. Exp Hematol. 2012;40(4):268–278. doi: 10.1016/j.exphem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Mossadegh-Keller N, Sarrazin S, Kandalla PK, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497(7448):239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]