Abstract
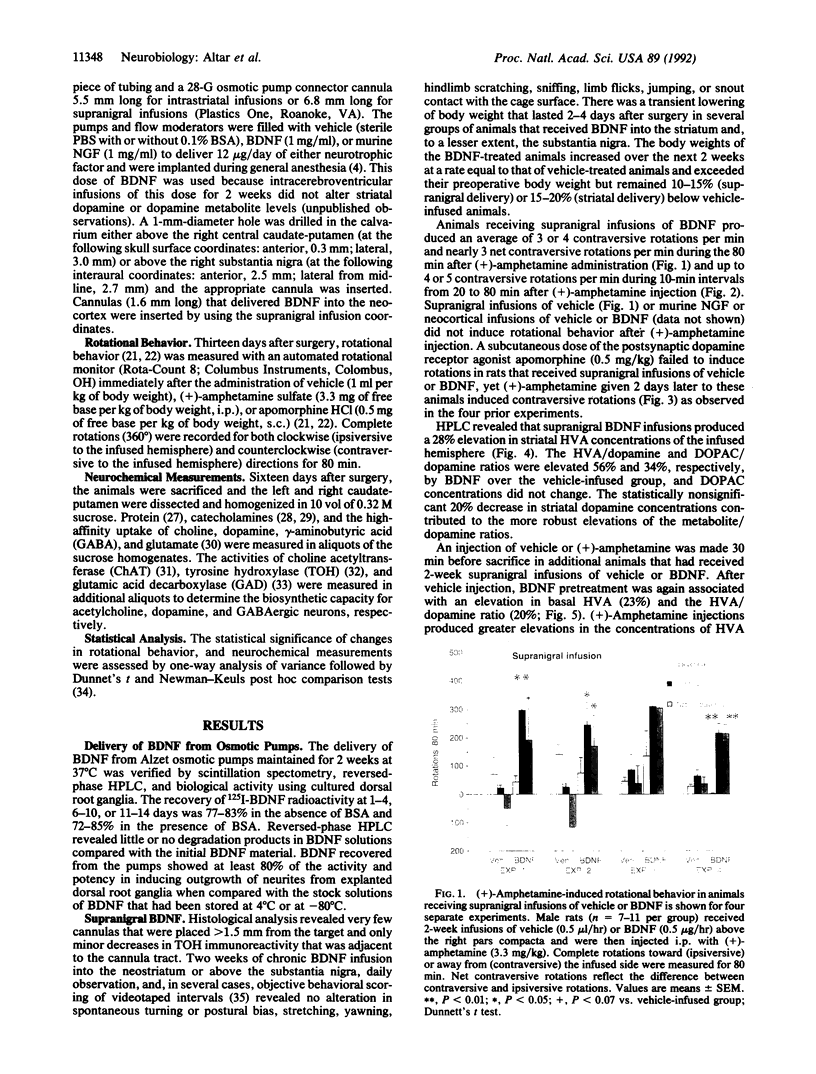
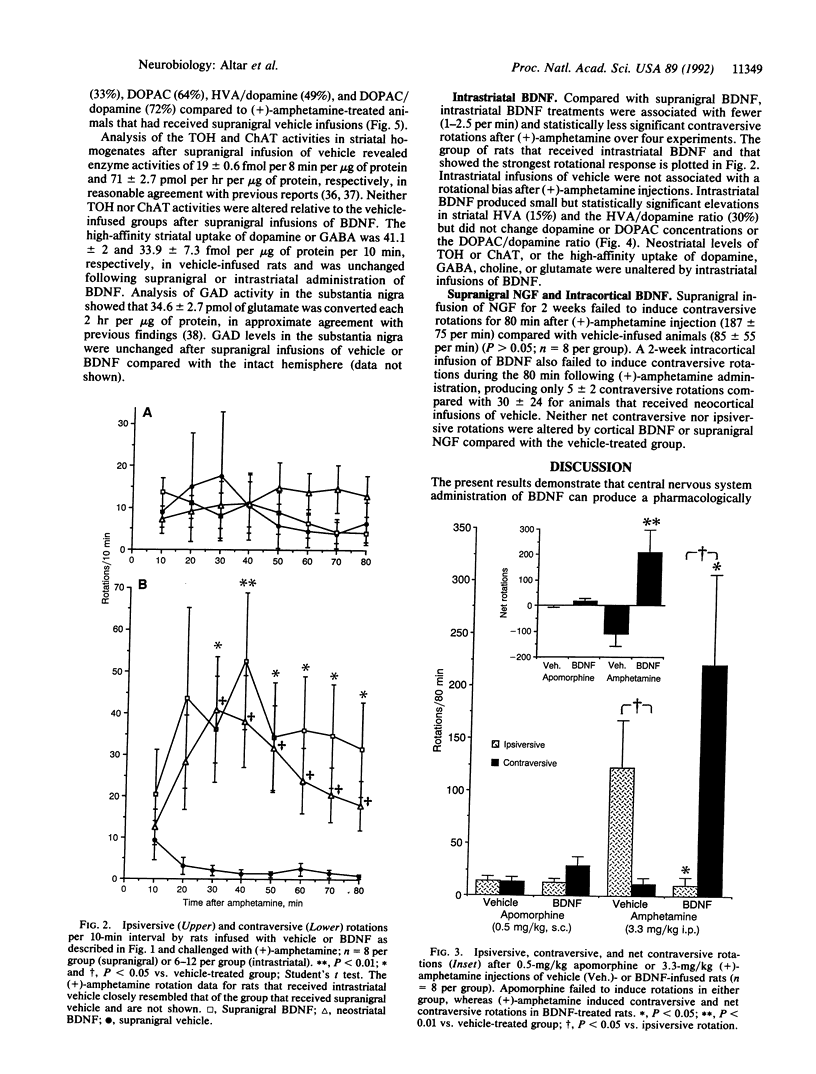
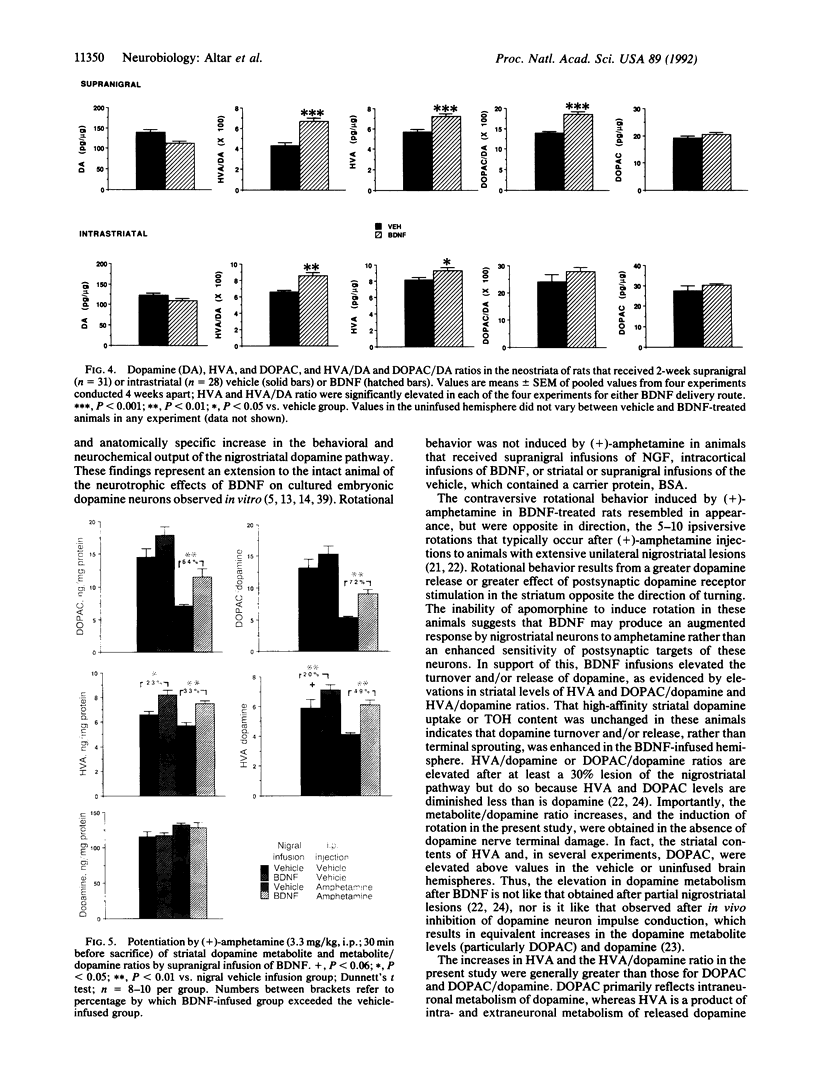
Brain-derived neurotrophic factor (BDNF), a member of the nerve growth factor (NGF)-related family of neutrophins, promotes the survival and differentiation of cultured nigral dopamine neurons. Two-week infusions of BDNF were made above the right pars compacta of the substantia nigra in adult rats. Systemic injection of these animals with (+)-amphetamine, a dopamine-releasing drug, induced 3 or 4 body rotations per minute directed away from the nigral infusion site. Neither supranigral NGF nor neocortical BDNF infusions induced rotational behavior. Systemic injections of the postsynaptic dopamine receptor agonist apomorphine did not induce rotations in these animals, demonstrating a presynaptic dopamine neuron locus for BDNF action. In support of this, neostriatal levels of the dopamine metabolite homovanillic acid (HVA) were elevated by 28%, and the HVA/dopamine and dihydroxyphenylacetic acid (DOPAC)/dopamine ratios were elevated by 56% and 34%, respectively, in the BDNF-infused brain hemisphere. BDNF augmented striatal concentrations of HVA and DOPAC and the metabolite/dopamine ratios to even greater extents after (+)-amphetamine injection, when peak rotational effects occurred. Intrastriatal infusions of BDNF produced fewer rotations per minute (1-2.5) after (+)-amphetamine and smaller elevations in HVA and the HVA/dopamine ratio (15% and 30%, respectively) than after supranigral delivery. Neither striatal dopamine, gamma-aminobutyric acid, nor acetylcholine high-affinity uptake or the synthetic enzymes for these neurotransmitters was altered by BDNF. These behavioral and neurochemical effects demonstrate an action of BDNF on dopamine neurons in vivo and are consistent with a potential role for BDNF in the treatment of Parkinson disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson A. L., Naujoks K., Thoenen H. Nerve growth factor-mediated enzyme induction in primary cultures of bovine adrenal chromaffin cells: specificity and level of regulation. J Neurosci. 1984 Jul;4(7):1771–1780. doi: 10.1523/JNEUROSCI.04-07-01771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson R. F., Alterman A. L., Barde Y. A., Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990 Sep;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Altar C. A., Marien M. R., Marshall J. F. Time course of adaptations in dopamine biosynthesis, metabolism, and release following nigrostriatal lesions: implications for behavioral recovery from brain injury. J Neurochem. 1987 Feb;48(2):390–399. doi: 10.1111/j.1471-4159.1987.tb04106.x. [DOI] [PubMed] [Google Scholar]
- Altar C. A., O'Neil S., Marshall J. F. Sensorimotor impairment and elevated levels of dopamine metabolites in the neostriatum occur rapidly after intranigral injection of 6-hydroxydopamine or gamma-hydroxybutyrate in awake rats. Neuropharmacology. 1984 Mar;23(3):309–318. doi: 10.1016/0028-3908(84)90192-8. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper D., Mytilineou C., Blum M. EGF enhances the survival of dopamine neurons in rat embryonic mesencephalon primary cell culture. J Neurosci Res. 1991 Oct;30(2):372–381. doi: 10.1002/jnr.490300213. [DOI] [PubMed] [Google Scholar]
- Date I., Notter M. F., Felten S. Y., Felten D. L. MPTP-treated young mice but not aging mice show partial recovery of the nigrostriatal dopaminergic system by stereotaxic injection of acidic fibroblast growth factor (aFGF). Brain Res. 1990 Aug 27;526(1):156–160. doi: 10.1016/0006-8993(90)90264-c. [DOI] [PubMed] [Google Scholar]
- Di Paolo T., Daigle M., Dupont A. Distribution of dopamine in 35 subregions of the rat caudate-putamen: a high performance liquid chromatography with electrochemical detection analysis. Can J Neurol Sci. 1982 Nov;9(4):421–427. doi: 10.1017/s0317167100044334. [DOI] [PubMed] [Google Scholar]
- DiStefano P. S., Friedman B., Radziejewski C., Alexander C., Boland P., Schick C. M., Lindsay R. M., Wiegand S. J. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992 May;8(5):983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Engber T. M., Chase T. N. Dextromethorphan does not protect against quinolinic acid neurotoxicity in rat striatum. Neurosci Lett. 1988 Dec 19;95(1-3):269–274. doi: 10.1016/0304-3940(88)90669-6. [DOI] [PubMed] [Google Scholar]
- Engele J., Schubert D., Bohn M. C. Conditioned media derived from glial cell lines promote survival and differentiation of dopaminergic neurons in vitro: role of mesencephalic glia. J Neurosci Res. 1991 Oct;30(2):359–371. doi: 10.1002/jnr.490300212. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Hartikka J., Hefti F. Development of septal cholinergic neurons in culture: plating density and glial cells modulate effects of NGF on survival, fiber growth, and expression of transmitter-specific enzymes. J Neurosci. 1988 Aug;8(8):2967–2985. doi: 10.1523/JNEUROSCI.08-08-02967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F., Hartikka J., Eckenstein F., Gnahn H., Heumann R., Schwab M. Nerve growth factor increases choline acetyltransferase but not survival or fiber outgrowth of cultured fetal septal cholinergic neurons. Neuroscience. 1985 Jan;14(1):55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Hefti F., Melamed E., Wurtman R. J. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980 Aug 11;195(1):123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988 Jan 21;331(6153):261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Bak I. J., Hassler R., Kim J. S., Wagner A. L-glutamate decarboxylase and choline acetyltransferase activity in the substantia nigra and the striatum after surgical interruption of the strio-nigral fibres of the baboon. Exp Brain Res. 1974 Jan 31;19(2):217–227. doi: 10.1007/BF00238536. [DOI] [PubMed] [Google Scholar]
- Knusel B., Michel P. P., Schwaber J. S., Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990 Feb;10(2):558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B., Winslow J. W., Rosenthal A., Burton L. E., Seid D. P., Nikolics K., Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Onali P., Olianas M. C. Stimulation of dopamine synthesis and activation of tyrosine hydroxylase by phorbol diesters in rat striatum. Life Sci. 1987 Mar 23;40(12):1219–1228. doi: 10.1016/0024-3205(87)90242-6. [DOI] [PubMed] [Google Scholar]
- Otto D., Unsicker K. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci. 1990 Jun;10(6):1912–1921. doi: 10.1523/JNEUROSCI.10-06-01912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzoli G., Zecchinelli A., Ricciardi S., Burke R. E., Fahn S., Scarlato G., Carenzi A. Intraventricular infusion of epidermal growth factor restores dopaminergic pathway in hemiparkinsonian rats. Mov Disord. 1991;6(4):281–287. doi: 10.1002/mds.870060403. [DOI] [PubMed] [Google Scholar]
- Refshauge C., Kissinger P. T., Dreiling R., Blank L., Freeman R., Adams R. N. New high performance liquid chromatographic analysis of brain catecholamines. Life Sci. 1974 Jan 16;14(2):311–322. doi: 10.1016/0024-3205(74)90061-7. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Otten U., Agid Y., Thoenen H. Nerve growth factor (NGF) in the rat CNS: absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979 Jun 8;168(3):473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spina M. B., Squinto S. P., Miller J., Lindsay R. M., Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem. 1992 Jul;59(1):99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Tassin J. P., Cheramy A., Blanc G., Thierry A. M., Glowinski J. Topographical distribution of dopaminergic innervation and of dopaminergic receptors in the rat striatum. I. Mictoestimation of [3H] dopamine uptake and dopamine content in microdiscs. Brain Res. 1976 May 7;107(2):291–301. doi: 10.1016/0006-8993(76)90227-4. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U., Arbuthnott G. W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970 Dec 18;24(3):485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. L., Altar C. A. Dopamine release in vivo from nigrostriatal, mesolimbic, and mesocortical neurons: utility of 3-methoxytyramine measurements. Pharmacol Rev. 1988 Sep;40(3):163–187. [PubMed] [Google Scholar]