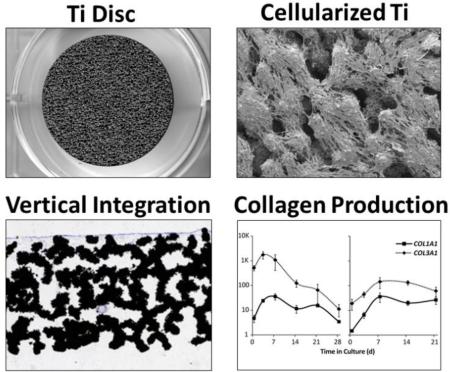
Abstract
Integration of porous metal prosthetics, which restore form and function of irreversibly damaged joints, into remaining healthy bone is critical for implant success. We investigated the biological properties of adipose-tissue derived mesenchymal stromal/stem cells (AMSCs) and addressed their potential to alter the in vitro microenvironment of implants. We employed human AMSCs as a practical source for musculoskeletal applications, because these cells can be obtained in large quantities, are multipotent, and have trophic paracrine functions. AMSCs were cultured on surgical-grade porous titanium discs as a model for orthopedic implants. We monitored cell/substrate attachment, cell proliferation, multipotency, and differentiation phenotypes of AMSCs upon osteogenic induction. High-resolution scanning electron microscopy and histology revealed that AMSCs adhere to the porous metallic surface. Compared to standard tissue culture plastic, AMSCs grown in the porous titanium microenvironment showed differences in temporal expression for genes involved in cell cycle progression (CCNB2, HIST2H4), extracellular matrix production (COL1A1, COL3A1), mesenchymal lineage identity (ACTA2, CD248, CD44), osteoblastic transcription factors (DLX3, DLX5, ID3) and epigenetic regulators (EZH1, EZH2). We conclude that metal orthopedic implants can be effectively seeded with clinical-grade stem/stromal cells to create a pre-conditioned implant.
Keywords: mesenchymal stem cell, biomaterial, gene expression, extracellular matrix, scanning electron microscopy
Graphical abstract
INTRODUCTION
Prosthetic joint replacements improve patient mobility by mitigating arthritis-associated pain, traumatic injury, tumor resection, and genetic defects [1]. The occurrence of such procedures has increased dramatically: cumulative total hip arthroplasty and total knee arthroplasty procedures exceed more than 1 million per year in the US [2, 3], and are projected to increase 4-fold by the year 2030 [4, 5]. Even low rates of prosthetic joint failures produce a significant burden on health care systems [5], and failures related to poor osseointegration (i.e., bone growth into the porous implant surface) can lead to long term patient disability. Implant failure commonly leads to absent, damaged, or fractured local bone. These complications create a compelling need for rapid and successful osseointegration that increases implant durability and clinical performance.
To prevent prosthetic joint failures, implants are optimized for high strength-to-weight ratios, improving biological fixation [6, 7]. Advancements in metallic biomaterials include composing implants of multiple alloyed and composite metals, such as tantalum, titanium and chromium, and surface modifications that increase the surface area available for cell attachment. However, to meet the increasing demands of aging patients, additional strategies are required to improve outcomes for patients with poor bone quality and/or complex reconstructive requirements. As a corollary to advancements in orthopedic implant design, adult tissue-derived stromal/stem cells are being investigated, because such cells retain trophic paracrine functions that may benefit the cellular microenvironment of healing tissues [8, 9]. Cell transplantations permit customization of implant components that may be further investigated using animal models of osseointegration [10].
To address the clinical need of improving implantation, the use of mesenchymal stromal/stem cells in adult reconstructive surgery merits additional exploration. Of particular interest are adipose-tissue derived mesenchymal stromal/stem cells (AMSCs); cells that can be harvested in large quantities while retaining their multi-lineage differentiation potential (e.g., into osteogenic, chondrogenic and adipogenic cells) [11]. According to Crespo-Diaz et al. [12], the yield of mesenchymal stromal cells per ml volume of adipose tissue is approximately 80,000-110,000, which is nearly 10-fold greater than an equivalent volume of bone marrow. For this investigation, we define AMSCs as mesenchymal progenitor cells of stromal origin (adipose tissue) with multi-lineage differentiation potential. However, the phenotype of proliferating AMSCs may change as cells become adapted to plastic-adherence and such phenotypic alterations may compromise their multi-lineage capabilities. Differentiation of AMSCs toward the osteoblast lineage, which results in extracellular matrix (ECM) production and mineralization, has been well-studied on tissue culture polystyrene (tc-PS) [12-14]. Therefore, a key question is whether AMSCs can be adapted to materials used for orthopedic implantation to generate a biologically favorable microenvironment for osseointegration.
Results presented in previous studies indicate that a number of mesenchymal and/or osteogenic cell types are capable of surviving, proliferating and differentiating on select metallic substrates [15-30]. In this study, we cultured clinical-grade AMSCs on surgical-grade porous structured titanium (ps-Ti) currently used in reconstructive joint arthroplasty. Our main findings are that: (i) ps-Ti is a suitable in vitro cell culture substrate for AMSCs, (ii) temporal expression of mRNAs for bone-related collagenous and non-collagenous ECM proteins upon osteogenic induction of AMSCs suggest positive changes to the implant microenvironment, and (iii) select histone modifying enzymes may act as suitable implant enhancement reagents due to their consistent patterns of regulation among cell donors.
METHODS
Porous structured titanium
ps-Ti discs (3 mm height; 25 mm diameter) comprised of alloyed titanium, aluminum, vanadium, and trace elements (Ti6Al4V) were provided by Stryker Orthopedics (Mahwah, NJ). The detailed fabrication method used in the manufacture of these discs, laser sintered additive manufacturing, has been described elsewhere [31]. Using an EOS 280 laser sintering machine, a powder of titanium alloy is melted by a computer-guided laser, which constitutes beads into solid form. This process is then continued layer-by-layer until a fully customizable, porous, three-dimensional metal shape is constructed. The resulting materials have the following parameters: strut size of 185.7 ± 8.4 μm, pore size of 408.6 ± 89.5 μm, overall porosity of 65.2 ± 3.1 %, yield strength of 176.13 ± 1.00 Mpa, an average modulus of elasticity of 3.48 ± 0.26 GPa, and a random pore distribution [31]. The surface roughness of this material was 81.372 +/− 6.297 μm, as measured at five independent points on one of the ps-Ti discs using a Zygo Newview white light interferometer (Zygo Corporation, Middlefield, CT). The constructs used in this study were designed to mimic the in vivo architectural environment of trabecular bone. A vacuum furnace was used to relieve any residual stresses from the laser sintering, and a cleaning process of passivation and gamma sterilization was performed to establish functional equivalency to current medical devices that are similarly fabricated.
Mesenchymal cells from adipose tissue
Human AMSCs were isolated from adipose tissue samples (i.e., lipo-aspirates) obtained from three de-identified healthy donors with written individual consent and institutional approval (Mayo Clinic Institutional Review Board, Rochester, MN). These cells have been in routine use at our institute specifically for clinical trial applications and have been extensively tested for cell surface markers, RNAseq transcriptome profiles and multi-lineage potential [12-14]. Cell isolation and culture conditions have previously been described in detail [12, 32, 33]. In brief, cells are isolated following fat tissue digestion with 0.075% Type I collagenase (≥125 units per mg dry weight; Worthington Biochemicals, Lakewood, NJ) for 1.5 h at 37°C. Adipocytes, which are more buoyant than mesenchymal stromal cells, were removed by low speed centrifugation (400 g for 5 min). The resulting stromal vascular fraction was rinsed with phosphate buffered saline (PBS) and strained (70 μm cell strainer; BD Biosciences), while erythrocytes were eliminated by lysis (154 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Mesenchymal stromal cells in the resulting fraction were expanded in Advanced MEM (Life Technologies, Grand Island, NY), 5% human platelet lysate (PLTMax; Mill Creek Life Sciences, Rochester, MN), 2 mM Glutamax (Life Technologies, Grand Island, NY), 2 U/ml heparin, 1% Penn-Strep (100 U/ml penicillin, 100 μg/ml streptomycin (Cellgro, Corning, NY)). Osteogenic supplements 50 ug/mL ascorbic acid (Sigma-Aldrich, St. Louis, MI), 10 mM β-glycerophosphatase (Sigma-Aldrich, St. Louis, MI), and 392.46 μg/mL dexamethasone (Sigma-Aldrich, St. Louis, MI) were added to the base medium on day 0 of experimentation. Previously published studies have shown that AMSCs grown for extended culture periods without inductive stimulus may detach or spontaneously differentiate into cells with connective tissue properties [13]. The key comparison in our present study is the phenotype of cells at the start of the experiment when cells adhere to titanium and the final biological state at the end of the osteoblastic time course.
During passivation, cells were released from T175 plates using 3mL of trypLE Express dissociation reagent (Life Technologies, Grand Island, NY) for 3 minutes at 37° C, followed by the addition of 12 mL of maintenance medium to stop dissociation. The remaining solutions containing the cells were centrifuged at 1100 rpm for 5 minutes and aspirated to form a cell pellet. Pellets were suspended in 30 mL of fresh osteogenic medium and plated in T175 cell culture plates. All cells used in experimentation were of passage 6 or 7, which is comparable to clinically administered cell populations, as they are capable of differentiation at later passage levels [12].
Cell sampling strategy
Cells were cultured in 6-well polystyrene plates (Corning Inc., Corning, NY). Each ps-Ti well was coated with poly 2-hydroxyethyl methacrylate (Sigma-Aldrich, St Louis, MO) to ensure growth of cells on the titanium discs and to prevent cell migration onto polystyrene. Standard two-dimensional tissue culture polystyrene (tc-PS) wells were used in parallel for comparison with ps-Ti experiments. To accomplish adsorption, cell suspensions of approximately 2.5 × 105 cells in 500 μL were applied to either the surface of ps-Ti discs (placed in the well of a 6-well plate) or tc-PS wells. An additional 500 μL of medium was added to the bottom of each well to prevent desiccation of the cells during adsorption. After 2-3 hours of adsorption in a standard incubator (37° C and 5% CO2), 3 mL of media were added to each well (day −1). Following adsorption, the cells were incubated for 24 h, and maintenance medium was replaced with osteogenic medium (day 0). Osteogenic media changes were performed every 3-4 days (i.e., twice weekly) throughout the 28-day duration of the study (Table 1; Supplementary Figure 1).
Table 1.
An overview of donor data and sampling for gene expression studies
| ID | 211 | 237 | 283 | |||
| Age | 41 | 60 | 54 | |||
| Gender | M | F | M | |||
| Disease | None | None | None | |||
| Harvest Site | Abdomen | Abdomen | Abdomen | |||
| Culture Treatment | tc-PS | ps-Ti | tc-PS | ps-Ti | tc-PS | ps-Ti |
| Day -1 (seeding/subconfluent) | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
| Day 0 (osteo. cocktail) | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
| Day 3 | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
| Day 7 | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
| Day 14 | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
| Day 21 | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
| Day 28 | ♣ | ♣ ♥ | ♣ | ♣ ♥ | ♣ | ♣ ♥ |
(♣ = RT-qPCR data; ♥ = SEM; tc-PS = tissue culture polystyrene; ps-Ti = porous structured titanium).
RNA Isolation & RT-qPCR
At each time point (days 0, 3, 7, 14, 21, and 28), cells from ps-Ti discs and tc-PS were lysed using Qiazol and further processed with the Qiagen miRNeasy Mini kit (Qiagen, Valencia, CA). Sampled RNA quantity (ng/ μL) and integrity (260/280 absorbance ratio) were measured using a Nanodrop 2000 (Thermo Fisher Scientific Inc, Minneapolis, MN). Reverse transcriptase-PCR reactions were performed in a total volume of 40 μL using the Superscript III RT kit (Invitrogen, Waltham, Massachusetts, USA.) Quantitative real-time PCR (RT-qPCR) reactions were prepared using the QuantiTect SYBR Green PCR kit (Qiagen) in a total volume of 10 μL with 0.05 μL of cDNA (10 ng/μL). Primer sequences used in qPCR reactions were selected as appropriate based on preliminary RNA-seq analyses conducted by our research group, and are listed in Table 2. Specifically, CCNB2 was selected as a marker of proliferation because it is specific for mitosis and therefore very clearly marks cells undergoing cell division, unlike cyclin D which is constitutively expressed in both cycling and non-cycling cells. Adiponectin was measured because the cells originate in adipose tissue, thereby making it important to determine the extent of differentiation toward the osteoblastic lineage. In our study, cells retain their adipose-tissue derived identity while becoming osteoblast-like.
Table 2.
Sequences for primers used in qPCR reactions
| Gene ID | Class / Biology | Forward Primer | Reverse Primer |
|---|---|---|---|
| ACAN | B / Cartilage & ECM | GTGCCTATCAGGACAAGGTCT | GATGCCTTTCACCACGACTTC |
| ACTA2 | B / Smooth muscle | AAAAGACAGCTACGTGGGTGA | GCCATGTTCTATCGGGTACTTC |
| ADIPOQ | B / Fat | AACATGCCCATTCGCTTTACC | TAGGCAAAGTAGTACAGCCCA |
| ALPL | B / Bone | ACTGGTACTCAGACAACGAGAT | ACGTCAATGTCCCTGATGTTATG |
| ATF1 | TF | CTGGAGTTTCTGCTGCTGTC | GGCAATGGCAATGTACTGTC |
| ATF4 | TF | ATGACCGAAATGAGCTTCCTG | CTGGAGAACCCATGAGGTTTG |
| BGN | B / ECM | GAGACCCTGAATGAACTCCACC | CTCCCGTTCTCGATCATCCTG |
| CCNB2 | B / Cell cycle | CCGACGGTGTCCAGTGATTT | TGTTGTTTTGGTGGGTTGAACT |
| CD248 | B / Cell surface | ATCGCAGCCAACTATCCAGAT | TTCCAGGCAAATGAGTGGTGG |
| CD44 | B / Cell surface | CTGCCGCTTTGCAGGTGTA | CATTGTGGGCAAGGTGCTATT |
| COL1A1 | B / Bone | GTAACAGCGGTGAACCTGG | CCTCGCTTTCCTTCCTCTCC |
| COL3A1 | B / Bone | TTGAAGGAGGATGTTCCCATCT | ACAGACACATATTTGGCATGGTT |
| DCN | B / Cartilage | ATGAAGGCCACTATCATCCTCC | GTCGCGGTCATCAGGAACTT |
| DLX3 | TF | TACCCTGCCCGAGTCTTCTG | GCCCGAGTAGTAATCGTGCTG |
| DLX5 | TF | AGCTACGCTAGCTCCCTACCACC | GGTTTGCCATTCACCATTCTCAC |
| EZH1 | ER / Me writer | GACATGCTATTGAAGGCAACA | GACACCATTCTCAGGGAACAT |
| EZH2 | ER / Me writer | GACGGCTTCCCAATAACAGTA | AGTCCCTGCTTCCCTATCACT |
| FOSL1 | TF | GCCGCCCTGTACCTTGTATCT | CAGTGCCTCAGGTTCAAGCA |
| GAPDH | Norm / Housekeeping | ATGTTCGTCATGGGTGTGAA | TGTGGTCATGAGTCCTTCCA |
| HIST2H4A | B / Cell cycle | AGCTGTCTATCGGGCTCCAG | CCTTTGCCTAAGCCTTTTCC |
| IBSP | B / Bone | GAACCTCGTGGGGACAATTAC | CATCATAGCCATCGTAGCCTTG |
| ID1 | TF | CTGCTCTACGACATGAACGG | GAAGGTCCCTGATGTAGTCGAT |
| ID3 | TF | GAGAGGCACTCAGCTTAGCC | TCCTTTTGTCGTTGGAGATGAC |
| KLF10 | TF | CTTCCGGGAACACCTGATTTT | GCAATGTGAGGTTTGGCAGTATC |
| MEF2C | TF | TCGTGGAGACGTTGAGAAAG | TTCTTGTTCAATGCGGAATC |
| MGP | B / ECM | TCCGAGAACGCTCTAAGCCT | GCAAAGTCTGTAGTCATCACAGG |
| MSX2 | TF | ATGGCTTCTCCGTCCAAAGG | TCGTCGGGCGAAAACAAGTC |
| NKX3-1 | TF | CCCACACTCAGGTGATCGAG | GAGCTGCTTTCGCTTAGTCTT |
| PRRX1 | TF / MSC phenotype | CAGGCGGATGAGAACGTGG | AAAAGCATCAGGATAGTGTGTCC |
| RUNX1 | TF | CTGCCCATCGCTTTCAAGGT | GCCGAGTAGTTTTCATCATTGCC |
| RUNX2 | TF | TGGTTACTGTCATGGCGGGTA | TCTCAGATCGTTGAACCTTGCTA |
| RUNX3 | TF | AGGCAATGACGAGAACTACTCC | CGAAGGTCGTTGAACCTGG |
| SATB2 | TF | GTGCCCGGATGGTTACACC | CTTCCTACAAATATGGCGGCAT |
| SOX4 | TF | AGCGACAAGATCCCTTTCATTC | CGGGGTAGTCAGCCATGTG |
| SOX9 | TF | TGTATCACTGAGTCATTTGCAGTGT | AAGGTCTGTCAGTGGGCTGAT |
| SP1 | TF | CAGGTGCAAACCAACAGATTA | GCTGGAGTAGGTTTGGCATAG |
| SUV39H1 | ER / Me writer & reader | CCTGCCCTCGGTATCTCTAAG | ATATCCACGCCATTTCACCAG |
| SUV39H2 | ER / Me writer & reader | TCTATGACAACAAGGGAATCACG | GAGACACATTGCCGTATCGAG |
| TRPS1 | TF / MSC phenotype | ATGACACTCCTGTTGGGTACT | CGTGCTGCTTGCCATAATGTT |
(B = biomarker; TF = transcription factor; ER = epigenetic regulator; Me = methylation; MSC = mesenchymal stromal/stem cell).
Quantification measurements (Ct) were conducted using a CFX384 Real-time System c1000 Touch Thermal Cycler (BioRad, Hercules, California). Transcript levels measured by RT-qPCR were normalized to the housekeeping gene GAPDH using the 2ΔΔCt method. Additionally, the combined matrix of all mRNA expression values in multiple differentiation time-courses was log-transformed, row-centered and standardized to account for biological changes in GAPDH gene expression.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was conducted once on a naked ps-Ti disc (without cells), as well as cell-seeded ps-Ti discs at each time point (days 0, 3, 7, 14, 21, and 28). Discs were immersed in Trump's fixative (pH of ~7.3) to preserve cell attachment. The following treatments were performed to rinse, fix and desiccate each sample: (i) PBS (2x), (ii) water (2x); (iii) 10% ethanol; (iv) 30% ethanol; (v) 50% ethanol; (vi) 70% ethanol; (vii) 90% ethanol; (viii) 95% ethanol; (ix) 100% ethanol (2x). Discs were then placed in a critical point dryer while still in 100% ethanol and subsequently attached to an aluminum stub by double-sided conductive tape. Final preparation included sputter-coating for 90 s with gold-palladium. In total, images at low (30x), medium (200x), and high (1300x) magnification were obtained for each sample (Table 1; Supplementary Figure 1).
Sectioning & Staining
To measure three-dimensional growth, AMSCs from a fourth donor were seeded onto ps-Ti discs for 14 days. To preserve the state of cells attached to the ps-Ti disc, they were rinsed in PBS, transferred to neutral buffered formalin (10%) for 24 hours and then placed in 70% ethanol. Each disc was then embedded in acrylic resin (methyl methacrylate monomer; Fisher Scientific) and cut into full-width sagittal sections 200 μm thick using a diamond-tipped band saw (Exakt Technologies, Oklahoma City, OK). To highlight cell formation, two histological procedures, Sanderson's rapid bone stain (Dorn & Hart Microedge, Villa Park, IL) and modified Gomori's stain (Sigma-Aldrich, St. Louis, MO), were conducted on representative AMSC samples from day 14 (i.e., cell donor 258). Modified Gomori's staining involves soaking the slides in a mixture of Weigert's hematoxylin followed by a rinse with distilled water, a 20 minute soak with Gomori stain, a rinse with 1% acetic acid (2x), and dehydration. Stained images were captured using a Sony 3CCD DSP instrument. ImageJ, an open-source NIH image analysis software program, was used to evaluate three-dimensional AMSC integration (i.e. depth of penetration) into pores of the ps-Ti scaffold.
RESULTS
Orthopedic implant devices normally function to restore joint mobility and relieve pain, but some implants fail. Our goal was to coat ps-Ti with AMSCs and monitor their attachment to the ps-Ti substrate, proliferation and survival, as well as their ability to differentiate along the osteogenic lineage and initiate bone-related ECM production. This strategy is warranted because successfully combined application of implants and AMSCs may reduce the rate of primary arthroplasty failure and improve the outcomes of revision procedures.
Surface adherence and pore integration
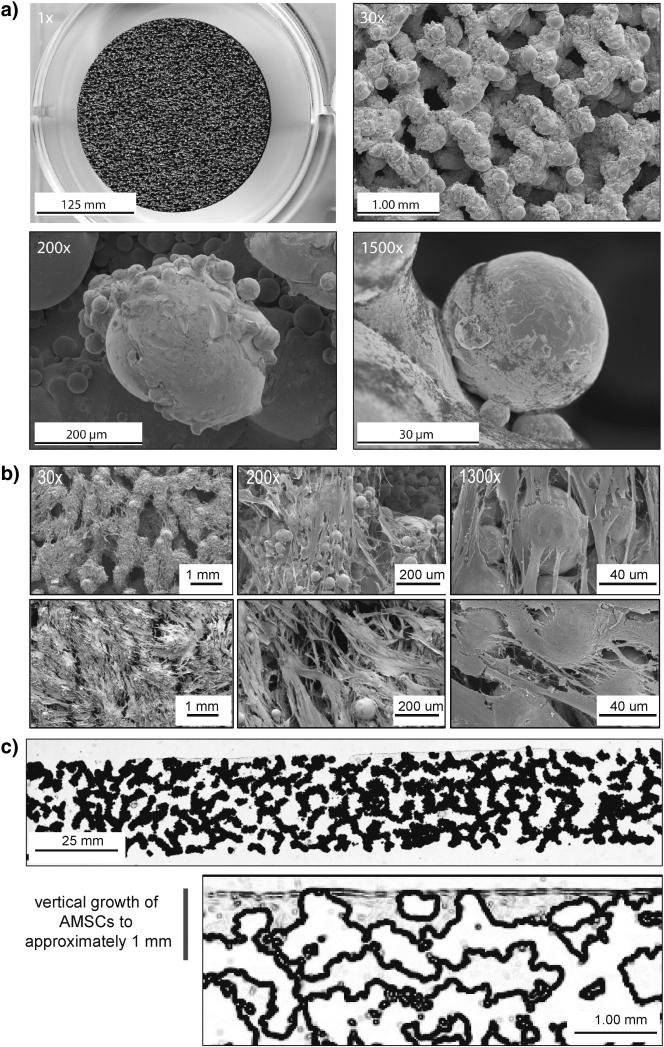
AMSCs retain the intrinsic ability to become osteogenic upon induction with appropriate tissue culture cocktails [13]. Thus, as a possible strategy to improve implant osseointegration, we considered whether autogenic or allogeneic AMSCs are viable when seeded onto orthopedic devices prior to implantation. We first examined the attachment of cells to the horizontal and vertical surfaces of metallic discs. Throughout our studies, AMSCs were initially seeded in the presence of normal maintenance medium (day −1 to day 0). Cells were subsequently cultured in osteogenic medium to promote production of an osteoblast-like extracellular matrix and avoid spontaneous differentiation into less defined fibroblastic phenotypes, as previously noted [13]. ps-Ti has a complex three-dimensional structure with macro-, micro-, and nano-scale features (e.g., struts, pores, and beads; Fig. 1a) that are suitable for the adherence of AMSCs (Fig. 1b). Vertically, AMSCs migrated into the pores of ps-Ti discs, as evidenced by a cross-sectional analysis showing positive staining of cell growth and/or migration to a depth of approximately 1 mm after 14 days (Fig. 1c). Thus, ps-Ti discs support the three-dimensional growth of AMSCs.
Figure 1.
(a) Scanning electron microscopy images of porous structured titanium (TiAl6V4) at 1x, 30x, 200x, and 1500x. (b) SEM images after 7 days in culture at 30x, 200x, and 1300x. (c) Histology slide of Sanderson's rapid bone stain demonstrating vertical penetration into pores of ps-Ti to an approximate depth of 1 mm. Samples were taken from donor AMSC-258 after 14 days. Close-up image of Sanderson's Rapid Bone Stain (Collagen = Grey).
Phenotypic variation in cell growth
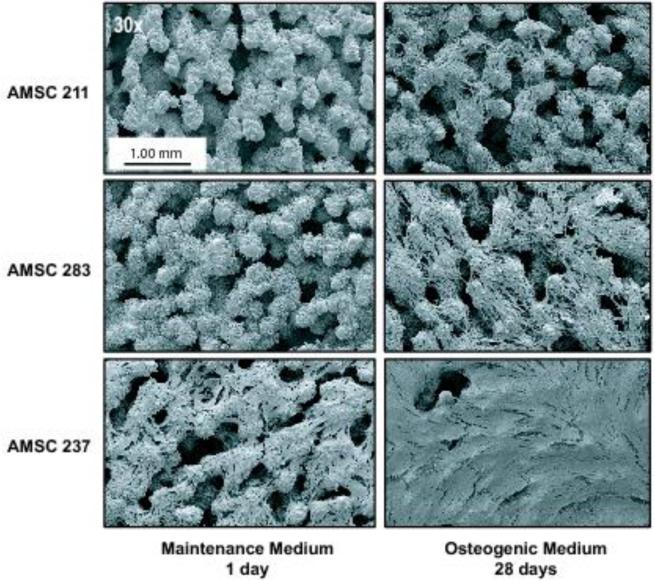
Next, we examined whether osteogenic AMSCs from several different patients (Table 1) display the same adherence pattern. Based on SEM analysis, AMSCs exhibit patient-to-patient (or sample-to-sample) variation in cellular attachment and lateral expansion on ps-Ti discs. SEM images revealed the lateral expansion of proliferating cells and complete coverage of the ps-Ti substrate. This expansion was observed for all patient donors after the initial day of seeding in normal maintenance media, as well as after subsequent culture in osteogenic media at, respectively, 3, 7, 14 and 28 days in culture (Fig. 1b). AMSCs from all three patients adhered to ps-Ti discs after 1 day, survived for at least 28-days, and migrated into interstitial spaces (Figs. 1c and 2). After 7 days, cells ceased proliferation and maximize ECM production (see below). In addition, cells at the beginning and end of experimental time points illustrate heterogeneity among patient cell donors.
Figure 2.
Scanning electron microscopy images (30x resolution) of highly porous structured titanium (TiAl6V4) seeded with adipose tissue derived mesenchymal stromal/stem cells (AMSCs) from three different human donors: after 1 day in standard maintenance medium (left), or 4 weeks in osteogenic medium (right).
We note that phenotypic variability in cell-cell or cell/ps-Ti contacts among cell populations was apparent after 7 days (Fig. 1b). Our SEM images exhibit variation in the extent of surface coverage. These differences are possibly related to cell number (i.e., at the end of the culturing period), random sampling within each cell population, and/or patient-to-patient heterogeneity. Irrespective of the differences observed by SEM, the consistent adherence and survival of AMSCs on the ps-Ti substrate suggests that they generate a potentially beneficial biological micro-texture (e.g., by expressing ECM proteins; see below).
ps-Ti supports robust cell proliferation and prolonged ECM mRNA production
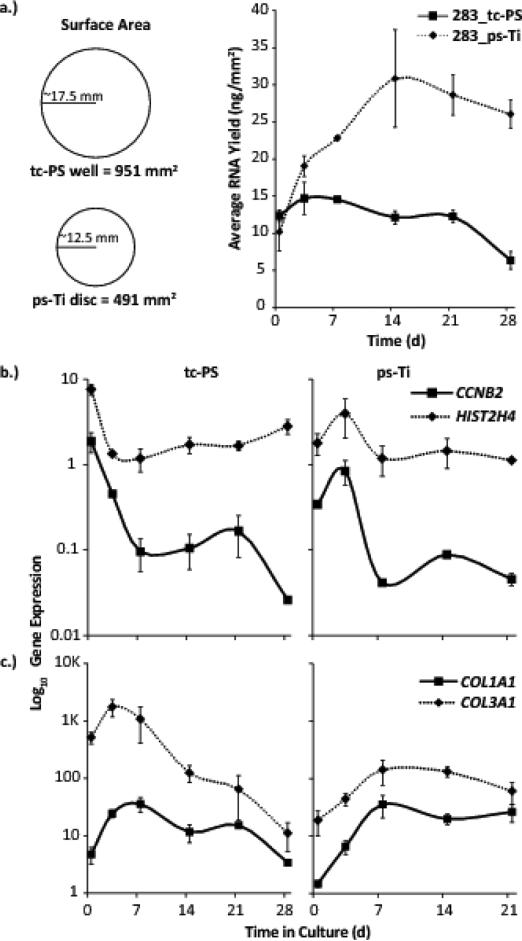
In addition to nutrients, gases and temperature, the number of cells supported by a culture substrate is directly proportional to the surface area available for colonization. Compared to normal tc-PS, ps-Ti is a three-dimensional substrate that permits expansion in the z-direction, and thus has an increased surface area. Consistent with this increased surface area, greater average RNA yields are evident for cells cultured in osteogenic media on ps-Ti compared to tc-PS. The increased RNA yields in osteogenic medium suggest that the cells are actively proliferating and/or generate more RNA per cell. Cells were not counted directly due to the technical difficulties of reliably extracting all cells that have invaded the interconnected pores of the porous Titanium material. While RNA yields increase on tc-PS until cells begin to reach confluence around day 3, RNA yields continue to rise until a maximum on day 14 for cells grown on ps-Ti discs, as cells colonize the additional surfaces provided by the three-dimensional architecture (Fig. 3a). The 3D surface area of the ps-Ti disc is higher due to the increased surface roughness and rugosity of the materials, although differences in RNA yield may also be caused by increased cell adhesion, proliferative potential and/or metabolic rate of the cells. Examination of RNA integrity by spectrophotometry revealed that RNA remains intact at all days during the 28-day culturing period, indicating that RNA samples at all time-points are primarily derived from living (or recently living) cells.
Figure 3.
(a) Average RNA Yield (ng/mm2) on porous structured Ti (ps-Ti) vs tissue culture polystyrene (tc-PS) for a representative donor (AMSC 283). RNA yields were standardized relative to the respective 2D surface areas of standard 6-well tissue culture plate (951 mm2) and ps-Ti discs (491 mm2). mRNA yields increased considerably by day 14 on ps-Ti compared to tc-PS samples. (b) Cell cycle markers (CCNB2, HIST2H4). (c) ECM production by collagenous bone-related proteins (COL1A1, COL3A1).
We tested whether cultured cells can assume one of its two fundamentally distinct biological cellular states (an actively proliferating self-renewing state or confluent metabolically active differentiated state). As a biomarker of cell cycle progression and proxy for proliferation, we measured expression levels of Cyclin B2 (CCNB2) throughout the 28-day time course for both ps-Ti and tc-PS samples. CCNB2 reaches its maximum expression on day 3 in cells grown on ps-Ti, while cells grown on tc-PS reach this maximum three days earlier at the start of the time course (day 0; Fig. 3b). As a representative mRNA for histone proteins (which compact newly replicating DNA during S phase in mitotically dividing cells), we analyzed a robustly expressed and the cell cycle controlled H4 variant (HIST2H4A). The HIST2H4A mRNA exhibits a similar pattern of initial down-regulation for cells cultured on tc-PS and an overall increase to a maximum on day 3 for cells cultured on ps-Ti (Fig. 3b).
We have previously shown that actively dividing AMSCs grown on tc-PS express mostly cytoskeletal and chromatin-related proteins, while high level expression of most ECM proteins appears to be restricted to non-dividing confluent cells [13]. Therefore, we anticipated that loss of cell cycle markers, which reflects the cessation of cell division of AMSCs on ps-Ti, would be followed by the production of mRNAs for ECM proteins during osteogenic differentiation. We measured expression of genes involved in ECM production, such as collagenous proteins (e.g., collagen type I α1 (COL1A1) and collagen type III α1 (COL3A1)). Maximal expression of COL1A1 and COL3A1 occur later in cells grown on ps-Ti (days 7-14) than tc-PS (days 3-7) (Fig. 3c), and also decrease more rapidly in cells grown on tc-PS. Taken together, RNA yields and mRNA expression results indicate that AMSCs grown on tc-PS tend to reach a maximum proliferative potential and start producing ECM sooner, but they achieve greater proliferation and sustain ECM production longer when seeded on ps-Ti.
Non-collagenous ECM production is not diminished by ps-Ti
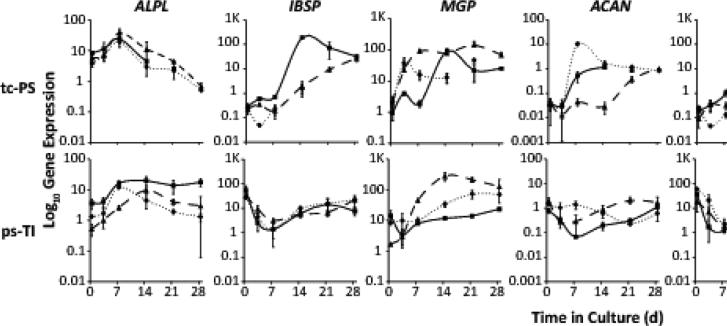
We tested whether osteogenic differentiation of mesenchymal stromal/stem cells on ps-Ti results in expression of non-collagenous ECM proteins. Due to increased RNA yield on ps-Ti discs, we predicted that production of mRNAs for ECM proteins would be prolonged. We measured expression levels of representative non-collagenous biomarkers. For all three patient cell populations grown on ps-Ti, phenotypic changes related to proliferation and ECM synthesis could be detected in patterns similar to those grown on tc-PS (Fig. 4). For example, mRNA expression levels of non-collagenous bone-related extracellular matrix proteins (alkaline phosphatase liver/bone/kidney (ALPL), integrin-binding sialoprotein (IBSP), matrix gla protein (MGP), aggrecan (ACAN), and adiponectin (ADIPOQ), biglycan (BGN), and decorin (DCN)) on ps-Ti were not diminished in comparison to tc-PS (Fig. 4). Also, the mRNA levels of each non-collagenous ECM protein followed unique temporal patterns and exhibited intricate differences between cells grown on tc-PS versus ps-Ti (Fig. 4). The main finding of these temporal gene expression profiles for non-collagenous ECM proteins is that production of mRNAs for ECM proteins by cells grown on ps-Ti is robust and follows temporal patterns distinct from cells grown on tc-PS.
Figure 4.
Production of genes related to ECM. Non-collagenous bone-related proteins: ALPL, IBSP, MGP, ACAN, ADIPOQ, and small leucine-rich proteoglycans: BGN, and DCN.
Mesenchymal identity is maintained on both culture substrates
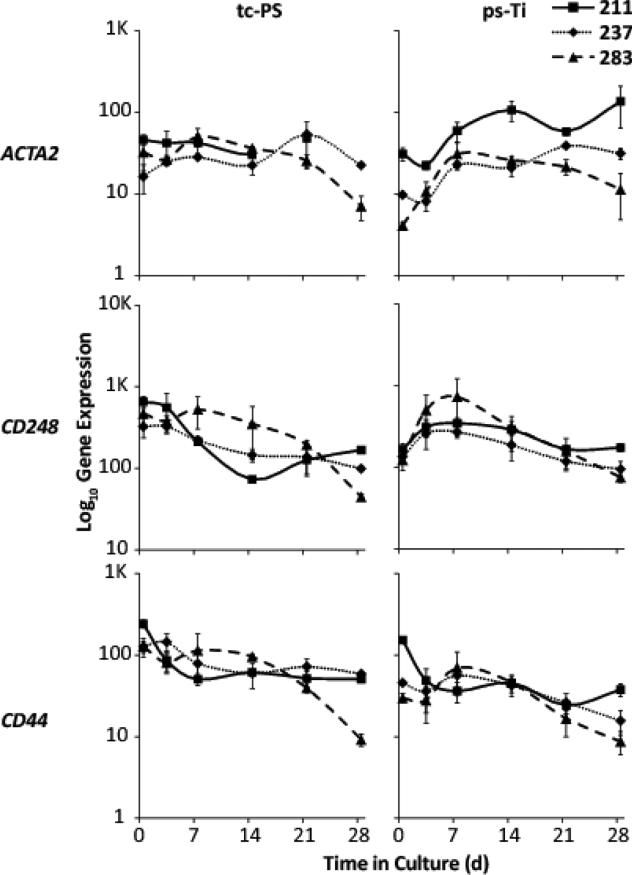
Three-dimensional substrates can change the cell surface and physical structure of mesenchymal stromal/stem cells. To verify the mesenchymal identity of AMSC populations grown on ps-Ti and tc-PS surfaces, we assessed gene expression levels of actin, alpha 2, smooth muscle, aorta (ACTA2), endosialin/Cluster of Differentiation 248 (CD248) and Cluster of Differentiation 44 (CD44). Expression levels were similar for all three patients in both microenvironments (Fig. 5). These results suggest that AMSCs maintain their mesenchymal identity independently of the culture substrate.
Figure 5.
Identity of AMSCs based on mesenchymal lineage markers: ACTA2, CD248, and CD44.
TFs targeted by BMP2 are differentially expressed in tc-PS and ps-Ti
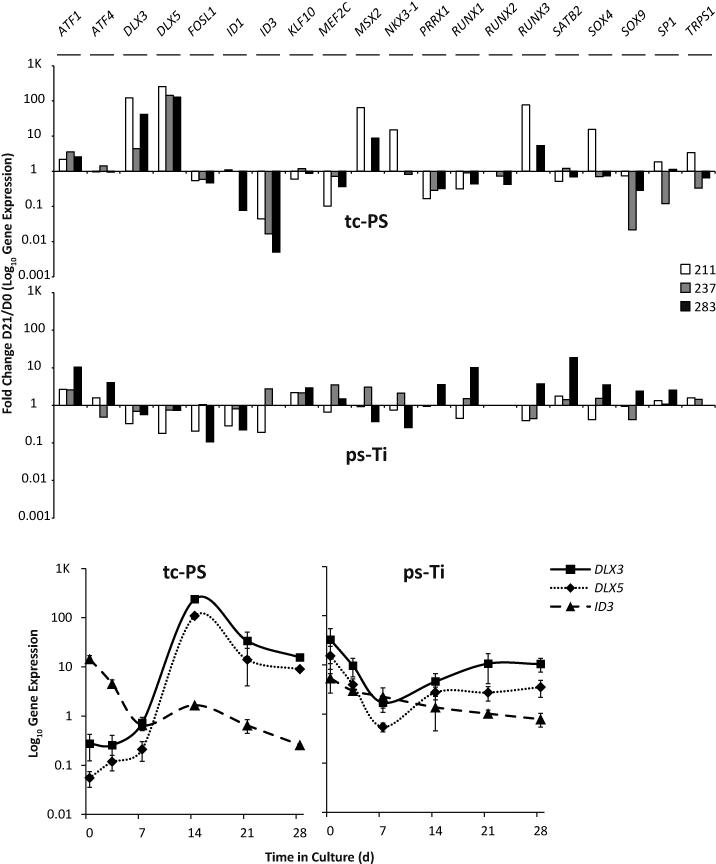
Phenotypic differences between cells grown on tc-PS and ps-Ti are evident from changes in gene expression that are controlled by transcription factors. To begin assessing gene regulatory events, we screened a large panel of transcription factors known to regulate the subcellular processes of mesenchymal stromal/stem cells. Within this panel, we found that the distal-less homeobox 3 (DLX3), and its paralog distal-less homeobox 5 (DLX5), increase over time in cells grown on tc-PS for all three patients, whereas, they decreased in cells grown on ps-Ti. Inhibitor of DNA binding 3 (ID3), a dominant negative helix-loop-helix protein, showed the opposite pattern of fold-change for cells cultured on tc-PS versus ps-Ti, consistent with findings for all three patients (Fig. 6). Interestingly, DLX3, DLX5 and ID3 are all important targets of bone morphogenetic protein 2 (BMP2). Thus, genes involved in BMP2 signaling (e.g., DLX3, DLX5 and ID3) appear to exhibit ps-Ti dependent changes in expression. Also of interest, the runt-related transcription factor 2 (RUNX2) is genetically required for bone and stimulated during osteoblast differentiation, but this marker is not unique for bone, and broadly expressed in mesenchymal cells. Although RUNX2 is robustly expressed throughout the time course, its levels do not appear to be modulated (i.e., fold-change indicated in Fig. 6).
Figure 6.
Screen of gene expression for 20 transcription factors (fold change of expression on D21 vs D0). Time course analysis of mRNA levels for DLX3, DLX5, and ID3 using AMSC 211.
Changes in expression of epigenetic regulators on ps-Ti discs
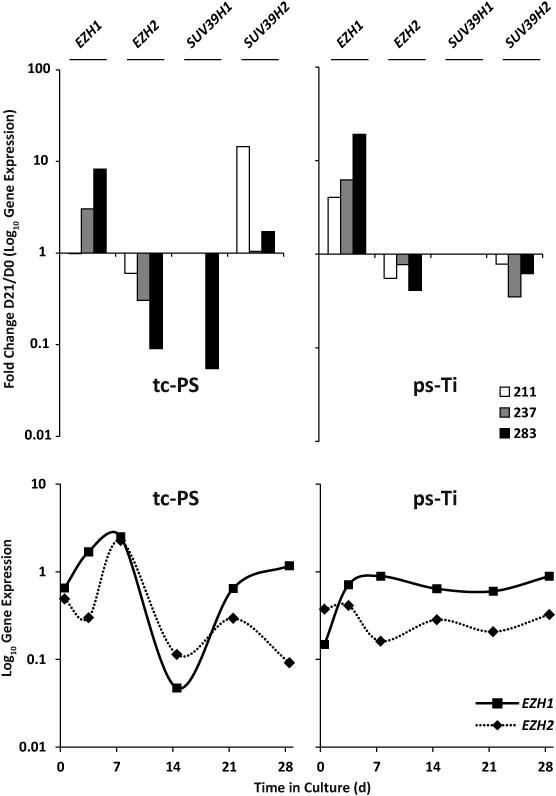
Adaptability of mesenchymal cells to in vitro culture substrates may be driven by epigenetic modifications controlled by histone modifying enzymes [13, 14]. To assess whether epigenetic modifications are associated with observed phenotypic differences among AMSCs cultured on tc-PS or ps-Ti, we performed a limited expression screen of epigenetic regulators based on previously published data regarding their important role in bone formation and homeostasis [34, 35]. We monitored mRNA levels for proteins known to influence the osteogenic differentiation of these cells [13] by either depositing transcriptionally repressive chromatin marks through trimethylation of histone H3 lysine 27 (H3K27) or lysine 9 (H3K9). Enhancers of Zeste 1 and Zeste 2 in the Polycomb Repressive Complex 2 Subunit (EZH1 & EZH2), which mediate and maintain cell pluripotency and plasticity through H3K27 methylation [34] show consistent patterns of up and down regulation, respectively, based on fold-change comparisons (Fig. 7). Suppressors of Variegation 3-9 Homolog 1 and Homolog 2 (Drosophila) (SUV39H1 & SUV39H2), which mediate transcriptional gene silencing through H3K9 methylation [35], were less consistently expressed among patients. Our results suggest selective differences in the expression of epigenetic regulators when cells are cultured on ps-Ti and tc-PS.
Figure 7.
Mini-screen of epigenetic regulators related to bone (EZH1, EZH2, SUV39H1, and SUV39H2) as fold change of expression on D21 vs D0. Time course data showing temporal changes in mRNA levels consistent with results of the analysis of selected epigenetic regulators on tc-PS versus ps-Ti for a representative patient (AMSC 211).
DISCUSSION
Complications in patients with major bone deficiencies, diabetes, osteopenia, osteoporosis, and/or impaired bone healing (e.g., when smoking or receiving chemotherapy/radiation) often lead to challenging primary and revision surgeries. These patients would indirectly benefit from in vitro validation of combining an FDA-approved implant material with human AMSCs prepared under Good Manufacturing Practice guidelines, if the technology progressed to animal model and human clinical trial. Among metal biomaterials (e.g., highly porous tantalum, trabecular metal), ps-Ti has a favorably high coefficient of friction and surface rugosity, while its material strength mimics the elastic modulus of bone. Therefore, our study was designed to investigate the potential for improving a currently used implant material by seeding AMSCs to ultimately achieve rapid and ubiquitous osseointegration. We uncovered the potential of this technique by revealing that human AMSCs can be successfully seeded and expanded on the metal, while preserving their physicochemical identity, and importantly, their osteogenic potential.
AMSCs attach to ps-Ti
To assess strategies for enhancing orthopedic implant osseointegration, we examined whether AMSCs are capable of adhering to ps-Ti surfaces. We found that these cells clearly have potential use as a coating material for orthopedic bone implants. We selected clinical-grade AMSCs grown in human platelet lysate because these cells are in ample supply, are capable of tri-lineage differentiation [12], and have trophic actions that may aid in recruiting other cells that regenerate bone [8]. The timing of lateral surface coverage expansions on the ps-Ti substrate varied depending on patient donor, yet ps-Ti supports three-dimensional substrate integration. Also on ps-Ti, AMSCs exhibited delayed cessation of proliferation, prolonged expression of mRNAs related to ECM production, and retained mesenchymal identity, suggesting therapeutic relevance in vivo.
When metallic implants interact with cells through focal adhesions, this contact should trigger integrin-dependent activation of kinase pathways. These kinases ultimately communicate with gene regulatory pathways by phosphorylating transcription factors and/or epigenetic regulators that function as molecular endpoint effectors in the nucleus. Stable production of known BMP2 targets (DLX5 and ID3), were observed in cells grown on ps-Ti. EZH1 and EZH2 exhibit differences in expression on ps-Ti compared to standard tc-PS, suggesting a potentially important role for the culture substrate to alter epigenetic regulation. Because transcription factors and epigenetic regulators control the osteoblast phenotype and bone-related ECM gene expression by altering molecular memory at the level of chromatin structure, we envision that modulating the activities of these gene regulatory factors by inhibition may permit beneficial manipulations of the osteogenic potential of ps-Ti-seeded AMSCs.
Optimizing cell growth and differentiation on ps-Ti
The rugosity of ps-Ti increases the three-dimensional surface area accessible to cells. Nevertheless, cell coverage of ps-Ti was observed on a simple disc as a model for stem cell growth on an orthopedic ps-Ti implant. Although ps-Ti exhibits considerable structural complexity by SEM, we show that AMSCs from three different patients are capable of adhering to ps-Ti and continuously cover the surface within two to three weeks. We also show that when AMSCs differentiate on ps-Ti, they produce high levels of mRNAs for bone-related collagenous and non-collagenous proteins (e.g., collagens and proteoglycans), as well as paracrine signaling ligands (e.g., cytokines and chemokines). Recent data from our group indicate that these bone-anabolic functions of AMSCs can be enhanced by inhibiting epigenetic enzymes [36]. Thus, it is feasible to design strategies to test the role of specific epigenetic inhibitors on expression of bone-related ECM proteins during osteogenic differentiation of AMSCs on ps-Ti in three-dimensional culture.
Trophic paracrine activities of AMSCs
AMSCs are trophically active, yet the specific trophic functions of cells attached to biomaterials are not well documented. Specifically, cells should alter the implant surface microenvironment by coating the ps-Ti surface with biosynthetically produced ECM proteins, and by secreting paracrine factors that will either recruit other mesenchymal progenitors or inactivate inflammatory cells [8]. Experimentally validating these scenarios would be a logical next step toward building on the results presented here. Likewise, some cells and surface coatings have an exogenous antibacterial action when attached to biomaterials. In the case of ps-Ti, this hypothesis is testable by co-culture experiments designed to measure the “race for the surface” by AMSCs in comparison to specific pathogenic bacteria [37].
Additional metallic biomaterials may positively interact with AMSCs to improve osseointegration (e.g., tantalum, cobalt chromium, nitinol, plastic polymers, and hydrogels), or materials with tethered paracrine factors (or other agents) to improve the microenvironment. By modulating culture conditions (e.g., oxygen tension), or using other mesenchymal cell types or sources it may be possible to further optimize cell culture conditions. Nevertheless, the results presented here provide the in vitro validation necessary for further in vitro experimentation and functional osseointegration modeling in vivo.
Conclusion
Innovative improvements in implant technologies are necessary to address surgical complications resulting from more than 4 million total hip and knee replacements expected by 2030 in the US [4]. Revision joint surgeries are sometimes necessary and associated with major bone loss and/or infection, thus reducing options for fixation of revision components. Responding to this pressing clinical need, we used clinical-grade mesenchymal stromal/stem cells to effectively coat a surgical-grade ps-Ti implant material. By concluding that ps-Ti implants can be biologically enhanced using AMSCs pre-committed to express the osteoblastic phenotype; we provide the preliminary information necessary for preclinical animal modeling, and by extension, preclinical FDA-approval for progression toward a human trial.
Supplementary Material
HIGHLIGHTS.
We modeled growth and differentiation of adipose-derived mesenchymal stromal cells (AMSCs) on orthopedic metallic implants
Electron microscopy and histology reveal that AMSCs adhere to porous titanium
AMSCs cultured on titanium exhibit altered cell cycle progression
Extracellular matrix production is enhanced on the titanium microenvironment
AMSCs maintain phenotypic identity as indicated by expression of key osteoblastic transcription factors and epigenetic regulators.
ACKNOWLEDGMENTS
We thank members of the van Wijnen and Dietz Laboratories, especially Matthew Getzlaf and Darcie Radel, as well as Scott Riester, Emily Camilleri and Janet Denbeigh for stimulating discussions and/or assistance with reagents and procedures. The Biomaterials Characterization and Quantitative Histomorphometry Core Laboratory core facility performed histological analysis, particularly Bob Brown, and Jim Herrick. We are grateful to Scott Gamb and the Electron Microscopy Core facility for their assistance with acquiring scanning electron microscopy images. We thank the Reconstructive Research and Development group at Stryker Orthopedics, particularly Brent Mitchell, Dale Swarts, and Mark Gruczynski for manufacturing biomaterials used in this study. Financial support for this work came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR049069 to Andre van Wijnen; T32 AR56950 to Eric Lewallen; and F32 AR066508 to Amel Dudakovic). We also appreciate the generous philanthropic support of William H. and Karen J. Eby, and the charitable foundation in their names. The following author disclosures are provided regarding affiliations that may be perceived to bias the presentation of this work: ABD has commercial interest in Mill Creek Life Sciences which manufactures the clinical grade commercial platelet lysate product used for maintaining adipose-tissue derived mesenchymal stem cells. RCC serves as the Vice President and General Manager of Reconstructive Research and Development, Stryker Orthopedics, 325 Corporate Drive, Mahwah, NJ 07430. DGL reports personal fees and other from Stryker, Pipeline Biomedical, Zimmer, and Ketai Medical Devices. In addition, DGL has patents on selected hip and knee implants with royalties paid by Zimmer, and is employed part time as the Medical Director for The American Joint Replacement registry.
ABBREVIATIONS
- AMSCs
adipose-tissue derived mesenchymal stromal/stem cells
- ECM
extracellular matrix
- tc-PS
tissue culture polystyrene
- ps-Ti
porous structured titanium
- PBS
phosphate buffered saline
- RT-qPCR
Quantitative real-time PCR
- SEM
Scanning electron microscopy
- CCNB2
Cyclin B2
- HIST2H4A
Histone Cluster 2, H4a
- COL1A1
collagen type I α1
- COL3A1
collagen type III α1
- ALPL
alkaline phosphatase liver/bone/kidney
- IBSP
integrin-binding sialoprotein
- MGP
matrix gla protein
- ACAN
aggrecan
- ADIPOQ
adiponectin
- BGN
biglycan
- DCN
decorin
- ACTA2
actin, alpha 2, smooth muscle, aorta
- CD248
endosialin/Cluster of Differentiation 248
- CD44
Cluster of Differentiation 44
- DLX3
distal-less homeobox 3
- DLX5
distal-less homeobox 5
- ID3
Inhibitor of DNA binding 3
- BMP2
bone morphogenetic protein 2
- RUNX2
Runt-Related Transcription Factor 2
- H3K27
histone H3 lysine 27
- H3K9
histone H3 lysine 9
- EZH1
Enhancers of Zeste 1 in the Polycomb Repressive Complex 2 Subunit
- EZH2
Enhancers of Zeste 2 in the Polycomb Repressive Complex 2 Subunit
- SUV39H1
Suppressor of Variegation 3-9 Homolog 1 (Drosophila)
- SUV39H2
Suppressor of Variegation 3-9 Homolog 2 (Drosophila)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Di Puccio F, Mattei L. Biotribology of artificial hip joints. World journal of orthopedics. 2015;6(1):77–94. doi: 10.5312/wjo.v6.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehil MS, Bozic KJ. Trends in total hip arthroplasty implant utilization in the United States. The Journal of arthroplasty. 2014;29(10):1915–8. doi: 10.1016/j.arth.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen LC, Lehil MS, Bozic KJ. Trends in Total Knee Arthroplasty Implant Utilization. The Journal of arthroplasty. 2014 doi: 10.1016/j.arth.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz S, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. The Journal of bone and joint surgery. American volume. 2007;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz SM, et al. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. The Journal of bone and joint surgery. American volume. 2014;96(8):624–30. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 6.Bobyn J, et al. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. Journal of Bone and Joint Surgery. 2004;86:123–129. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 7.Bobyn J, et al. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. journal of bone and Joint Surgery Br. 1999;81:907–914. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & molecular medicine. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eirin A, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewallen EA, et al. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Engineering Part B: Reviews. 2014;21(2):218–230. doi: 10.1089/ten.teb.2014.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crespo-Diaz R, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell transplantation. 2011;20(6):797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 13.Dudakovic A, et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. Journal of cellular biochemistry. 2014;115(10):1816–28. doi: 10.1002/jcb.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudakovic A, et al. Histone Deacetylase Inhibition Destabilizes the Multi-Potent State of Uncommitted Adipose-Derived Mesenchymal Stromal Cells. Journal of cellular physiology. 2015;230(1):52–62. doi: 10.1002/jcp.24680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benazzo F, et al. Trabecular titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. Journal of biomedical materials research. Part A. 2013 doi: 10.1002/jbm.a.34875. [DOI] [PubMed] [Google Scholar]
- 16.Gastaldi G, et al. Human adipose-derived stem cells (hASCs) proliferate and differentiate in osteoblast-like cells on trabecular titanium scaffolds. Journal of biomedical materials research. Part A. 2010;94(3):790–9. doi: 10.1002/jbm.a.32721. [DOI] [PubMed] [Google Scholar]
- 17.Blanco JF, et al. Titanium and tantalum as mesenchymal stem cell scaffolds for spinal fusion: an in vitro comparative study. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2011;20(Suppl 3):353–60. doi: 10.1007/s00586-011-1901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Peppo GM, et al. Free-form-fabricated commercially pure Ti and Ti6Al4V porous scaffolds support the growth of human embryonic stem cell-derived mesodermal progenitors. TheScientificWorldJournal. 2012;2012:646417. doi: 10.1100/2012/646417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ercan B, Webster TJ. The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials. 2010;31(13):3684–93. doi: 10.1016/j.biomaterials.2010.01.078. [DOI] [PubMed] [Google Scholar]
- 20.Findlay DM, et al. The proliferation and phenotypic expression of human osteoblasts on tantalum metal. Biomaterials. 2004;25(12):2215–2227. doi: 10.1016/j.biomaterials.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Gotman I, et al. Mesenchymal stem cell proliferation and differentiation on load-bearing trabecular Nitinol scaffolds. Acta biomaterialia. 2013;9(9):8440–8. doi: 10.1016/j.actbio.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Lopa S, et al. Orthopedic bioactive implants: Hydrogel enrichment of macroporous titanium for the delivery of mesenchymal stem cells and strontium. Journal of biomedical materials research. Part A. 2013;101(12):3396–403. doi: 10.1002/jbm.a.34649. [DOI] [PubMed] [Google Scholar]
- 23.Olivares-Navarrete R, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31(10):2728–35. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagomonyants KB, et al. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(4):609–16. doi: 10.1002/jor.21251. [DOI] [PubMed] [Google Scholar]
- 25.Welldon KJ, et al. Primary human osteoblasts grow into porous tantalum and maintain an osteoblastic phenotype. Journal of biomedical materials research. Part A. 2008;84(3):691–701. doi: 10.1002/jbm.a.31336. [DOI] [PubMed] [Google Scholar]
- 26.Hirota M, et al. High porous titanium scaffolds showed higher compatibility than lower porous beta-tricalcium phosphate scaffolds for regulating human osteoblast and osteoclast differentiation. Materials Science and Engineering: C. 2015;49:623–631. doi: 10.1016/j.msec.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Catauro M, et al. Biological response of human mesenchymal stromal cells to titanium grade 4 implants coated with PCL/ZrO2 hybrid materials synthesized by sol–gel route: in vitro evaluation. Materials Science and Engineering: C. 2014;45:395–401. doi: 10.1016/j.msec.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Chan CW, et al. Effect of laser treatment on the attachment and viability of mesenchymal stem cell responses on shape memory NiTi alloy. Materials science & engineering. C, Materials for biological applications. 2014;42:254–63. doi: 10.1016/j.msec.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Shen X, et al. Mesenchymal stem cell growth behavior on micro/nano hierarchical surfaces of titanium substrates. Colloids and Surfaces B: Biointerfaces. 2015;127:221–232. doi: 10.1016/j.colsurfb.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 30.Olivares-Navarrete R, et al. Coordinated regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by endogenous bone morphogenetic proteins. Bone. 2015;73:208–16. doi: 10.1016/j.bone.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen R. An Additive Manufacturing Technology Porous Metal Construct. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 34):267–267. [Google Scholar]
- 32.Mader EK, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11(20.10):1186. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoudifar N, Doran PM. Osteogenic differentiation and osteochondral tissue engineering using human adipose-derived stem cells. Biotechnology progress. 2013;29(1):176–85. doi: 10.1002/btpr.1663. [DOI] [PubMed] [Google Scholar]
- 34.Boyer LA, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 35.Guasconi V, et al. Preferential association of irreversibly silenced E2F-target genes with pericentromeric heterochromatin in differentiated muscle cells. Epigenetics. 2010;5(8):704–709. doi: 10.4161/epi.5.8.13025. [DOI] [PubMed] [Google Scholar]
- 36.Dudakovic A, et al. Epigenetic control of skeletal development by the histone methyltransferase Ezh2. Journal of Biological Chemistry. 2015;290(46):27604–27617. doi: 10.1074/jbc.M115.672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Getzlaf MA, et al. Multi-disciplinary antimicrobial strategies for improving orthopaedic implants to prevent prosthetic joint infections in hip and knee. Journal of orthopaedic research. 2015 doi: 10.1002/jor.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.