Abstract
Although genital HPV is the most prevalent STI in the US, rates of vaccination uptake among high-risk subgroups remain low. Investigations of vaccine compliance have mainly targeted mother-daughter dyads, which in some settings may prove difficult. This study examines an innovative culturally tailored, computer-delivered media-based strategy to promote HPV vaccine uptake. Data, inclusive of sociodemographics, sexual behaviors, knowledge, attitudes, and beliefs about HPV and vaccination were collected via ACASI from 216 African American adolescent females (ages 14–18 years) seeking services in family planning and STI public health clinics in metropolitan Atlanta. Data were obtained prior to randomization and participation in an interactive media-based intervention designed to increase HPV vaccination uptake. Medical record abstraction was conducted 7 month post-randomization to assess initial vaccine uptake and compliance. Participants in the intervention were more compliant to vaccination relative to a placebo comparison condition (26 doses vs. Seventeen doses; p=0.12). However, vaccination series initiation and completion were lower than the national average. Thorough evaluation is needed to better understand factors facilitating HPV vaccine uptake and compliance, particularly perceived susceptibility and the influence of the patient-provider encounter in a clinical setting.
Keywords: HPV, vaccination uptake, adolescent, media intervention
Introduction
Genital HPV is the most common sexually transmitted infection (STI) in the US, with about 14 million males and females newly infected annually.1 Over 80% of sexually active women are exposed to the virus within 3 to 4 y after coital initiation.2 As most females in the United States initiate sexual activity during adolescence, HPV is of particular concern in this age group.
HPV prevalence among adolescents is not uniform. Research consistently shows that among adolescent females, greater HPV prevalence is observed among low-income and minority populations,3,4 particularly African American female adolescents. One study, of a sample of predominantly African American adolescents 13–18 y of age, identified 70.7% infected with HPV.5 Another observed 64% HPV prevalence among a sample of predominantly African American adolescents 12–19 y of age.6 Further, 77% of that sample was infected with at least 1 high-risk HPV subtype, with HPV type 16 being the most prevalent (10.2%). The epidemiological research demonstrates that HPV prevalence is markedly higher for African American adolescents relative to other ethnic/racial groups. Prevention strategies directed at African American adolescents are urgently needed to reduce this marked and persistent health disparity.3,4
Despite a safe and effective vaccine to protect against HPV, vaccination rates are low, particularly among adolescents. Recommendations for HPV vaccination from the Advisory Committee on Immunization Practices (ACIP) are not sufficient, in and of themselves, to enhance vaccination rates, especially among populations that are most vulnerable to HPV infection like African American adolescents. Data from the 2006–2011 National Immunization Survey (NIS) for teens shows that only 53% of female adolescents 13–17 received one or more doses of the HPV vaccine. Further, only 34.8% of adolescents had completed the required 3 dose series.7 More recent data from the 2007–2012 NIS-teens shows that for the first time since the vaccine was approved, there has been no increase in series initiation and series completion rates among adolescent females in the United States.8
Several studies have also observed marked and persistent racial and socio-economic disparities in HPV vaccination initiation and series completion,7,9-14 with African American adolescents less likely than whites to initiate and complete the HPV vaccine series.11 The 2011 NIS-teens reported that only 56.0% of African American adolescents initiated the vaccine with only 31.7% completing the series. Series completion is lower than both whites (33.0%) and Hispanics (41.6%).7 This racial disparity is a serious public health concern as African American females are more likely to become infected with HPV, more likely to develop cervical cancer, and more likely to experience mortality from cervical cancer.10 Given such evidence, focused vaccination efforts on minority adolescents could reduce racial disparities in cervical cancer incidence being that cervical cancer is most prevalent among minority women.10 Thus, innovative, theoretically grounded intervention strategies are needed to enhance African American female adolescents' uptake of HPV vaccine.
Intervention innovation
A review of interventions to improve immunization rates found that multicomponent interventions were more effective than single-focus interventions.15 Interventions to enhance adoption of health-promoting behaviors have been effective – though of limited applicability in clinical venues. Unfortunately, it is often not feasible to implement or sustain these interventions in clinical venues attributable to a host of factors such as clinicians' lack of time, lack of awareness, lack of self-efficacy in health behavior counseling, lack of outcome expectations, and lack of reimbursement, as the most prominent barriers to providing clinician-delivered one-on-one health promotion education.16,17 Expecting clinicians to routinely provide in-depth advice and counseling during the typical brief outpatient visit is not realistic or sustainable.18 A new paradigm is required that overcomes these barriers and permits the dissemination, adoption, and sustainment of effective health promotion programs in clinical settings. One emerging innovative strategy to overcome many of the barriers to providing health promotion education in clinical venues is the use of computer-delivered programs.
Many interactive computer-delivered health programs use simulations, typically with limited branching based on users' decisions at critical junctures in the narrative. Simulations provide users an opportunity to practice choosing responses in realistic, videotaped scenarios. This opportunity to practice pairing responses with discriminative stimuli and obtaining immediate, realistic and safe feedback is superior to linear programs, which are played from beginning to end without user involvement.19
Technological interventions, such as computer-delivered programs, may also be particularly compelling for clinical venues attributable to: (1) cost being relatively low; (2) dissemination to large numbers of patients is possible; (3) information can be easily updated; (4) interventions can be tailored to be gender-, developmentally- and culturally-congruent to the particular patient population; (5) interventions can be readily incorporated into traditional clinical venues, as supplements in primary care settings; and (6) there is potential for greater adherence, engagement, and candor.20
Purpose of study
The primary purpose of the study was to conduct a randomized placebo-controlled trial to test the efficacy of a theory-based, multi-component computer-delivered media-based intervention, Girls OnGuard, designed to enhance the initial uptake of HPV4 and completion of the series. The main objectives were to (1) evaluate the efficacy of Girls OnGuard, relative to the health promotion comparison condition, in enhancing the initial uptake of the first dose of HPV4 and (2) evaluate the efficacy of Girls OnGuard, relative to the health promotion comparison condition, in enhancing compliance with receiving the second and third doses of HPV4. It was hypothesized that a larger proportion of adolescents in Girls OnGuard, relative to the health promotion comparison condition, would receive the first dose of the HPV4 vaccine and would complete a greater number of the 3 recommended doses of HPV4.
Theoretical framework
The Information-Motivation-Behavioral Skills (IMB) model provides a framework for understanding risk behavior and for constructing interventions to facilitate behavior change21,22 and has been applied to a wide range of behaviors across diverse populations.23 The IMB model asserts that individuals are likely to initiate and maintain positive health behaviors to the extent that they are well informed, motivated to act, and possess the requisite behavioral skills.24
Key IMB model constructs, specifically information, motivation, and behavioral skills, have been demonstrated to affect acceptance of HPV vaccination. In a qualitative study examining acceptability of HPV vaccination among young adult African American and Latina females, Scarinci et al25 found that 77.8% of African Americans had never heard about HPV. After a brief presentation about cervical cancer and HPV, African American females indicated that an HPV preventive vaccine would be acceptable; however, they expressed concerns about vaccine effectiveness and adverse side effects. Motivating factors for vaccination included: (1) receiving education/information about the vaccine, (2) affordable pricing, (3) good results in research trials, and (4) knowing others who had been vaccinated. Behavioral skills identified included using condoms, not having multiple male partners, and remaining abstinent; many were unaware that HPV vaccination was an effective prevention option. These findings suggest that unique intervention strategies should be developed based on the needs and perceptions of the target audience.
These findings are supported by 2 systematic reviews of predictors of HPV vaccine acceptance among parents and adolescents.26,27 These reviews found that often the majority of men and women had never heard of HPV. Although there is mixed evidence for the relationship between knowledge about HPV vaccination and HPV vaccine acceptance,27 several studies did identify greater knowledge about the benefits of HPV vaccination as a correlate of HPV vaccine acceptance. In terms of motivation, recent reviews identified several factors correlated with HPV vaccine acceptance among adolescents, including: (1) perceived likelihood of getting HPV or cervical cancer, (2) perceived severity of HPV infection, (3) perceived effectiveness of the HPV vaccine, (4) physician recommendation, (5) parental acceptance of HPV vaccination, and (6) school requirements.26,27
Both reviews noted prominent limitations of published acceptability research, specifically citing that much of the research is limited to small cross-sectional studies conducted with predominantly Caucasian samples.27 Most important, the majority of published acceptability studies were conducted prior to formal FDA approval and availability of the HPV4 vaccine; thus, the findings may be limited by the hypothetical nature of the HPV vaccine at that time.26
This study was a unique opportunity to apply IMB theoretical constructs to influence uptake of HPV4 vaccine among African American adolescents and determine actual vaccination rates through medical record abstraction, as opposed to participant's acceptance of vaccination.
Formative research
In addition to applying the IMB theoretical constructs to guide the development of the Girls OnGuard intervention, formative research was conducted in July and August of 2009. A combination of focus groups and individual interviews were conducted with mothers, health care providers, and adolescents to identify relevant knowledge, attitudes, and perceptions of HPV and HPV4 vaccination.28-30
Two focus groups were conducted with mothers of African American adolescent females, 13–18 y old, living in metropolitan Atlanta. Focus groups were audio-recorded, transcribed verbatim, and content analyzed for predominant themes. Mothers had some knowledge of HPV, but limited knowledge of the HPV4 vaccine and its side effects. They expressed a general need for more information on the vaccine, particularly its long-term effectiveness. While mothers preferred their daughters communicate with them about getting vaccinated, some mothers feared that their daughters' interest in vaccination was a signal of sexual activity. Overall, most mothers were receptive to vaccination; if their child's health care provider initiated the conversation and could clarify their questions about HPV4 vaccine.
Telephone elicitation interviews were conducted with health care providers who served the target population and individual in-person elicitation interviews were conducted with African American adolescent females aged 13–18 y All interviews were audio-recorded, transcribed verbatim, and content analyzed. Data collected from adolescents suggested an overall lack of understanding of HPV and HPV vaccination. The majority, however, were open-minded and interested in receiving additional information about both. While providers were seen as a trusted source of information, many inconsistencies were identified in terms of their HPV vaccination recommendations and counseling of adolescents.
Qualitative findings revealed potential motivators and barriers to vaccination that were later incorporated into the development of the Girls OnGuard interactive computer-delivered intervention. Predominant themes that emerged were used to apply IMB model constructs. Application of the IMB constructs that served as a benchmark to guide development of the Girls OnGuard interventions are presented in Figure 1.
Figure 1.

Application of IMB model constructs to guide development of the Girls OnGuard intervention.
Best practices in multimedia health education were also reviewed, and a 5-scene media-based intervention script was developed.31-35 Prior to production, theater testing was performed at a local adolescent health center in one of the study implementation counties to pre-test content and obtain post-exposure attitudes and preferences of the script. Other than several dialog edits and minor phrasing changes to enhance its “realism” for our target audience, adolescents perceived the content to be useful, practical, and relevant.
A novel reminder strategy to enhance adolescents' compliance with the recommended second and third doses of HPV4 vaccine was also developed. This element of Girls OnGuard builds on the Cochrane Review of patient reminder systems for immunization compliance.36,37 In addition to clinical efforts of offering appointment cards indicating the date of participants' next vaccination appointment, and physician reinforcement of the need to return for subsequent HPV4 vaccinations, key chains with a motivational health message that can be used to store a vaccine reminder card were given to participants. The application of these key chains was further modeled in the Girls OnGuard intervention. Prior to the start of the main trial, a small pilot test (N=10) was conducted to determine the feasibility of study procedures and to assess the acceptability of study completion time from the participant's perspective. We received positive feedback particularly in regards to time needed to complete the computer assessment and video(s) within the clinic setting.
Methodology
Sample
Participants were recruited in the waiting areas of 5 health clinics in metropolitan Atlanta between 2010 and 2012. To be eligible potential participants had to: (a) self-identify as an African American female; (b) be between 13–18 y of age at the time of enrollment; (c) be unmarried; (d) seeking reproductive or STI services at a participating study clinic; (e) report that they have not previously received HPV vaccine; and (f) provide written informed consent and the Health Insurance Portability and Accountability Act (HIPPA) Privacy Rule consent, when applicable. Adolescents who refused to provide written HIPAA consent could still participate but medical records could not be accessed to evaluate vaccination uptake; adolescents who refused to provide written informed consent were excluded. A total of 216 African American adolescent females were enrolled in Girls OnGuard. Of the 216 participants, 108 were randomized to the Girls OnGuard intervention group and 108 were randomized to the health promotion comparison condition (Fig. 2).
Figure 2.
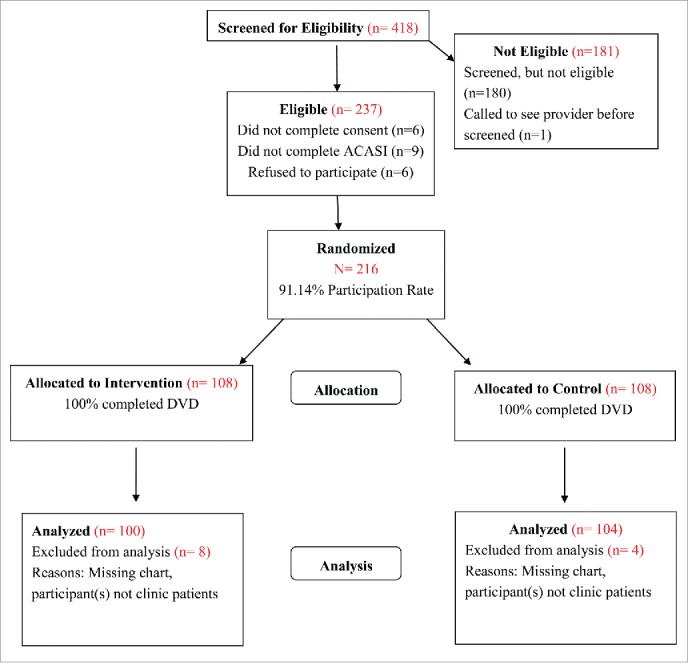
Consort diagram of Girls OnGuard study allocation.
Recruitment
Upon being approached, prospective participants were screened for eligibility. Eligible adolescents provided written informed consent and HIPPA, if applicable. Parental consent was not required, in accordance with Georgia law, as participants were seeking confidential services in a reproductive health clinic and HPV vaccination at participating clinics could be obtained without parental consent. All participants in this study received standard-of-care counseling according to clinic protocol. For participants who elected to receive the HPV vaccine, initial vaccination uptake occurred on the same day as study enrollment/randomization. All financial barriers were removed as the cost of the vaccine was covered under the Vaccines for Children (VFC) program. Participants received 10 dollars for participating in the study. The confidentiality of participants' data and identity was ensured. This study was accepted and approved by the Emory University Institutional Review Board.
Procedure
Following recruitment, eligibility screening, and consent, all participants completed an Audio Computer-Assisted Self-Interview (ACASI) survey. Research staff provided participants with a small (10 inch) laptop computer and headphones, described the brief, 15-minute ACASI survey, and permitted participants to ask questions before beginning. The ACASI survey assessed socio-demographics, sexual history, and knowledge, attitudes and beliefs about HPV and HPV4 vaccination.
Upon completion of the ACASI assessment, research staff randomized participants to the 2 study conditions. Randomization occurred after the ACASI survey as opposed to before to account for participants who may not stay for the duration of the clinic visit. Timing randomization in this way ensured we could compare participants and non-participants on baseline characteristics. Randomization was done using pre-packaged unmarked envelopes containing solid blue (intervention group) or purple (comparison group) slips of paper. The color coding was based on a randomization scheme that was created by computer algorithm, designed to eliminate bias in assigning participants to study conditions. Following randomization, participants watched a short video on the same laptop used to administer the ACASI.
Participants randomized into the Girls OnGuard intervention condition viewed a 12-minute interactive computer-delivered media presentation on HPV vaccination designed to enhance initial uptake and compliance of HPV4 and received a motivational keychain to store a vaccine reminder card (that was modeled in the video). Those randomized to the health comparison condition viewed a time-equivalent health promotion media presentation on physical activity and nutrition. Both videos were designed to be gender- and culturally-appropriate, beneficial and engaging. Study procedures were initiated and completed while participants waited in the clinic waiting area to receive health services. The entire process was approximately 30 minutes from the time of consent to completion of study procedures. All clinic providers were blind to study treatment conditions and provided standard of care counseling to all participants in accordance with clinic protocol.
There were no follow-up assessments incorporated into the study procedure, as this was a purposive method to minimize reactivity and reporting bias, such as social desirability bias. At 7 months post-randomization, medical record abstraction was initiated for each participant by research staff and designated clinic personnel. Medical records were reviewed to identify which participants received HPV4 vaccination, how many HPV4 doses were received, and the date (day/month) of receipt of each vaccination. In addition, clinic records were reviewed to identify any STI diagnosis/treatment during this time.
Measures
The quantitative data examined in this study was collected using ACASI and medical chart abstraction 7 mo post randomization. The ACASI measured constructs identified in the research literature and our formative research that might be associated with the uptake of the HPV4 HPV vaccine: sociodemographic variables, sexual history, HPV/cervical cancer knowledge, HPV vaccine acceptability and effectiveness, and normative beliefs. Selection of measures for inclusion on the ACASI was guided by a number of factors, including: (1) relevance of the construct for influencing HPV4 vaccination; (2) use of the measure with similar populations; and (3) the underlying theoretical framework.
Sociodemographics
The ACASI measured adolescents' age, education, living situation, whether anyone in the household received government assistance, if the participant was employed, and current health insurance coverage. The ACASI also assessed whether any family member had been diagnosed with cancer (in general) or cervical cancer (specifically), and whether they had personally been tested (Pap smear) or told by a clinician they have HPV infection.
Sexual history
The ACASI measured adolescent's sexual history using items developed by the Girls OnGuard research team. These items assessed: sexual debut, age of sex partners, number of male sex partners in 3 months prior to assessment and lifetime number of male sex partners, frequency of vaginal sex and condom use in the last 3 months, pregnancy and STI history, alcohol/drug use prior to sex, and relationship history including number of casual male sex partners. Many of these items were developed by the researchers' previous STI/HIV research and have been used extensively with same-age African American girls attending similar clinical venues.
Awareness of HPV and cervical cancer
Knowledge about HPV was assessed using an 11-item scale, with true-false responses, developed by Kahn et al.38 Items were recoded with correct responses receiving a score of “1” and all others “0.” Items were summed to create a composite score reflecting the total number of correct responses.
Perceived susceptibility and perceived severity of HPV and cervical cancer
Participants completed a set of questions assessing perceptions of risk and seriousness of HPV and cervical cancer. The subscale on perceptions of risk was comprised of 10 items and perceived seriousness of cervical cancer was comprised of 12 items that were adapted from Ingledue et al.39 and Marlow et al.40 Participants rated responses on a 4-point Likert scale from “strongly agree” to “strongly disagree.” The Ingledue et al.39 statements were developed for an older age group, however, over a 2-week period, high test-retest reliability coefficients were reported: .90 for knowledge and .95 for perceptions. A composite summed score was created by adding individual items. Individual questions were coded/recoded so that higher numbers indicated a greater perceived susceptibility/severity of HPV and cervical cancer.
Perceptions about vaccines in general and the HPV vaccine
Seven items assessed general perceptions about vaccines (3 items from Gerend et al.41 and 4 items modified from Marlow et al.).42 Examples of statements include: “Vaccines are the most effective way to prevent disease” and “I am concerned about possible bad side effects of any vaccine.” Perceptions about the HPV vaccine was measured using 4 items adapted from Marlow et al.40,42 Participants rated responses on a 4-point Likert scale from “strongly agree” to “strongly disagree.” Responses were coded/recoded and then summed to yield separate composite scores for general vaccine perceptions and HPV vaccine specific perceptions where higher scores indicated more negative perceptions.
Risk compensation
Risk compensation was assessed using one item developed by the research team: “If I get vaccinated with the HPV vaccine, it will protect me against other sexually transmitted infections (for example, Chlamydia, gonorrhea, etc.).” Participants rated responses on a 4-point Likert scale from “strongly agree” to “strongly disagree.”
HPV vaccine acceptability
Participants completed a set of items from 2 related scales, one by Gerend et al.41 and another by Zimet et al.43 Participants completed a modified 5-item measure assessing HPV vaccine acceptability/likelihood of vaccination. Responses were rated on a 6-point Likert scale from “very unlikely” to “very likely.” Cronbach's α was 0.90.
Normative beliefs
Participants were assessed using 4 items on who they believe would be supportive of them receiving the HPV vaccine. Participants responded to statements as either “agree” or “disagree.” These items were developed by the Girls OnGuard research team and were modified from previously used items with similar populations.
Primary outcome
The primary study outcome to assess the efficacy of the Girls OnGuard intervention was receipt of the first dose of the HPV4 vaccine. Clinic records were reviewed to identify whether and when (day/month) participants received HPV4 vaccination. A secondary outcome was participants' STI incidence during the 7 mo post randomization period. A Medical Record Abstraction Form (MRAF) was developed with the assistance of clinic staff to document this information. The use of medical record abstraction was selected as the primary outcome measure to provide an objective and quantifiable measure of HPV4 vaccine uptake that avoided biases associated with self-report.
Analysis
Statistical power was originally calculated based on an absolute difference of 10% (20% of girls in Girls OnGuard relative to 10% of girls in the control condition would initiate vaccination). Using this projected effect estimate and setting the Type 2 error rate to .05 (2-tail test) and power = .80, required a sample size of 400 adolescents. Descriptive statistics were used to evaluate the distribution of participants with regards to variables assessed in the ACASI survey and primary/secondary study outcomes. Summary statistics for all measures were computed separately for participants in the Girls OnGuard intervention and health promotion comparison condition. Generation of these statistics also served as the last of our ongoing quality control and quality assurance activities in data management, during which we identified and corrected any data that may have resulted from management error. Bivariate analyses were conducted to test variables for possible inclusion in regression models. Possible confounding factors were also assessed to identify if there were differences between the 2 trial conditions on key variables (i.e., attitude about HPV vaccination). SPSS software, version 21 was used to perform the analyses.
Results
Descriptive comparisons
Of the 216 participants, the average age at enrollment was 16 y (M = 16.47, SD = 1.50). Most attended school and completed at least the ninth grade (20.4%), lived with their mother only (53.7%), received at least one form of public assistance (47.7%), and 44.9% reported Medicaid as their current form of health insurance. Approximately one-third of participants reported a family history of cancer (39.3%), with only 8 adolescents reporting a history of cervical cancer in their family (3.7%). Approximately 40% of participants reported ever having a pap smear with 16% of those reporting an abnormal result. Two participants who reported having an abnormal pap smear indicated they received a positive HPV diagnosis (Table 1).
Table 1.
Participant sociodemographic characteristics
| Participant Characteristic | Comparison N=108 n (%) | Intervention N=108 n (%) | Total N=216 n (%) | p value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age (M, SD) | 16.68 (1.44) | 16.26 (1.54) | 16.47 (1.50) | 0.04* |
| Clinic | 0.38 | |||
| Carroll | 0 (0.0) | 1 (0.9) | 1 (0.5) | |
| Clayton | 11 (10.2) | 11 (10.2) | 22 (10.2) | |
| DeKalb | 72 (66.7) | 71 (65.7) | 143 (66.2) | |
| Fulton | 25 (23.1) | 22 (20.4) | 47 (21.8) | |
| Planned Parenthood | 0 (0.0) | 3 (2.8) | 3 (1.4) | |
| Education Level | 0.39 | |||
| Less than 8thgrade | 5 (4.6) | 8 (7.4) | 13 (6.0) | |
| 8thgrade | 13 (12.0) | 12 (11.1) | 25 (11.6) | |
| 9thgrade | 19 (17.6) | 25 (23.1) | 44 (20.4) | |
| 10thgrade | 12 (11.1) | 18 (16.7) | 30 (13.9) | |
| 11thgrade | 21 (19.4) | 21 (19.4) | 42 (19.4) | |
| 12thgrade | 16 (14.8) | 12 (11.1) | 28 (13.0) | |
| High school grad or GED | 22 (20.4) | 12 (11.1) | 34 (15.7) | |
| Living Environment | 0.25 | |||
| Live alone | 0 (0.0) | 4 (3.7) | 4 (1.9) | |
| Live with both parents | 24 (22.2) | 22 (20.4) | 46 (21.3) | |
| Live with their mother | 56 (51.9) | 60 (55.6) | 116 (53.7) | |
| Live with their father | 4 (3.7) | 7 (6.5) | 11 (5.1) | |
| Live with their boyfriend | 6 (5.6) | 2 (1.9) | 8 (3.7) | |
| Live with other relative | 13 (12.0) | 9 (8.3) | 22 (10.2) | |
| Other | 5 (4.6) | 4 (3.7) | 9 (4.2) | |
| Received Public Assistance | 0.12 | |||
| No | 50 (46.3) | 53 (49.1) | 113 (52.3) | |
| Welfare (TANF, SSI) | 10 (9.3) | 8 (7.4) | 18 (8.3) | |
| Food stamps | 49 (45.4) | 50 (46.3) | 99 (45.8) | |
| WIC | 13 (12.0) | 13 (12.0) | 26 (12.0) | |
| Section 8 housing | 4 (3.7) | 6 (5.6) | 10 (4.6) | |
| Currently Employed | 20 (18.5) | 14 (13.0) | 34 (15.7) | 0.65 |
| Health Insurance | 0.19 | |||
| Private | 8 (7.4) | 11 (10.2) | 19 (8.8) | |
| Medicaid | 54 (50.0) | 43 (39.8) | 97 (44.9) | |
| GA CHIP | 3 (2.8) | 0 (0.0) | 3 (1.4) | |
| No insurance | 18 (16.7) | 20 (18.5) | 38 (17.6) | |
| Don't know | 25 (23.1) | 34 (31.5) | 59 (27.3) | |
| Family history of any cancer | 44 (40.7) | 40 (37.0) | 84 (39.3) | 0.65 |
| Family history of cervical cancer | 5 (4.6) | 3 (2.8) | 8 (3.7) | 0.49 |
| Ever had a Pap smear | 51 (47.2) | 43 (39.8) | 94 (43.5) | 0.27 |
| Had previous Pap smear+ | 16 (31.4) | 11 (25.6) | 27 (28.7) | 0.54 |
| Ever had abnormal Pap smear | 7 (13.7) | 8 (18.6) | 15 (16.0) | 0.52 |
| >Ever positive HPV diagnosis | 0 (0.0) | 2 (4.7) | 2 (2.1) | 0.12 |
Significant difference between study conditions, p<.05; +Indicates previous Pap smear before most recent Pap smear.
Over 75% of the sample was sexually active, with the average age of sexual debut 14 y of age (M = 14.63, SD = 2.17). Most reported having male sex partners who were the same age (56.1%) or older (34.1% within 2–3 y of their own age) and reported having an average of 4 lifetime male sexual partners (M = 4.04, SD = 4.83). Over half of all participants reported being in a current romantic relationship (63%) and usually waiting over a month before initiating sex in the relationship (52.9%). Of those in relationships, the average length was 11 months (M = 11.18, SD = 11.64). Although this may seem long by adolescent standards, sexual concurrency was also prevalent. Of those reported to be in a relationship, 17% also believed their main boyfriend or partner was also having sex with another person, and 15.3% of participants self-reported having a casual male partner in addition to a main partner. Of those reporting a casual male partner in addition to their main partner, 33.3% also believed their casual male partner was having sex with other girls/women.
Approximately 22% of participants reported ever testing positive for an STI, and of those, the majority reported only one positive test (74.5%). Over half (53.7%) of those sexually active reported using a condom at last sex. Adolescents reported having sex on average 6 times in the past 90 d (M = 6.20, SD = 9.99) yet reported using condoms less than half of those times (M = 2.84, SD = 3.85). Approximately 38% reported a history of oral sex and 7.9% reported anal sex. However, use of protective measures such as condoms or dental dams was infrequent (See Table 2).
Table 2.
Participant sexual and relationship history
| Participant Characteristic | Comparison N=108 n (%) | Intervention N=108 n (%) | Total N=216 n (%) | p value |
|---|---|---|---|---|
| Sexual History | ||||
| Ever vaginal sex | 82 (75.9) | 82 (75.9) | 164 (75.9) | 1.00 |
| Age at first sex (M, SD) | 15.01 (1.59) | 14.26 (2.58) | 14.63 (2.17) | 0.03* |
| Usually have sex with | 0.56 | |||
| Men | 79 (96.3) | 77 (93.9) | 156 (95.1) | |
| Women | 0 (0.0) | 1 (1.2) | 1 (0.6) | |
| Both men and women | 3 (3.7) | 4 (4.9) | 7 (4.3) | |
| Typical age of sex partners | 0.44 | |||
| Much younger (4+ yrs) | 1 (1.2) | 0 (0.0) | 1 (0.6) | |
| Younger (2–3 yrs) | 3 (3.7) | 2(2.4) | 5 (3.0) | |
| Same age | 49 (59.8) | 43 (52.4) | 92 (56.1) | |
| Older (2–3 yrs) | 23 (28.0) | 33 (40.2) | 56 (34.1) | |
| Much older (4+ yrs) | 6 (7.3) | 4 (4.9) | 10 (6.1) | |
| Lifetime vaginal sex partners (M, SD) | 3.89 (2.76) | 4.20 (6.27) | 4.04 (4.83) | 0.69 |
| Vaginal sex partners in past 90 d (M, SD) | 1.41 (1.31) | 1.34 (.93) | 1.38 (1.14) | 0.68 |
| Ever pregnant | 27 (32.9) | 16 (19.5) | 43 (26.2) | 0.05+ |
| Condom use at last sex | 47 (57.3) | 41 (50.0) | 88 (53.7) | 0.35 |
| Protection used at last sex (other than condom) | 0.58 | |||
| Pill/Patch/Depo/Ring | 20 (24.4) | 19 (23.2) | 39 (23.8) | |
| Withdrawal | 9 (11.0) | 11 (13.4) | 20 (12.2) | |
| Other | 12 (14.6) | 9 (11.0) | 21 (12.8) | |
| None | 47 (57.3) | 47 (57.3) | 94 (57.3) | |
| # Times vaginal sex in past 90 d (M, SD) | 6.96 (11.67) | 5.43 (7.96) | 6.20 (9.99) | 0.33 |
| # Times condoms used in past 90 d (M, SD) | 2.83 (3.44) | 2.85 (4.25) | 2.84 (3.85) | 0.99 |
| # Times vaginal sex while high/drunk past 90 d (M, SD) | 0.93 (2.97) | 0.24 (1.15) | 0.59 (2.28) | 0.07 |
| Ever anal sex | 15 (13.9) | 2 (1.9) | 17 (7.9) | p<0.01 |
| Age at first anal sex (M, SD) | 15.67 (2.26) | 14.50 (3.53) | 15.53 (2.32) | 0.52 |
| # Times anal sex in past 90 d (M, SD) | 2.47 (4.02) | 0.50 (0.71) | 2.23 (3.82) | 0.51 |
| # Times condoms used for anal sex past 90 d (M, SD) | 1.40 (1.58) | 0 (0.0) | 1.27 (1.56) | 0.42 |
| Ever performed oral sex | 38 (35.2) | 45 (41.7) | 83 (38.4) | 0.33 |
| Age when first performed oral sex (M, SD) | 16.13 (1.88) | 15.6 (1.89) | 15.84 (1.89) | 0.20 |
| Lifetime oral sex partners (M, SD) | 1.95 (1.89) | 2.82 (6.00) | 2.42 (4.60) | 0.39 |
| # Times had oral sex in past 90 d (M, SD) | 2.97 (4.04) | 4.11 (8.05) | 3.59 (6.52) | 0.43 |
| # Times used condom/dental dam for oral sex past 90 d (M, SD) | 0.83 (1.75) | 0.94 (2.00) | 0.89 (1.87) | 0.82 |
| Ever tested positive for STI infection | 22 (20.4) | 25 (23.1) | 47 (21.9) | 0.57 |
| Number of positive STI diagnoses | 0.99 | |||
| Once | 16 (72.7) | 19 (76.0) | 35 (74.5) | |
| Twice | 4 (18.2) | 4 (16.0) | 8 (17.0) | |
| Three times | 1 (4.5) | 1 (4.0) | 2 (4.3) | |
| Four or more times | 1 (4.5) | 1 (4.0) | 2 (4.3) | |
| Relationship Characteristics | ||||
| Currently in romantic relationship | 67 (62.0) | 69 (63.9) | 136 (63.0) | 0.59 |
| Length of current relationship in months (M, SD) | 11.07 (10.58) | 11.29 (12.66) | 11.18 (11.64) | 0.92 |
| Time frame for first sex in relationship | 0.44 | |||
| Haven't had sex yet | 15 (22.4) | 16 (23.2) | 31 (22.8) | |
| Within a month | 14 (20.9) | 8 (11.6) | 22 (16.2) | |
| Less than a month | 4 (6.0) | 7 (10.1) | 11 (8.1) | |
| More than a month | 34 (50.7) | 38 (55.1) | 72 (52.9) | |
| Believes partner has had sex with another person | 11 (16.4) | 12 (17.4) | 23 (16.9) | 0.88 |
| Has current casual sex partner | 11 (10.2) | 22 (20.4) | 33 (15.3) | 0.04* |
| Believes casual partner has had vaginal sex with another person | 4 (36.4) | 7 (31.8) | 11 (33.3) | 0.79 |
Significant difference between study conditions, p<.05; + Difference in study conditions approached significance at .05 level.
Based on the 11-item scale assessing knowledge of HPV and cervical cancer, participants on average got less than half correct (M = 4.61, SD = 2.16). Participants did recognize that certain types of HPV cause cancer (53.2%), HPV is transmitted through sexual intercourse (50.0%), and a person may be infected with HPV and not know it (79.2%). Conversely, 84.3% believed incorrectly or were unsure that HPV could be cured with antibiotics.
Among study participants, only 18.9% believed “they were at risk for getting HPV” and 38.9% were “worried about getting HPV.” Additionally, 25% believed that it was possible that they may get HPV in the future. Almost 80% of participants believed they have the ability to avoid HPV infection, would be upset if they had HPV (84.7%), and agreed that acquiring HPV would be more difficult to have a long-term sexual partner (73.6%). Further, 83.4% believed HPV can have serious negative health consequences, be extremely harmful (83.8%), and is life-threatening (76.4%).
Individual perceptions related to cervical cancer were similar to those related to HPV infection, although fewer participants perceived “they were at risk for cervical cancer” (19.5%) and “worried about getting cervical cancer” (41.2%) than those who believed they were not at risk. The majority believed their chances of getting cervical cancer were not high (90.7%), that they have the ability to avoid it (68.1%), but that getting cervical cancer is among the most serious of all diseases they could imagine (69.9%). (See Table 3 for perceived susceptibility/severity item frequencies by condition).
Table 3.
Perceived susceptibility and severity of HPV and cervical cancer by study condition
| Perceived Susceptibility and Severity Assessment Items | Comparison N=108 n (%) | Intervention N=108 n (%) | Total N=216 n (%) | p value |
|---|---|---|---|---|
| Perceived Susceptibility of HPV | ||||
| My chances of getting HPV in the future are low. | 69 (63.9) | 54 (50.0) | 123 (56.9) | 0.21 |
| I worry about getting HPV. | 42 (38.9) | 42 (38.9) | 84 (38.9) | 0.75 |
| I believe I am at risk for getting HPV. | 17 (15.7) | 24 (22.2) | 41 (19.0) | 0.04* |
| It is possible that I may get HPV in the future. | 20 (18.5) | 34 (31.5) | 54 (25.0) | 0.08 |
| I have the ability to avoid HPV infection. | 89 (82.4) | 83 (76.9) | 172 (79.6) | 0.32 |
| Perceived Susceptibility of Cervical Cancer | ||||
| I worry about getting cervical cancer. | 38 (35.2) | 51 (47.2) | 89 (41.2) | 0.05+ |
| I believe that I am at risk for developing cervical cancer. | 17 (15.7) | 25 (23.1) | 42 (19.4) | 0.01* |
| All women have an equal chance of developing cervical cancer; it is beyond my personal control. | 55 (50.9) | 70 (64.8) | 125 (57.9) | 0.08 |
| My chances of getting cervical cancer are high. | 11 (10.2) | 9 (8.3) | 20 (9.3) | 0.41 |
| I have the ability to avoid cervical cancer. | 73 (67.6) | 74 (68.5) | 147 (68.1) | 0.42 |
| Perceived Severity of HPV | ||||
| Having HPV would be upsetting to me. | 88 (81.5) | 95 (88.0) | 183 (84.7) | 0.13 |
| Having HPV would make it difficult for me to get a long-term sexual partner. | 79 (73.1) | 80 (74.1) | 159 (73.6) | 0.96 |
| I believe that HPV can have serious negative health consequences. | 88 (81.5) | 92 (85.2) | 180 (83.3) | 0.73 |
| I believe that HPV can be extremely harmful. | 91 (84.3) | 90 (83.3) | 181 (83.8) | 0.24 |
| I believe that HPV causes cervical cancer. | 86 (79.6) | 84 (77.8) | 170 (78.7) | 0.81 |
| HPV is a life-threatening infection. | 85 (78.7) | 80 (74.1) | 165 (76.4) | 0.81 |
| I believe HPV is curable with proper medical treatment. | 81 (75.0) | 84 (77.8) | 165 (76.4) | 0.82 |
| Perceived Severity of Cervical Cancer | ||||
| All women who develop cervical cancer must have their uterus removed. | 23 (21.3) | 26 (24.1) | 49 (22.7) | 0.77 |
| Among the diseases that I can imagine, getting cancer of the cervix is among the most serious. | 77 (71.3) | 74 (68.5) | 151 (69.9) | 0.92 |
| No one dies anymore of cervical cancer. | 11 (10.2) | 7 (6.5) | 18 (8.3) | 0.25 |
| Cervical cancer is often curable with early detection and proper medical treatment. | 74 (68.5) | 82 (75.9) | 156 (72.2) | 0.32 |
Significant difference between study conditions, p<.05; + Difference in study conditions approached significance at .05 level.
Participants' perceptions about vaccines in general were relatively neutral (M = 10.16, SD = 2.76, range = 0-21), indicating neither extreme positive nor negative views. Participants' perceptions about the HPV vaccine, more specifically, were more positive (lower values indicated more positive views, M = 4.76, SD = 1.72, range 0–12), possibly suggesting greater acceptability of the HPV vaccine versus vaccines in general. Approximately 35% of participants mistakenly agreed that if vaccinated with the HPV vaccine they would be protected against other STIs.
When assessing acceptability of the HPV vaccine, 76.3% of participants reported they would “try to get more information about the HPV vaccine,” 63% reported they would be “likely to get the HPV vaccine if a healthcare provider offered it to them in the next 12 months,” and 26.9% reported they would be “likely to actually get the HPV vaccine today” (on the day of study enrollment).
Few significant differences were observed between study conditions (Tables 2 and 3). Participants in the comparison condition were younger at the time of enrollment (M = 16.68, SD = 1.44) (although both averaged approximately 16 y of age), reported ever being pregnant (32.9%, n = 27/82) and ever having anal sex (13.9%, n = 15/108). Those in the intervention condition were younger at first sex (M = 14.26, SD = 2.58) and more reported having casual male sex partners. Participants assigned to the intervention condition also had a higher perceived susceptibility/severity composite score (MI = 32.22, SD = 5.83 vs. MC = 30.42, SD = 6.56; p = .03).
Independent from the composite score, 2 individual items reached statistical significance and one item was marginally significant (Table 3). Intervention participants, in particular, reported believing they were at risk for HPV and that they were at risk for developing cervical cancer more than comparison participants (p < .05). Intervention participants also reported worrying about getting cervical cancer (p = .05) and that they would be more “likely to get the HPV vaccine today” as compared to those in the comparison condition (34.3% vs. Nineteen.4%, respectively, p = .01).
Primary outcome results
Seven months post-randomization medical records were reviewed to assess initial HPV vaccine uptake and dosage compliance. Approximately 12% of all study participants (n = 24) received the first dose of HPV vaccine, with an equal number of participants in the intervention and comparison conditions (n = 12 in each condition). Of those who received the first dose of the vaccine, more intervention participants were compliant with the series (n = 8/12 received dose 2, n = 6/12 received doses 2 and 3 vs. those in the comparison condition, n = 3/12 received dose 2 and n = 2/12 received doses 2 and 3). The intervention group included more participants who completed the vaccine series (26 doses vs. Seventeen doses in the comparison group respectively; p = .12.
Discussion
This study is among the first to evaluate a brief, computer-administered culturally- and gender-tailored intervention to enhance HPV4 uptake among a vulnerable population at high-risk for HPV acquisition and transmission. Using a randomized placebo controlled design, we have demonstrated the feasibility of a computer-administered, media-based intervention. Although not statistically significant, we observed greater uptake of HPV4 vaccine in the intervention condition relative to the comparison condition.
Overall vaccine uptake was low. This is consistent with recent literature and national trends highlighting the prevailing racial/ethnic disparities in HPV vaccination11,44 as African American and Hispanic adolescents remain less likely to initiate HPV vaccination compared to whites.45 Variation across ethnic groups has been linked to factors such as poor quality of health care, decreased access to health care or lack of a “medical home” and high vaccine cost.46-49 Unique to this study, we eliminated these barriers and created a brief, targeted intervention in a clinical setting within communities of color, provided direct and immediate access to the vaccine, and removed cost barriers through the Vaccines for Children program. In addition, any clinic-specific administrative fees associated with vaccination were also eliminated, making vaccine initiation and series completion free-of-charge. Why then was vaccine uptake poor?
Our findings corroborate and extend those observed by Gelman and colleagues44 suggesting that structural factors, such as cost and access to the vaccine, may not explain poor vaccine uptake. Specifically, Gelman et al.44 examined how access to health care affects the association between HPV vaccine initiation and race/ethnicity; after adjusting for sociodemographics and health care measures, a disparity in vaccination acceptance persisted, with African American adolescents less likely to be vaccinated. And unlike Hispanics, Gelman et al.44 noted, the disparity in HPV vaccination among African Americans was not attributable to differential access to health care.
Importantly, this underscores that there may be other salient barriers to HPV vaccination for this population. We observed that while most teens have a low perceived susceptibility of HPV infection, which may help explain our low rates of vaccination, over 80% believed that “their provider would think it was a good idea to get vaccinated.” Moreover, from our formative work, teens discussed how they viewed the provider as someone “they trust and look to for medical guidance.”30 The patient-provider interaction and providers' recommendation for HPV vaccination may be one of the strongest influences forecasting HPV vaccine uptake among African American adolescents.50-52 The empirical literature suggests that while there is a positive association between providers' recommendation and uptake of HPV vaccine across ethnic groups (white, African American, and Hispanic), the association is strongest for African Americans.53 Thus, providers can be influential in dispelling common myths and misconceptions surrounding the HPV vaccine and promote positive attitudes toward vaccination, which may reduce the observed disparities in HPV vaccination uptake.
As with all studies, some inherent limitations should be noted. One factor impeding our data analysis in evaluating the effect of the intervention to enhance vaccine uptake and compliance was limited sample size. Changes in bus routes midway through the recruitment phase of the study adversely impacted patient throughput at the primary clinics. As a result, it is possible that participants could have received subsequent vaccinations at other clinics that were not study sites, and therefore would have been missed during medical record abstraction. This was a limitation, as we were unable to track vaccine doses received from non-participating clinics.
Similarly, participant recruitment was conducted during normal clinic hours (9am-5pm), which encompass much of an adolescent's school day. As the study eligibility criteria specified that adolescents between 13–18 y of age at time of trial enrollment were eligible for inclusion, many of our “eligible” participants were attending school, further limiting our participant pool. As part of our screening process, we also did not assess whether adolescents were planning to get the HPV vaccine as part of their visit to the clinic on the day they were recruited. This was not part of our exclusion criteria. As such, we may have undermined the true effectiveness of our intervention, as there were some adolescents who already planned to get vaccinated, even prior to viewing the computer-delivered intervention (as indicated through the ACASI survey; 26.9% respectively). Another potential limitation was the brevity of the computer-administered intervention. The entire media program was 12 minutes in duration; designed specifically not to interfere or hinder patient flow at the clinics. However, as the girls are young and may be less familiar with HPV and HPV4, an intervention of longer duration may be needed to clearly convey relevant information so that adolescents could make an informed decision about vaccination.
It was also unclear what the most influential factor was for intervention participants deciding to receive vaccination. Although the difference in vaccine uptake between study conditions was not significant, it would have been advantageous had our study incorporated a factorial design to disaggregate specific components of the intervention and comparison videos to determine whether parts of the videos may have been more impactful than others. Additionally, there is a possibility that our study groups were not equivalent, even with a standardized randomization procedure. The intervention group reported being more likely to accept the HPV vaccine today and had higher perceived HPV susceptibility/severity scores for HPV and cervical cancer risk. Both factors could have influenced the likelihood of being more vaccine compliant relative to the comparison group. More intervention participants also tested STI-positive in the 7 months post-randomization. Thus, they likely had supplementary medical encounters and therefore, may have had more opportunities to be vaccinated.
The research team provided information to clinic providers through an in-service presentation prior to study implementation. The research team was also careful to maintain fidelity when recruiting and administering intervention. However, what the research team could not control were the patient-provider interactions. Substantial variability may exist between and within clinics with respect to patient-provider interactions. As the trial design did not request a follow-up participant interview, the tenor and substance of the patient-provider interaction remains a “black box.” It is unclear if providers, in the course of their interaction with patients in the exam room, offered counseling to encourage (or discourage) HPV vaccination. While our media intervention did include a scene modeling an adolescent getting vaccinated by her provider in positive patient-provider interaction, it is possible that more visual emphasis was needed from the provider's perspective to build trust and increase credibility. The low acceptance rate is particularly concerning given that there is an established state policy that allows adolescents receiving STI sexual health services to consent to HPV vaccine without parental permission, and recommends providers inform and refer adolescents for HPV vaccination.
Conclusions
We designed a brief media-based computer-administered HPV vaccine enhancement intervention that was implemented in the course of routine provision of clinical care to African American adolescent girls as they sought sexual health services. The feasibility of the intervention was established by the high participation rate observed (91%). Adolescents also completed audio computer-administered interviews and permitted investigators to access their medical records to confirm receipt of HPV vaccination. However, vaccine uptake was poor. While there was a noticeable difference favoring the intervention condition, in terms of total number of vaccine doses received; this difference did not achieve statistical significance.
Perhaps the major limitation, if it is indeed a study limitation, was our inability to monitor patient-provider interactions as this may have had a profound impact on whether adolescents elected to be vaccinated. Limitations notwithstanding, the study suggests that brief clinic-based vaccine promotion interventions are feasible, acceptable to patients and clinic providers, and can be implemented with fidelity without adversely impacting normal clinic operations, procedures and patient flow. Further research, elaborating on these methods (including adding a follow-up assessment), expanding the duration of the intervention, and adding more emphasis on the intervention messages from the viewpoint of a medical provider, may be important in enhancing HPV vaccine uptake.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Special thanks to the Girls OnGuard participants, graduate student research assistants, collaborating clinic sites, and clinical staff.
Authors' Contributions
Ralph J. DiClemente—secured funding, designed the study methodology, developed the intervention and wrote the manuscript. Colleen Crittenden Murray—assisted in the development of the intervention, served as Project Director, performed data analyses, and co-wrote the manuscript. Tracie Graham—assisted in the development of the intervention, served as Study Coordinator, and edited the manuscript. Julia Still—assisted with study recruitment and edited the manuscript. All authors have approved the final draft.
Funding
This research was supported by funding from a grant, IISP ID# 37287, from Merck, Inc.., awarded to the Principal Investigator, Ralph J. DiClemente, PhD.
References
- 1.Centers for Disease Control and Prevention Fact sheet—Incidence, prevalence, and cost of sexually transmitted infections in the United States. Available online: http://www.cdc.gov/std/stats/sti-estimates-fact-sheet-feb-2013.pdf [accessed 03.October.13] [Google Scholar]
- 2.Moscicki AB. HPV infections in adolescents. Dis Markers 2007; 23(4): 229-234; PMID:17627058; http://dx.doi.org/ 10.1155/2007/136906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunne EF, Unger ER, Sternberg M, et al.. Prevalence of HPV infection among females in the United States. JAMA 2007; 297: 813-819; PMID:17327523; http://dx.doi.org/ 10.1001/jama.297.8.813 [DOI] [PubMed] [Google Scholar]
- 4.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high risk human papillomavirus infection. Obstet Gynecol 2007; 110(1): 87-95; PMID:17601901; http://dx.doi.org/ 10.1097/01.AOG.0000266984.23445.9c [DOI] [PubMed] [Google Scholar]
- 5.Moscicki AB. Impact of HPV infection in adolescent populations. J Adolesc Health 2005; 37(6): S3-S9; PMID:16310138; http://dx.doi.org/ 10.1016/j.jadohealth.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 6.Tarkowski TA, Koumans EH, Sawyer M, Pierce A, Black CM, Papp JR, Unger ER. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004; 189(1): 46-50; PMID:14702152; http://dx.doi.org/ 10.1086/380466 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . National and state vaccination coverage among adolescents ged 13–17 Years- United States, 2011. MMWR 2012; 61(34): 671-677; PMID:22932301 [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013 - United States. MMWR 2013; 62(29): 591-595; PMID:23884346 [PMC free article] [PubMed] [Google Scholar]
- 9.Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Matern Child Health J 2013; 17(5): 879-885; PMID:22729660; http://dx.doi.org/ 10.1007/s10995-012-1066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings T, Zimet GD, Brown D, Tu W, Yang Z, Fortenberry JD, Shew ML. Reduction of HPV infections through vaccination among at-risk urban adolescents. Vaccine 2012; 30: 5496-5499; PMID:22750043; http://dx.doi.org/ 10.1016/j.vaccine.2012.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine 2012; 30(24): 3546-3556; PMID:22480928; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.063 [DOI] [PubMed] [Google Scholar]
- 12.Niccolai LM, Mehta NR, Hadler JL. Racial/Ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med 2011; 41(4): 428-433; PMID:21961471; http://dx.doi.org/ 10.1016/j.amepre.2011.06.032 [DOI] [PubMed] [Google Scholar]
- 13.Gelman A, Nikolajski C, Schwarz EB, Borrero S. Racial disparities in awareness of the human papillomavirus. J Womens Health (Larchmt) 2011; 20(8): 1165-1173; PMID:21668381; http://dx.doi.org/ 10.1089/jwh.2010.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins RB, Brogly SB, Adams WG, Freund KM. Correlates of human papillomavirus vaccination rates in low-income, minority adolescents: A multicenter study. J Womens Health (Larchmt) 2012; 21(8): 813-820; PMID:22860770; http://dx.doi.org/ 10.1089/jwh.2011.3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shefer A, Briss P, Rodewald L, Bernier R, Strikas R, Yusuf H, Hinman AR. Improving immunization coverage rates: an evidence-based review of the literature. Epidemiol Rev 1999; 21(1): 96-142; PMID:10520476; http://dx.doi.org/ 10.1093/oxfordjournals.epirev.a017992 [DOI] [PubMed] [Google Scholar]
- 16.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282(15): 1458-1465; PMID:10535437; http://dx.doi.org/ 10.1001/jama.282.15.1458 [DOI] [PubMed] [Google Scholar]
- 17.Carpiano RM, Flocke SA, Frank SH, Stange KC. Tools, teamwork, and tenacity: an examination of family practice office system influences on preventive service delivery. Prev Med 2003; 36(2): 131-140; PMID:12590987; http://dx.doi.org/ 10.1016/S0091-7435(02)00024-5 [DOI] [PubMed] [Google Scholar]
- 18.Stange KC, Zyzanski SJ, Jaen CR, et al.. Illuminating the ‘black box’. J Fam Pract 1998; 46: 377-389; PMID:9597995 [PubMed] [Google Scholar]
- 19.Zhang D, Zhou L, Briggs RO, Nunamaker JFJ. Instructional video in e-learning: Assessing the impact of interactive video on learning effectiveness. Information & Management 2006; 43(1): 15-27; http://dx.doi.org/ 10.1016/j.im.2005.01.004 [DOI] [Google Scholar]
- 20.Robinson TN, Patrick K, Eng TR, Gustafson D. An evidence-based approach to interactive health communication: A challenge to medicine in the information age. Science panel on interactive communication and health. JAMA 1998; 280(14): 1264-1269; PMID:9786378; http://dx.doi.org/ 10.1001/jama.280.14.1264 [DOI] [PubMed] [Google Scholar]
- 21.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull 1992; 111(3): 455-474; PMID:1594721; http://dx.doi.org/ 10.1037/0033-2909.111.3.455 [DOI] [PubMed] [Google Scholar]
- 22.Fisher WA, Fisher JD. A general social psychological model for changing AIDS risk behavior In The Social Psychology of HIV Infection, Pryor JB, Reeder GD, Eds.; Lawrence Erlbaum Associates, Inc., Hillsdale, NJ, England, 1993; pp. 127-153 [Google Scholar]
- 23.Fisher WA, Fisher JD. The information-motivation-behavioral skills model as a general model of health behavior change: Theoretical approaches to individual-level change In Social Psychological Foundations of Health, Suls J, Wallston K, Eds., Blackwell Publishers, United Kingdom, 2003; pp. 82-106 [Google Scholar]
- 24.Hammer J, Fisher J, Fitzgerald P. When two heads aren't better than one: AIDS risk behavior in college-age couples. J Appl Soc Psychol 1996; 26(5): 375-97. [Google Scholar]
- 25.Scarinci IC, Garces-Palacio IC, Partridge EE. An examination of acceptability of HPV vaccination among African American women and Latina immigrants. J Womens Health (Larchmt) 2007; 16(8): 1224-1233; PMID:17937576; http://dx.doi.org/ 10.1089/jwh.2006.0175 [DOI] [PubMed] [Google Scholar]
- 26.Zimet GD, Liddon N, Rosenthal SL, Lazcano-Ponce E, Allen B. Chapter 24: Psychosocial aspects of vaccine acceptability. Vaccine 2006; 24(Suppl 3): S3/201-209; PMID:16950008; http://dx.doi.org/ 10.1016/j.vaccine.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 27.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev Med 2007; 45(2–3): 107-114; PMID:17628649; http://dx.doi.org/ 10.1016/j.ypmed.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 28.Murray CC, Graham T, DiClemente RJ, Wingood GM. Mother may I? Maternal attitudes and perceptions surrounding HPV vaccination from African-American mothers living in the southern United States. Proceedings of the 26th International Papillomavirus Conference, Montreal, Canada, July 2010 [Google Scholar]
- 29.Murray CC, Graham T, DiClemente RJ, Wingood GM. Developmental strategies for a targeted, multimedia intervention to enhance HPV vaccination uptake in a southern US city. Proceedings of the 26th International Papillomavirus Conference, Montreal, Canada, July 2010 [Google Scholar]
- 30.Graham T, Murray CC, DiClemente RJ, Wingood GM. Acceptability of the HPV vaccine among African-American mothers in the southern US. Proceedings of the 138th annual meeting of the American Public Health Association, Denver, CO, November 2010 [Google Scholar]
- 31.Beffa-Negrini PA, Cohen NL, Miller B. Strategies to motivate students in online learning environments. J Nutr Educ Behav 2002; 34(6): 334-340; PMID:12556272; http://dx.doi.org/ 10.1016/S1499-4046(06)60116-4 [DOI] [PubMed] [Google Scholar]
- 32.Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. Am J Health Behav 2003; 27 Suppl 3(3): S227-232; PMID:14672383; http://dx.doi.org/ 10.5993/AJHB.27.1.s3.6 [DOI] [PubMed] [Google Scholar]
- 33.Herek GM, Gillis JR, Glunt EK, Lewis J, Welton D, Capitanio JP. Culturally sensitive AIDS educational videos for African American audiences: Effects of source, message, receiver, and context. Am J Community Psychol 1998; 26(5): 705-743; PMID:9861691; http://dx.doi.org/ 10.1023/A:1022157914906 [DOI] [PubMed] [Google Scholar]
- 34.Ward ML. Wading through the stereotypes: Positive and negative associations between media use and black adolescents' conceptions of self. Dev Psychol 2004; 40(2): 284-294; PMID:14979767; http://dx.doi.org/ 10.1037/0012-1649.40.2.284 [DOI] [PubMed] [Google Scholar]
- 35.Majundar B, Roberts J. AIDS awareness among women: The benefit of culturally sensitive educational programs. Health Care Women Int 1998; 19(2): 141-153; PMID:9526334; http://dx.doi.org/ 10.1080/073993398246476 [DOI] [PubMed] [Google Scholar]
- 36.Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA, Rodewald LE. Effect of patient reminder/recall interventions on immunization rates: A review. JAMA 2000; 284(14): 1820-1827; PMID:11025835; http://dx.doi.org/ 10.1001/jama.284.14.1820 [DOI] [PubMed] [Google Scholar]
- 37.Szilagyi PG, Schaffer S, Barth R, Shone LP, Humiston SG, Ambrose S, Averhoff F. Effect of telephone reminder/recall on adolescent immunization and preventive visits: Results from a randomized clinical trial. Arch Pediatr Adolesc Med 2006; 160(2): 157-163; PMID:16461871; http://dx.doi.org/ 10.1001/archpedi.160.2.157 [DOI] [PubMed] [Google Scholar]
- 38.Kahn JA, Rosenthal SL, Hamann T, Bernstein DI. Attitudes about human papillomavirus vaccine in young women. Int J STD AIDS 2003; 14: 300-306; PMID:12803935; http://dx.doi.org/ 10.1258/095646203321605486 [DOI] [PubMed] [Google Scholar]
- 39.Ingledue K, Cottrell R, Bernard A. Women's knowledge, perceptions, and preventive behaviors regarding human Papillomavirus infection and cervical cancer. Am J of Health Stud 2004; 19: 28-34 [Google Scholar]
- 40.Marlow LAV, Waller J, Wardle J. The impact of human papillomavirus information on perceived risk of cervical cancer. Cancer Epidemiol Biomarkers Prev 2009; 18(2): 373-376; PMID:19190156; http://dx.doi.org/ 10.1158/1055-9965.EPI-08-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerend MA, Lee SC, Shepherd JE. Predictors of human papillomavirus vaccination acceptability among underserved women. Sex Trans Dis 2007: 34(7): 468-471 [DOI] [PubMed] [Google Scholar]
- 42.Marlow LAV, Forster AS, Wardle J, Waller J. Mothers' and adolescents' beliefs about risk compensation following HPV vaccination. J Adolesc Health 2009; 44(5): 446-451; PMID:19380091; http://dx.doi.org/ 10.1016/j.jadohealth.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 43.Zimet GD, Mays RM, Winston Y, Kee R, Dickes J, Su L. Acceptability of Human Papillomavirus immunization. Journal of Women's Health & Gender-Based Medicine 2000; 9(1): 47-50; PMID:10718505; http://dx.doi.org/ 10.1089/152460900318957 [DOI] [PubMed] [Google Scholar]
- 44.Gelman A, Miller E, Schwarz EB, Akers AY, Jeong K, Borrero S. Racial disparities in human papillomavirus vaccination: Does access matter?. J Adolesc Health 2013; 53(6): 756-762; PMID:23992645; http://dx.doi.org/ 10.1016/j.jadohealth.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention . National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR 2011; 60(33): 1117-1123; PMID:21866084 [PubMed] [Google Scholar]
- 46.Sadigh G, Dempsey AF, Ruffin M 4th, Resnicow K, Carlos RC. National patterns in human papillomavirus vaccination: An analysis of the national survey of family growth. Hum Vaccin Immunother 2012; 8(2): 234-242; PMID:22414967; http://dx.doi.org/ 10.4161/hv.18456 [DOI] [PubMed] [Google Scholar]
- 47.Williams WW, Lu PJ, Saraiya M., Yankey D., Dorell C, Rodriguez JL, et al.. Factors associated with human papillomavirus vaccination among young adult women in the United States. Vaccine 2013; 31(28): 2937-2946; PMID:23643629; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agency for Healthcare Research and Quality Chapter 10: Priority populations. National Healthcare Disparities Report 2011. Available online: http://www.ahrq.gov/qual/nhdr11/chap10.htm#racial [accessed 03.February.14] [Google Scholar]
- 49.Mulye TP, Park MJ, Nelson CD, et al.. Trends in adolescent and young adult health in the United States. J Adolesct Health 2009; 45: 8-32; PMID:19541245; http://dx.doi.org/ 10.1016/j.jadohealth.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 50.Lau M, Lin H, Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children's Health. Vaccine 2007; 30: 3112-3120; PMID:22425179; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.034 [DOI] [PubMed] [Google Scholar]
- 51.Sanders Thompson VL, Arnold LD, Notaro SR. African American parents' HPV vaccination intent and concerns. J Health Care Poor Underserved 2012; 23: 290-301; PMID:22643477; http://dx.doi.org/ 10.1353/hpu.2012.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamlish T, Clarke L, Alexander KA. Barriers to HPV immunization for African American adolescent females. Vaccine 2012; 30: 6472-6478; PMID:22910288; http://dx.doi.org/ 10.1016/j.vaccine.2012.07.085 [DOI] [PubMed] [Google Scholar]
- 53.Yitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US national immunization survey. Am J Public Health 2013; 103: 164-173; PMID:22698055; http://dx.doi.org/ 10.2105/AJPH.2011.300600 [DOI] [PMC free article] [PubMed] [Google Scholar]


