Abstract
Current clinically available treatments for rheumatoid arthritis (RA) fail to cure the disease or unsatisfactorily halt disease progression. To overcome these limitations, the development of therapeutic DNA vaccines and boosters may offer new promising strategies. Because type II collagen (CII) as a critical autoantigen in RA and native chicken type II collagen (nCCII) has been used to effectively treat RA, we previously developed a novel therapeutic DNA vaccine encoding CCII (pcDNA-CCOL2A1) with efficacy comparable to that of the current “gold standard”, methotrexate(MTX). Here, we systemically evaluated the safety and immunogenicity of the pcDNA-CCOL2A1 vaccine in normal Wistar rats. Group 1 received only a single intramuscular injection into the hind leg with pcDNA-CCOL2A1 at the maximum dosage of 3 mg/kg on day 0; Group 2 was injected with normal saline (NS) as a negative control. All rats were monitored daily for any systemic adverse events, reactions at the injection site, and changes in body weights. Plasma and tissues from all experimental rats were collected on day 14 for routine examinations of hematology and biochemistry parameters, anti-CII IgG antibody reactivity, and histopathology. Our results indicated clearly that at the maximum dosage of 3 mg/kg, the pcDNA-CCOL2A1 vaccine was safe and well-tolerated. No abnormal clinical signs or deaths occurred in the pcDNA-CCOL2A1 group compared with the NS group. Furthermore, no major alterations were observed in hematology, biochemistry, and histopathology, even at the maximum dose. In particularly, no anti-CII IgG antibodies were detected in vaccinated normal rats at 14 d after vaccination; this was relevant because we previously demonstrated that the pcDNA-CCOL2A1 vaccine, when administered at the therapeutic dosage of 300μg/kg alone, did not induce anti-CII IgG antibody production and significantly reduced levels of anti-CII IgG antibodies in the plasma of rats with established collagen-induced arthritis (CIA). This is the first study demonstrating the safety and immunogenicity of a DNA vaccine encoding CCII for treating RA in normal rats. These results may support the use of this novel therapeutic DNA vaccine for the treatment of RA in the future.
Keywords: chicken type II procollagen, immunogenicity, rheumatoid arthritis, safety, therapeutic DNA vaccine
Abbreviations
- CCOL2A1
chicken type II procollagen gene
- CCII
chicken type II collagen
- RA
rheumatoid arthritis
- CIA
collagen induced arthritis
- MTX
methotrexate
- DMARDs
disease modifying antirheumatic drugs
- Tr
CD4+CD25+ regulatory T
- Ts
CD8+CD28−suppressor T
- Th1
CD4+CD28+ Th1
- ELISA
enzyme-linked immunosorbent assay
- WBC
white blood cell
- RBC
red blood cell
- OD
optical density
Introduction
Rheumatoid arthritis (RA) is one of the most common chronic inflammatory/autoimmune diseases and is characterized by chronic synovial inflammation and hyperplasia, resulting in progressive destruction of diseased cartilage and joints, severe disability, and shortened lifespan.1,2 Basic and clinical studies have demonstrated that the occurrence and development of RA are attributed to the failure of both CD4+CD25+ regulatory T (Tr) and CD8+CD28−suppressor T (Ts) cells to control autoreactive CD4+CD28+ Th1 (Th1) and auto-antibody-producing B cells.1,3-5 Although the exact origin and pathogenesis of RA is still unclear, research investigating the pathophysiology of RA has revealed various targets for the development of new antirheumatic therapies.6,7 However, currently available treatments for RA are still largely palliative rather than curative, do not satisfactorily halt disease progression, and are associated with significant side effects, including infections, anorexia, dyspepsia, tumor development, and immune regulatory disruption.8,9 Therefore, there is renewed enthusiasm for the development of new strategies aimed at re-establishing homeostasis toward environmental and self-antigens. Thus, the development of therapeutic DNA vaccines and boosters may allow for the recovery of immunological tolerance through the induction of both Tr and Ts cells.5,10,11
In theory, the ideal goal for treating RA should aim to interfere with the harmful autoreactive immune responses elicited by Th1 cells and B cells, while at the same time not altering normal immune responses necessary for combating infectious agents or inducing generalized immune suppression. Thus, the development of therapeutic DNA vaccines with these above-mentioned advantages may offer new promising strategies for the treatment of RA.5,10,11 Because type II collagen (CII) is as a critical autoantigen in RA, and native chicken type II collagen (nCCII) has been effectively used for the treatment of RA,12,13 we previously developed a novel therapeutic DNA vaccine encoding CCII (termed pcDNA-CCOL2A1).11 Strikingly, our study clearly demonstrated that single injection of the pcDNA-CCOL2A1 vaccine alone could induce potent immune tolerance against experimental RA, suggesting that this vaccine may have therapeutic applications in the treatment of RA in humans. More importantly, the pcDNA-CCOL2A1 vaccine alone seemed to be as effective as the current “gold standard” treatment, methotrexate (MTX).11
Therefore, because the safety and immunogenicity of vaccines are critical for their clinical use, we systemically examined the safety and immunogenicity of the pcDNA-CCOL2A1 vaccine in normal Wistar rats following a single intramuscular injection dose at the maximum dose (3 mg/kg).
Results
Immunization of normal rats with the pcDNA-CCOL2A1 vaccine did not cause systemic or local adverse events
In contrast to the control rats, the general health status (feed intake, activity, and behavior) of the vaccinated rats did not change, even at the maximum dosage of 3 mg/kg pcDNA-CCOL2A1. Throughout the course of this study, no animals died, and no abnormal clinical signs were observed in the rats receiving 3 mg/kg vaccine, clearly indicating that vaccination with pcDNA-CCOL2A1 was safe and well-tolerated (Table 1). As shown in Table 1, no vaccination site reactions, pain upon palpation, or injection sites welling were observed in either group. Furthermore, no adverse respiratory or somatomotor activity was detected in the vaccinated rats. In particular, no significant differences in body weights were found between unvaccinated and vaccinated rats at 14 d after vaccination (male group 318.2 ± 6.7 versus 317.9 ± 12.3 and female group 243.6 ± 8.5 vs. 234.0 ± 3.2, respectively; P > 0.05). Therefore, we could assume that the approximate lethal dose of the pcDNA-CCOL2A1vaccine in both male and female rats may be higher than 3 mg/kg. Taken together with our previous results (using a therapeutic dose of 300 μg/kg), these data demonstrated that the pcDNA-CCOL2A1 vaccine did not cause systemic or local adverse events in the vaccinated rats.11
Table 1.
Vaccination effect of normal rats with pcDNA-CCOL2A1 vaccine at the maximum doses of 3 mg/kg on general health status and behavior on 14 days after vaccination (n=6)*
| Control |
Vaccine |
|||
|---|---|---|---|---|
| Clinical symptoms | male | female | male | female |
| Fur shedding and pilo-erection | − | − | − | − |
| Injection site swelling | − | − | − | − |
| Sneeze and cough | − | − | − | − |
| Ecphysesis | − | − | − | − |
| Tears and eyes purulent sectretion | − | − | − | − |
| Instability of gait | − | − | − | − |
| Diarrhea | − | − | − | − |
| Skin ulcer | − | − | − | − |
| Pain upon palpation | − | − | − | − |
| Convulsive | − | − | − | − |
| Mortality | − | − | − | − |
The vaccinated rats and the control rats were observed for changes in clinical appearance. No significant changes were detected at 0 h, 1 h, 2 h, 3 h, 5 h, 1 day, 2 day, 4 day, 6 day, 8 day, 10 day, 12 day after vaccination(data not shown). This data is representative of 3 experiments. Three separate experiments yielded similar results. −: No corresponding clinical symptoms, +: Presenting corresponding clinical symptoms.
Immunization of normal rats with the pcDNA-CCOL2A1 vaccine did not cause changes in hematological or biochemical parameters
To investigate the potential adverse effects of the pcDNA-CCOL2A1 vaccine at the maximum dosage of 3 mg/kg on the hematological and biochemical parameters of vaccinated normal rats, we systemically examined various routine clinical parameters. Hematology results are summarized and presented in Table 2. Based on a significance level of 0.05, there were no statistically significant differences between the 2 groups. Similarly, no biochemical parameters, including liver, renal, and cardiac functions, were significantly affected by the vaccination (Table 3).
Table 2.
Vaccination effect of normal rats with pcDNA-CCOL2A1 vaccine at the maximum doses of 3 mg/kg on hematological clinical rountine parameters on 2 nd week after vaccination (n = 6, ± s)
| parameters | Vaccine | Control |
|---|---|---|
| WBC(×109 cells/L) | 18.16±4.91 | 17.68±4.84 |
| RBC(×109 cells/L) | 7.94±0.40 | 7.54±0.35 |
| MCHC(g/L) | 162.83±5.81 | 157±9.36 |
| Platelets(×109 cells/L) | 634.00±61.78 | 539.8±82.80 |
| Neutrophil(×109 cells/L) | 1.65±0.58 | 1.80±0.77 |
| Lymphocytes(×109 cells/L) | 15.86±4.49 | 15.33±4.10 |
| Monocytes(×109 cells/L) | 0.55±0.21 | 0.38±0.17 |
| Eosinophils(×109 cells/L) | 0.11±0.02 | 0.17±0.17 |
| Basophils(×109 cells/L) | 0.00±0.00 | 0.00±0.00 |
Parameters are listed in SI units; WBC: white blood cells, RBC: red blood cell, MCHC: mean corpuscular.
*P < 0.05 compared with control. These data are representative of 3 experiments. Three separate experiments yielded similar results.
Table 3.
Vaccination effect of normal rats with pcDNA-CCOL2A1 vaccine at the maximum doses of 3 mg/kg on serum biochemistry rountine parameters on 2nd week after vaccination (n = 6, ± s)
| Parameters | Vaccine | Control | |
|---|---|---|---|
| GLU(mmol/L) | 8.02±2.30 | 10.71±5.42 | |
| Renal function | BUN(mmol/L) | 8.91±1.14 | 9.19±1.38 |
| Cre(μmol/L) | 37.58±7.47 | 42.17±9.29 | |
| UA(μmol/L) | 317.67±82.74 | 278.5±93.10 | |
| Liver function | ALT(IU/L) | 75.17±34.53 | 57.83±9.20 |
| TP(g/L) | 80.52±3.24 | 77.30±4.78 | |
| Albumin(g/L) | 33.42±3.25 | 30.93±3.10 | |
| Glubulin(g/L) | 47.10±5.15 | 46.37±2.40 | |
| TBIL(μmol/L) | 2.82±0.46 | 3.13±0.80 | |
| DBIL(μmol/L) | 0.57±0.33 | 0.47±0.18 | |
| TBA(μmol/L) | 26.10±9.29 | 16.66±3.40 | |
| Heart function | ALP(IU/L) | 344.50±130.96 | 291.8±105.36 |
| AST(mmol/L) | 252.17±127.47 | 197.17±64.40 | |
| Blood lipids | triglyceride(mmol/L) | 2.79±1.03 | 3.53±0.94 |
| TC(mmol/L) | 2.07±0.11 | 1.92±0.09 | |
| HDL(mmol/L) | 1.52±0.13 | 1.36±0.05 | |
| LDL(mmol/L) | 0.21±0.07 | 0.20±0.07 |
Parameters are listed in SI units; GLU: blood glucose; BUN: blood urea nitrogen; Cre: creatinine; UA: uric acid; ALT: alanine aminotransferase; TP: total protein; TBIL: total bilirubin; DBIL: direct bilirubin; TBA: total bile acid; ALP: alkaline phosphatase; AST: aspartate aminotransferase; TC: total cholesterol. HDL: high-density lipoprotein; LDL: low-density lipoprotein.
*P < 0.05 compared with control. These data are representative of 3 experiments. Three separate experiments yielded similar results.
Immunization of normal rats with the pcDNA-CCOL2A1 vaccine did not cause histopathological changes
Gross examination showed that all tissues from vaccinated rats were normal in both size and appearance. Inspection of histological sections from the heart, liver, spleen, lung, kidney, thymus and ankle joint demonstrated no obvious differences between the vaccinated and the unvaccinated rats (Fig. 1). After vaccination with pcDNA-CCOL2A1 at the maximum dosage of 3 mg/kg, no focal mononuclear cell infiltrates were observed in the connective tissue among the heart muscle fibers. The livers of vaccinated rats showed a normal lobular architecture with an intact central vein and portal tracts. The splenic tissue from the vaccinated rats showed normal red and white pulp. No focal degeneration of the bronchial epithelium was detected, and exudate (mononuclear and polymorphonuclear leukocytes) was absent from the bronchial lumen of the lungs of the vaccinated rats. The kidneys from the vaccinated rats showed normal histological structures of the glomeruli and renal tubules in the cortical and medullary tissues. Vaccination with pcDNA-CCOL2A1 did not trigger changes in lymphoreticular cells in the thymus of the vaccinated rats. Moreover, ankle joints of the vaccinated rats were the same as these of normal rats, with no swelling of the ankle joints, no inflamed synovium, no inflammatory cell infiltration within the joint space and synovial lining, no synovial angiogenesis or pannus, and no thickening of the synovial membrane.
Figure 1.
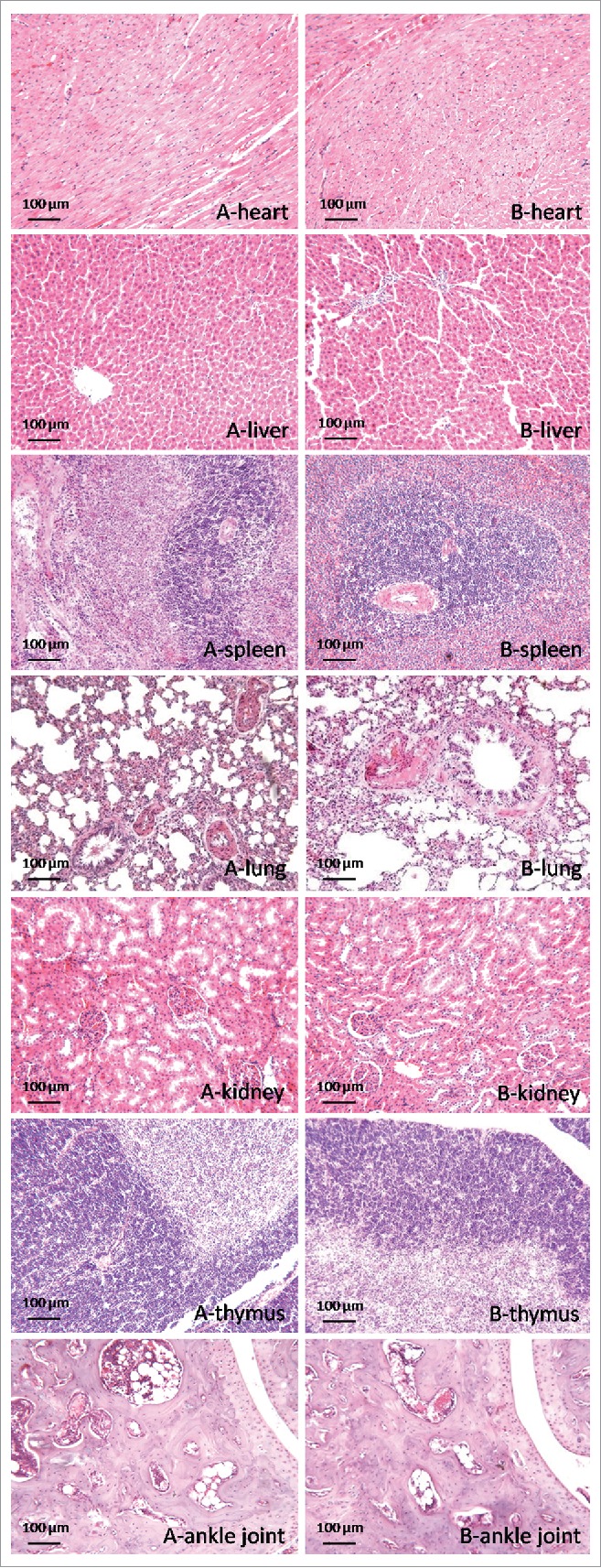
Histopathological analysis of various tissues from normal rats vaccinated with pcDNA-CCOL2A1 at a maximum dosage of 3 mg/kg on day 14 after a single intramuscular injection into the hind leg. H&E staining, original magnification: 200×. (A) Samples from the control rats. (B) Samples from the vaccinated normal rats. These data are representative of 3 experiments. Three separate experiments yielded similar results.
Immunization of normal rats with the pcDNA-CCOL2A1 vaccine did not stimulate anti-CII antibody production
The plasma levels of anti-CII IgG antibodies are considered the most reliable marker for arthritic severity.11,14 Our previous study also showed that administration of the pcDNA-CCOL2A1 vaccine at the therapeutic dose of 300 μg/kg did not induce the production of anti-CII IgG antibodies in normal rats from day 3 to day 35 (data not shown). Thus, we used ELISA to investigate effects of the pcDNA-CCOL2A1 vaccine at a maximum dosage of 3 mg/kg on plasma anti-CII IgG antibody levels on day 14 after vaccination. Consistent with our previous results using the therapeutic dose of 300 μg/kg, vaccination of normal rats with 3 mg/kg pcDNA-CCOL2A1 did not induce the production of anti-CII IgG antibodies, including anti-rat CII antibodies or anti-chicken CII antibodies, as compared to the control group (Table 4). The levels of anti-rat CII antibodies in the vaccinated group were slightly lower than those in the control group but there were no significant difference. Taken together, these data provided direct evidence that a single intramuscular injection of the pcDNA-CCOL2A1 vaccine at the maximum dosage of 3mg/kg did not induce the production of anti-CII antibodies.
Table 4.
Vaccination effect of normal rats with pcDNA-CCOL2A1 vaccine at the maximum doses of 3mg/kg on plasma production of anti-CII IgG antibodies on 2nd week after vaccination
| Anti-CII antibodies | Rat-IgG Anti-Rat | Rat-IgG Anti-chicken |
|---|---|---|
| NC | 0.1077±0.0565 | <0.01 |
| DNA vaccine | 0.07355 ±0.0558 | <0.01 |
Each plasma was not diluted, and the values were expressed as mean units of activity (units/ml, n = 6, +/−s). Statistical analysis was compared with control. P > 0.05 compared with control. These data are representative of 3 experiments. Three separate experiments yielded similar results.
Discussion
This is the first study demonstrating the safety and immunogenicity of a DNA vaccine containing the eukaryotic expression vector pcDNA-CCOL2A1 (encoding CCII), which we successfully developed for the treatment of RA,11 in vaccinated normal rats at the maximum dosage of 3 mg/kg. Our results clearly indicated that the pcDNA-CCOL2A1 vaccine was safe and well-tolerated; compared with the control group (receiving normal saline only), no abnormal clinical signs or mortality were observed in the vaccinated rats, even at the maximum dosage. These results may have important implications for use of this novel therapeutic DNA vaccine in the treatment of RA.
In this study, we monitored the general health status of rats after administration of the vaccine and observed the potential adverse effects of the pcDNA-CCOL2A1 vaccine on histological changes in various tissues. Our results clearly indicated that the pcDNA-CCOL2A1 vaccine (3 mg/kg) was generally safe and well-tolerated, with no observed clinical symptoms during the entire experimental period. No histological changes were found in the heart, liver, spleen, lungs, kidneys, and thymus. There were also no significant changes in markers of hepatic, renal, or cardiac function, and routine hematological clinical parameters remained normal during the course of the investigation. These results revealed that vaccination of normal rats with the pcDNA-CCOL2A1 vaccine did not adversely affect the normal physiological functions and metabolism of rats, suggesting that the vaccine has no toxic side effects on body's important organs. Now that pcDNA-CCOL2A1 vaccine have had the safety profiles, there are good reasons to support the vaccine to be progressed into phase I human trial in the near future. We chose the dose of 3 mg/kg to test the safety and immunogenicity of the pcDNA-CCOL2A1 vaccine for several reasons. First, the dose of 3 mg/kg was as 10 times that of the effective therapeutic dose of 300 μg/kg, which has been shown to be safe and well-tolerated, without inducing any abnormal clinical symptoms.11 Second, the dose of 300 μg/kg has been proven to be as effective as the current “gold standard” treatment, MTX, in halting CIA development and progression.11 In addition, our previous results clearly demonstrated that the pcDNA-CCOL2A1 vaccine has a small pharmaceutical window, which is coincident with many immunosuppressive agents, such as Tacrdimus (FK506) and cyclosporine A (CsA), that are the most widely prescribed therapeutics for various types of organ transplantation and autoimmune diseases.11 As we all know that the therapeutic or pharmaceutical window is an important index for estimating drug dosage which can treat disease effectively while staying within the range of safety. Theoretically, a narrow therapeutic window is a characteristic of therapies based on oral tolerization.
One of the most striking findings from this study was that a single intramuscular injection of pcDNA-CCOL2A1 vaccine at the maximum dosage of 3 mg/kg did not cause the production of anti-CII antibodies. This result was consistent with our recent finding that 300 μg/kg pcDNA-CCOL2A1 did not stimulate production of anti-CII IgG antibodies in normal rats during the experimental period from day 3 to day 35.15 Remarkably, these findings were inconsistent with decades of established knowledge on vaccinations, including DNA vaccinations. According to their mechanism of action, vaccinations must induce production of corresponding protective antibodies and/or protective T-cells, particularly in the case of DNA vaccines.16-18 Indeed, this effect has become an important indicator of the quality and effectiveness of the vaccine during development and production. However, our results were directly opposed to this; in fact, administration of pcDNA-CCOL2A1 at 300 μg/kg or 3 mg/kg did not induce anti-CII antibody production, and the therapeutic dose (300 μg/mg) actually caused a marked reduction in plasma anti-nCII antibodies in collagen-induced arthritic rats.11 It is clear from these data that the exact mechanisms through which this DNA vaccine functions are not yet known. One possible explanation is that unlike DNA vaccines to immunize against infectious agents, which was predictable, the truly important part of this DNA vaccines is not immunization of autoimmune diseases but rather the specific modulation of the immune system. The precise tweaking for unbalanced immune system provide by DNA vaccines may provided the opportunity to treat or even to cure autoimmune diseases.10 These interesting effects are currently being explored further in our laboratory.
Higher levels of anti-CII IgG antibodies in the plasma are considered the most reliable marker of arthritic severity.5,6,11 Anti-CII IgG antibodies have been shown to induce pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-8, when incorporated in immune complexes in vitro.14 Therefore, effectively reducing or lowering the concentration of anti-CII IgG antibodies in plasma from patients with RA has become an important factor for determining the curative effect of the drug. Low-dose MTX and anti-TNF-α are the best examples of this concept.6,19-21 MTX is a traditional folate antagonist and disease-modifying antirheumatic drug that can be administered weekly either alone or as combination therapy and is widely accepted as the gold standard in RA treatment. In our previous study, we found that low-dose MTX therapy alone significantly reduced, but did not eliminate, plasma levels of anti-CII IgG antibodies.6 TNF-α antagonists, such as infliximab (IFX), etanercept (ETN), adalimumab (ADA), golimumab (GOLI), and certolizumab pegol (CZP), have also been widely used as an immunotherapy strategy for the treatment of RA.22,23 Similarly, anti-TNF-α therapy has been shown to downregulate the production of anti-CII IgG antibodies.19-21 However, we cannot explain the lack of anti-CII IgG antibody production in normal rats following administration of our pcDNA-CCOL2A1 vaccine, despite the ability of the vaccine to reduce the concentration of anti-CII IgG antibodies in established CIA rats. One possible explanation is that the pcDNA-CCOL2A1 vaccine may only induce production of nonhydroxylated, nonglycosylated CCII;11 this may affect the 3-dimensional configuration of CCII, leading to formation of non-triple helical, nonfunctional CCII protein due to lack of sufficient prolyl-4-hydroxylase (P4H) and glycosylase activities.11,24,25 Thus, these proposed structural changes may markedly affect the antigenicity of CII conformational epitopes, suppressing the induction of B-lymphocyte immune recognition and response activities. As a result, vaccinated normal rats may not be able to produce anti-CII IgG antibodies. In contrast, the resulting CCII protein may be used as a tolerogenic and may be recognized by T-lymphocytes, particularly CD4+CD25+Tr cells and CD8+CD28- Ts cells, thereby producing immune tolerance.11,26 These findings are also supported by similar findings demonstrating that 2 separate synthetic peptides, CII 181–209 and CII 245–270, which are not glycosylated or hydroxylated, are tolerogenic in CIA.27,28 In particular, our recent results have also demonstrated that nonhydroxylated and nonglycosylated recombinant rcCTE1–2 can induce a potent tolerogenic response in the CIA mouse model,6 providing further experimental evidence for our explanation.
In conclusion, the present study suggested that vaccination of normal rats with the pcDNA-CCOL2A1 vaccine at a maximum dosage of 3 mg/kg was safe and well-tolerated. Moreover, this vaccine did not induce any abnormal clinical signs or adverse effects on normal physiological functions, including hematology and metabolism, and exhibited no immunogenicity. Further detailed biosecurity studies, including analysis of the dynamic profile, biodistribution, stability, and genomic integration, are currently underway to determine the potential clinical applicability of the pcDNA-CCOL2A1 vaccine.
Materials and Methods
pcDNA-CCOL2A1 vaccine
The eukaryotic pcDNA-CCOL2A1 expression vector were previously constructed in our laboratory containing the 4837-bp full-length cDNA encoding chicken type II procollagen (GenBank databases nos.AY046949 and AF452711) with deletion of the N-propeptides, signal peptide sequence, and Kozak consensus sequence.11 The resulting recombinant plasmid, pcDNA-CCOL2A1, was produced in Escherichia coli, purified using Endo-free Mega-prep kits (Qiagen, Valencia, CA, USA), and used here to study its safety and immunogenicity. DNA purified with EndoFree Plasmid Kits contains only negligible amounts of endotoxin(<0.1 EU/μg plasmid DNA).
Animals
Inbred female/male Wistar rats (4–6 weeks old) were obtained from the Animal Breeding Center of the Academy of Military Medical Sciences (Beijing, China) and maintained under specific pathogen-free conditions. The experiments were performed under the supervision and guidelines of the Academy of Military Medical Sciences Animal Welfare Committee. Rats were randomly divided into 2 groups, with 6 rats each in the acute toxicity group and the control group. Each group included 3 male rats and 3 female rats. The vaccinated group received a single intramuscular injection of the pcDNA-CCOL2A1 vaccine (3 mg/kg) into the hind leg on day 0. The control group was treated with the same volume of saline.
General safety analysis
Throughout the course of this study, the vaccinated rats and the control rats were observed for changes in their general health status and behavior.29 Mortality and systemic adverse events were continuously monitored at 0 h,1 h, 2 h, 3 h, 5 h, and 1 day after vaccination, then once per day from day 2 to day 14. The body weights of all experimental rats were measured on days 0 and 14 post-vaccination.
Evaluation of hematological and biochemistry parameters
Routine hematological parameters, including hemoglobin (HGB) levels, white blood cell (WBC) counts, and red blood cell (RBC) counts, were measured on an automatic hematology cell-counter (MS 4, Melet Schloesing Laboratories, Cergy-Pontoise Cedex, France). Routine blood biochemistry parameters were measured on anautomatic analyzer (BT 2000, Biotechnica Instruments, Rome, Italy).
Histopathological examination
Fourteen days after vaccination, all experimental rats were euthanized, and the heart, liver, spleen, lungs, kidneys, and thymus were collected and fixed in 10% neutral buffered formalin for 7 d. Tissue sections were processed for routine paraffin embedding, and hematoxylin and eosin staining was performed following decalcification. Sections were analyzed microscopically at 200× magnification to determine histopathological changes. All sections were analyzed by researchers who were blinded to the treatment of the rats.5,11
Measurement of plamsa anti-CII antibody levels
The plasma samples were collected from both vaccinated and unvaccinated rats on day 14 after vaccination. The levels of plasma anti-CII antibodies were measured by enzyme-linked immunosorbent assay (ELISA).5,6,26 In order to more specifically and more accurately determine whether anti-CII antibodies were produced in vaccinated normal rats with a maximum dosage of 3mg/kg, we uesed simultaneously 2 types of commercially available ELISA kits (Rat-IgG Anti-rat Type II Collagen Antibody and Rat-IgG Anti-chicken Type II Collagen Antibody ELISA Kits, Chondrex, USA). In this test assay, 8-well strips coated with type II collagen were mixed with rat plasma samples to allow specific binding of anti-CII antibodies. The secondary antibody (peroxidase-conjugated goat anti-rat IgG) was added, followed by addition of peroxidase, inducing the reaction with OPD-Urea H2O2. The standard curves for 2 types of anti-CII antibodies
were quantified and established by 7 standard positive control samples (containing 0.25–16 units/ml antibody) in each experiments according to the kit specifications. According to the instruction manual that the tested plasma need to be diluted so that the measured optical density (OD) values were between the linear range of 0.2 and 0.8. The presented values were calculated by multiplying the plasma dilution with the measured antibody levels. However, the results from our preliminary experiments clearly showed that the plasma levels of anti-CII antibodies in vaccinated normal rats with a maximum dosage of 3mg/kg were too low to be diluted. So the plasma samples from both vaccinated and unvaccinated rats were not diluted in our formal experiments.
Statistical analysis
Data were analyzed using SPSS13.0 software. The differences between groups were tested by Student's t-tests. Differences with P values of less than 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported in part by a grant from the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No.2009ZX09103–624 and No.2015GKS-072/139 to Xi YZ). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McInnes IB, Schett G. Mechanisms of disease:The pathogenesis of rheumatoid arthritis. NEJM 2011; 365:2205-2219; PMID:22150039; http://dx.doi.org/ 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 2.Holmdahl R, Malmström V, Burkhardt H. Autoimmune priming, tissue attack and chronic inflammation - the three stages of rheumatoid arthritis. Eur J Immunol 2014; 44:1593-9; PMID:24737176; http://dx.doi.org/ 10.1002/eji.201444486 [DOI] [PubMed] [Google Scholar]
- 3.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med 2004; 10:801-5; http://dx.doi.org/ 10.1038/nm0804-801 [DOI] [PubMed] [Google Scholar]
- 4.Von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol 2003; 3:223-32; PMID:12658270; http://dx.doi.org/ 10.1038/nri1029 [DOI] [PubMed] [Google Scholar]
- 5.Xue H, Liang F, Liu N, Song XQ, Yuan F, Luo Y, Zhao X, Long J, Sun YY, Xi YZ. Potent antirheumatic activity of a new DNA vaccine targeted to B7-2/CD28 costimulatory signaling pathway in autoimmune arthritis. Human Gene Therapy 2011; 22:65-76; PMID:20695769; http://dx.doi.org/ 10.1089/hum.2010.110 [DOI] [PubMed] [Google Scholar]
- 6.Song XQ, Liang F, Liu N, Luo Y, Yuan F, Xue H, Tan LX, Long J, Zhao X, Sun YY, Xi YZ. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+Treg cells and inducing Th1 toTh2 shift in both cells and cytokines. Biomedicine & Pharmacotherapy 2010; 64:463-71; PMID:20359858; http://dx.doi.org/ 10.1016/j.biopha.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol 2014; 66:775-82; PMID:24757129; http://dx.doi.org/ 10.1002/art.38323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramiro S, Gaujoux-Viala C, Nam JL, Smolen JS, Buch M, Gossec L, van der Heijde D, Winthrop K, Landewé R. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014; 73:529-35; PMID:24401994; http://dx.doi.org/ 10.1136/annrheumdis-2013-204575 [DOI] [PubMed] [Google Scholar]
- 9.Gaujoux-Viala C, Nam J, Ramiro S, Landewé R, Buch MH, Smolen JS, Gossec L. Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014; 73:510-5; PMID:24395555; http://dx.doi.org/ 10.1136/annrheumdis-2013-204588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallon S. DNA vaccines: Treatment options for autoimmune diseases. Microbiol Mol Gen 2008; 4:99-103 [Google Scholar]
- 11.Song XQ, Liang F, Liu N, Luo Y, Xue H, Yuan F, Tan LX, Sun YY, Xi CX, Xi YZ. Construction and characterization of a novel DNA vaccine that is potent antigen-specific tolerizing therapy for experimental arthritis by increasing CD4+CD25+Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Vaccine 2009; 27:690-700; PMID:19095031; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.090 [DOI] [PubMed] [Google Scholar]
- 12.Trentham DE, Dynesius-Trentham RA, Orav EJ. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993; 261:1727-30; PMID:8378772; http://dx.doi.org/ 10.1126/science.8378772 [DOI] [PubMed] [Google Scholar]
- 13.Xu SQ, Liu S, Zhang W, Shen J, Liu HX, Wang RC. A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Research & Therapy 2008; 11:R180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill J, Rowley MJ, Nandakumar KS, Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol 2008; 18:429-41; PMID:18521704; http://dx.doi.org/ 10.3109/s10165-008-0080-x [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Long J, Yun S, Zhang Z, Jin J, Yu K, Hao L, Dai D, Ding L, Tan L, et al.. Evaluation of Humoral and Cellular Immune Responses to a DNA Vaccine Encoding Chicken Type II Collagen for Rheumatoid Arthritis in Normal Rats. Human Vaccine & Immunther 2015; 11:938-45; http://dx.doi.org/ 10.1080/21645515.2015.1010977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas S, Luxon BA. Vaccines based on structure-based design provide protection against infectious diseases. Expert Rev Vaccines 2013; 12:1301-11; PMID:24090172; http://dx.doi.org/ 10.1586/14760584.2013.840092 [DOI] [PubMed] [Google Scholar]
- 17.Coban C, Kobiyama K, Aoshi T, Takeshita F, Horii T, Akira S, Ishii KJ. Novel strategies to improve DNA vaccine immunogenicity. Curr Gene Ther 2011; 11:479-84; PMID:22023477; http://dx.doi.org/ 10.2174/156652311798192815 [DOI] [PubMed] [Google Scholar]
- 18.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis 2011; 53:296-302; PMID:21765081; http://dx.doi.org/ 10.1093/cid/cir334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu MY, Xiangbin Wang XB, Yin CC, Zhong Zhang Z, Lin Q, Yongsu ZHhen YS, Huang HL. Targeting TNF-α with a tetravalent mini-antibody TNF-TeAb. Biochem J 2007; 406:237-46; PMID:17472572; http://dx.doi.org/ 10.1042/BJ20070149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee P, Wu B, Mayton L, Kim S H, Robbins P D, Wooley P H. TNF receptor gene therapy results in suppression of IgG2a anticollagen antibody in collagen induced arthritis. Ann Rheum Dis 2003; 62:707-14; PMID:12860724; http://dx.doi.org/ 10.1136/ard.62.8.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler D M, Malfait A M, Maini R N, Brennan F M, Feldmann M. Anti-IL-12 and anti-TNF antibodies synergistically suppress the progression of murine collagen-induced arthritis. Eur J Immunol 1999; 29:2205-12; PMID:10427983; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199907)29:07%3c2205::AID-IMMU2205%3e3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- 22.Navarro-Millán I, Sattui SE, Curtis JR. Systematic review of tumor necrosis factor inhibitor discontinuation studies in rheumatoid arthritis. Clin Ther 2013; 35:1850-61; http://dx.doi.org/ 10.1016/j.clinthera.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, Xu S. TNF inhibitor therapy for rheumatoid arthritis. Biomed Rep. 2013; 1:177-84; PMID:24648915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med 2001; 33:7-21; PMID:11310942; http://dx.doi.org/ 10.3109/07853890109002055 [DOI] [PubMed] [Google Scholar]
- 25.Nokelainen M. Recombinant human collagens: Characterization of type II collagen expressed in insect cells and production of types I-III collagen in the yeast pichia pastoris. Acta Universitatis OuluensisD Medica 2000; 604:1-70 [Google Scholar]
- 26.Xi CX, Tan LX, Sun YP, Liang F, Liu N, Xue H, Luo Y, Yuan F, Sun YY, Xi YZ. A novel recombinant peptide containing only two T–cell tolerance epitopes of chicken type II collagen that suppresses collagen-induced arthritis. Molecular Immunology 2009; 46:729-37; PMID:19041137; http://dx.doi.org/ 10.1016/j.molimm.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 27.Myers LK, Seyer JM, Stuart JM, Terato K, David CS, Kang AH, T cell epitopes of type II collagen that regulate murine collagen-induced arthritis. J Immunol 1993; 151:500-5; PMID:7686947 [PubMed] [Google Scholar]
- 28.Myers LK, Brand DD, Ye XJ, Cremer MA, Rosloniec EF, Bodo M, Myllyharju J, Helaakoski T, Nokelainen M, Pihlajaniemi T, et al.. Characterization of recombinant type II collagen: arthritogenicity and tolerogenicity in DBA/1 mice. Immunology 1998; 95:631-9; PMID:9893056; http://dx.doi.org/ 10.1046/j.1365-2567.1998.00637.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, Liang F, Liu N, Xue H, Song XQ, Yuan F, Long J, Zhao X, Sun YY, Xi YZ. Potent control of acute graft-versus-host disease lethality after immunization with a novel DNA vaccine targeted to B7-1/CD28 costimulatory signaling pathway. J Immunother 2013; 36:82-92; PMID:23377669; http://dx.doi.org/ 10.1097/CJI.0b013e31827a6e3e [DOI] [PubMed] [Google Scholar]


