Abstract
During the past decade, a number of H5 subtype influenza vaccines have been developed and tested in clinical trials, but most of them induced poor serum antibody responses prompting the evaluation of novel vaccination approaches. One of the most promising ones is a “prime-boost” strategy, which could result in the induction of prompt and robust immune responses to a booster influenza vaccine following priming with homologous or heterologous vaccine strains. In our study we evaluated immunogenicity of an adjuvanted A(H5N1) inactivated influenza vaccine (IIV) in healthy adult subjects who received A(H5N2) live attenuated influenza vaccine (LAIV) 1.5 years earlier and compared this with a group of naïve subjects. We found that priming with A(H5N2) LAIV induced a long-lasting B-cell immunological memory against influenza A(H5N1) virus, which was brought on by more prompt and vigorous antibody production to a single dose of A(H5N1) IIV in the primed group, compared to the naïve controls. Thus, by day 28 after the first booster dose, the hemagglutination inhibition and neutralizing (MN) antibody titer rises were 17.2 and 30.8 in the primed group, compared to 2.3 and 8.0 in the control group, respectively. The majority (79%) of the primed individuals achieved seroprotective MN antibody titers at 7 days after the first dose of the IIV. All LAIV-primed volunteers had MN titers ≥1:40 by Day 28 after one dose of IIV, whereas only 58% subjects from the naïve control group developed similar immune responses at this time point. The second A(H5N1) IIV dose did not increase the immune response in the LAIV-primed group, whereas 2 doses of IIV were required for naïve volunteers to develop significant immune responses. These findings were of special significance since Russian-based LAIV technology has been licensed to WHO, through whom the vaccine has been provided to vaccine manufacturers in India, China and Thailand — countries particularly vulnerable to a pandemic influenza. The results of our study will be useful to inform the development of vaccination strategies in these countries in the event of a pandemic
Keywords: clinical trial, H5 avian influenza viruses, inactivated influenza vaccine, live attenuated influenza vaccine, memory immune response, prime-boost strategy, safety
Introduction
Influenza pandemics occur on an average of 3 or 4 times per century, spread rapidly and affect entire continents or the whole world resulting in significant morbidity and high mortality in all population groups.1 Highly pathogenic avian influenza viruses (HPAIV) may lead to a pandemic if they overcome the species barrier and developed the ability to be transmitted among humans. Even though no steady transmissions of HPAIV from person to person have been recorded to this point, new genetic variations could likely enable these viruses to propagate efficiently in the human population. Due to this threat, a number of subtype A pre-pandemic vaccines have already been developed (H5, H7, H2), and some of them have already passed clinical testing.2-6 Unfortunately, most of the H5 vaccines induced poor serum antibody responses when compared to seasonal influenza vaccines.7 Therefore, novel vaccination approaches have been sought,4,5,7 one of the most promising ones is a “prime-boost” strategy, which could result in the induction of prompt and robust immune responses to a booster influenza vaccine following priming with homologous or heterologous vaccine strains8-11
Recently the World Health Organization (WHO) has recognized the advantages of using live attenuated influenza vaccine (LAIV) over inactivated vaccine (IIV) if a pandemic breaks out.1,12 These advantages include: high culture yield, easier down-stream processing, faster quality control release, needle-free administration, and cross-reactivity of the induced immune responses.13-20 In addition, LAIVs induce mucosal immunity in the upper respiratory tract, which may contribute to limiting virus replication and spread.21,22 which could potentially result in herd immunity 19
Talaat et al. and Babu at al. recently reported studies in which inactivated influenza vaccines were administered to subjects previously primed with MedImmune's LAIVs.23,24 While these LAIV recipients showed poor immune response to the initial vaccination, the responses were significantly boosted by A(H5N1) and A(H7N7) inactivated influenza vaccines administered 52 to 56 months later, even though the boosting doses used were suboptimal. These findings were evident as early as 7 days post-IIV.
In a previous study we evaluated an A(H5N2) LAIV candidate developed by the Institute of Experimental Medicine, St Petersburg, Russia.25 Two intranasal doses of the vaccine or placebo were administered intranasally 4 weeks apart to 29 and 10 subjects, respectively (September - October 2012). The vaccine was well tolerated and not associated with any clinically significant events. The rates of serologic and mucosal antibody responses were encouraging and supported further exploration of the immunologic profile elicited by vaccination with A(H5N2) LAIV, in particularly, with respect to the induction of long term memory. With that in mind we invited all reachable participants from the original study and an additional group of 24 naïve subjects to receive 2 doses of licensed inactivated A(H5N1) vaccine in order to investigate if the A(H5N2) LAIV candidate resulted in efficient long-lasting priming of the immune system. The study differed in a number of ways from the one described by Talaat et al.:23 1) the LAIV had a different attenuated backbone, 2) a shorter time interval elapsed between prime and boost vaccinations (1.5 versus 5 years), 3) 2 lower dose vaccinations of adjuvanted IIV were employed, and 4) additional immunological assays were applied to study mechanisms underlying immune responses to primary immunization with LAIV
Materials and Methods
Vaccine
The IIV used in this study was OrniFlu® prepared from the NIBRG-23 vaccine virus strain. One vaccine dose (0.5 ml) contained 15 µg of influenza A(H5N1) virus hemagglutinin (HA), adjuvanted with aluminum hydroxide. Two doses of the vaccine were administered intramuscularly 28 days apart to all study subjects
Study design and study subjects
The study was an open-label trial and evaluated the immunogenicity of the A(H5N1) IIV in healthy adult subjects who received 2 doses of A(H5N2) LAIV or placebo 28 days apart in a previous double blind placebo-controlled study conducted at the Institute of Influenza in September and October 2012, as well as additional naïve subjects who did not participate in the previous study
The study was approved by the Ministry of Health and Social Development of the Russian Federation (Moscow, Russia), the Research Institute of Influenza Ethics Committee (St. Petersburg, Russia.), and Western IRB (on behalf of PATH, USA). It was conducted in compliance with the Declaration of Helsinki. The clinical trial was registered on http://www.clinicaltrials.gov/ under the identifier NCT02153671
A total of 19 subjects who received 2 doses of A(H5N2) LAIV (primed group) were recruited to participate in the prime-boost study 1.5 years later. An additional group of 24 naïve subjects (control group), including 5 who received placebo in the original study were recruited and immunized with 2 doses of the A(H5N1) IIV. Inclusion/exclusion criteria for the additional group of naïve volunteers mirrored those utilized in the initial study.25
All subjects were consented for participation and screened for eligibility through medical history review and physical examination. They were tested for serologic evidence of chronic viral infection with human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) and underwent clinical chemistry and hematology laboratory examination. Female participants underwent urine test for pregnancy. Fully eligible subjects received 2 doses of the inactivated subunit adsorbed influenza vaccine 28 days apart via intramuscular route. Collection of serum, urine, and PBMC samples for safety monitoring and immunological testing took place at days 0, 7, 28, and 56. Subjects were asked to closely watch for and report any adverse events occurring the first 6 days after immunization, and followed for any reactions and adverse events occurring within 7 and 28 days after each vaccination
Assessment of serum antibody immune responses
Immune responses to the A(H5N1) IIV were primarily assessed by hemagglutination inhibition (HAI), microneutralization (MN) assays, immunoglobulin A (IgA) and immunoglobulin G (IgG) antibodies in serum samples collected prior to vaccination and on Days 7 and 28 after the first dose, and at day 28 after the second (referred as Day 56 hereafter). The following H5 antigens were tested to evaluate the breadth of the response: i) A/17/turkey/Turkey/05/133 (H5N2) (17/t/Tur (H5N2)); ii) A/turkey/Turkey/5/05(H5N1) PR8-based candidate vaccine virus (NIBRG-23 (H5N1)); iii) A/Indonesia/5/2005 (H5N1) PR8-based candidate vaccine virus (Indo (H5N1)); and iv) A/17/duck/Potsdam/86/92 (H5N2) (d/Pot (H5N2)).
HAI tests were performed on serum samples with the conventional WHO-recommended assays.26 Sera were pretreated with receptor destroying enzyme (RDE, Denka Seiken, Japan) and tested against 4 HA units of several H5 antigens using horse red blood cells. A four-fold or greater antibody rise in titer was considered to be a seroconversion.26
Serum specimens were tested for neutralizing antibodies against 17/t/Tur (H5N2) and Indo (H5N1) viruses by MN using Madin–Darby Canine Kidney cells as described in Rowe et al..27 Titers of neutralizing antibodies were expressed as reciprocal of the greatest dilution giving a neutralization of 50% on the cytopathic effects of the virus in the tissue culture (TCID50).
Detection of anti-HA IgA and IgG antibodies was carried out by indirect enzyme-linked immunosorbent assay (ELISA) according to the procedure described earlier.28 Briefly, 16 HA units of sucrose-purified virus antigen was used to coat ELISA plates in a volume of 100 µl. Two-fold dilutions of sera were prepared starting from 1:10 (for IgA antibody) and 1:100 (for IgG antibody) and added to the coated wells, followed by incubation with the horseradish peroxidase-conjugated goat anti-human IgA or IgG. Antibody titer was defined as the last dilution with optical density (OD) values at least twice exceeding mean values of OD measured in control wells. A post-vaccination increase of antibody titers 4-fold and higher as compared with baseline values was considered a seroconversion.
Avidity of anti-influenza serum IgA and IgG antibody
An avidity analysis of serum IgA and IgG antibodies was performed as described in.29 with some modifications as follows: serum samples (diluted 1:100 for IgG and 1:10 for IgA antibody analysis) were incubated on the antigen coated plated in triplicates, treated with urea (5 mol/L for 5 minutes for IgA and 8 mol/L for 10 minutes for IgG antibody) at room temperature before washing and followed by incubation with the horseradish peroxidase-conjugated goat anti-human IgA or IgG. The avidity index (AI) was defined as the ratio of the mean optical density at 450 nm (OD450) with urea to that without urea × 100. A ≥15% increase in the AI value was considered significant.
Determination of IgA and IgG antibodies in lymphocyte supernatants (ALS)
This was performed in accordance with the procedure described by He et al. for quantifying plasmablast-derived polyclonal antibody with some modifications.30 Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from blood specimens collected from study participants by conventional method at Days 0, 7, 28, and 56 of the study. The isolated cells were incubated over 4 days at 37°C in a 5% CO2 atmosphere and supernatants were collected by low-speed centrifugation. The supernatant samples were stored at −20°C until analysis by IgA and IgG ELISA as described above with the exception that the undiluted samples were used to prepare 2-fold dilutions.
Quantification of IFN–γ producing CD8+ memory T–cells
This was performed by flow cytometry. PBMCs were isolated on gradient, washed, and stored in liquid nitrogen until the analysis. Analysis of virus specific T cells was performed by conventional intracellular cytokine (IFN-γ) staining after in vitro stimulation of cells at a 12 MOI (multiplicity of infection) dose of purified vaccine virus as described in Rudenko et al..28
Statistical Analyses
Statistical analysis of the data was performed by Statistica 6 and GraphPad Prizm 5 software using the Wilcoxon Matched Pairs Test, Mann Whitney U-test, Friedman ANOVA, and Fisher exact test (2–tailed). The study included 19 primed and 24 control subjects, which allowed detecting significant differences between the 2 groups by nonparametric analyses with statistical power of 99.9%.31
Results
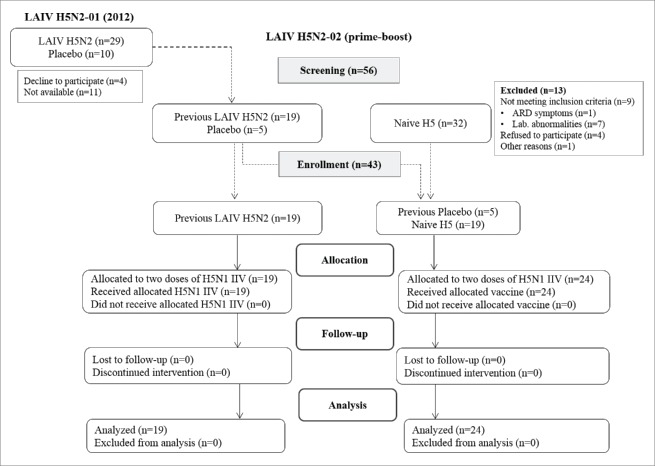
Figure 1 summarizes the design of the prime-boost study. Of the 29 volunteers who received 2 doses of the A(H5N2) LAIV in 2012, only 19 subjects were available for the enrollment. All of them were screened and found to be eligible. In addition, 24 H5 naïve volunteers were enrolled in this study as a control group, including 5 subjects from the placebo group of the 2012 study. The enrollment process, the number of eligible participants and the reasons for the exclusion from the study are shown on Figure 1. Demographic characteristics of the enrolled subjects are given in Table 1.
Figure 1.
Study flow chart.
Table 1.
Demographic characteristics of subjects enrolled in the prime-boost study
| Age (years) |
|||||
|---|---|---|---|---|---|
| Group | N | Mean | Variations | Race | Gender |
| Control (naive) | 24 | 30.3 | 20 – 48 | White: 24 (100%) | Males – 12 (50.0%) Females – 12 (50.0%) |
| Primed with A(H5N2) LAIV | 19 | 30.8 | 20 – 51 | White: 19 (100%) | Males – 12 (63.2%) Females – 7 (36.8%) |
| Total | 43 | 30.5 | 20 – 51 | White: 43 (100%) | Males – 24 (55.8%) Females – 19 (44.1%) |
Safety
The A(H5N1) IIV was well tolerated by both study groups, suggesting that previous exposure to the A(H5N2) LAIV did not increase reactogenicity of the A(H5N1) IIV given intramuscularly 1.5 years later. The detailed summary on adverse reactions detected during the study can be found in supplementary material.
Immune Status of Volunteers Prior to Vaccination
Since the majority of these volunteers had immune responses detected in one or more immunological assays after 2 doses of the A(H5N2) LAIV,25 we had an opportunity to estimate the longevity of these responses. HAI and serum IgA antibody titers decreased over the 1.5 years in the primed subjects and exhibited no significant differences with the control group at baseline (Fig. 2). On the other hand, MN and serum IgG antibody levels were significantly higher in individuals primed with A(H5N2) LAIV in 2012 as compared to control (naïve) subjects (p = 0.0009 for MN and p = 0.0411 for IgG antibody). Interestingly, the MN antibodies remained at the same levels as were detected 4 weeks after the second dose of LAIV 1.5 years earlier. 25
Figure 2.
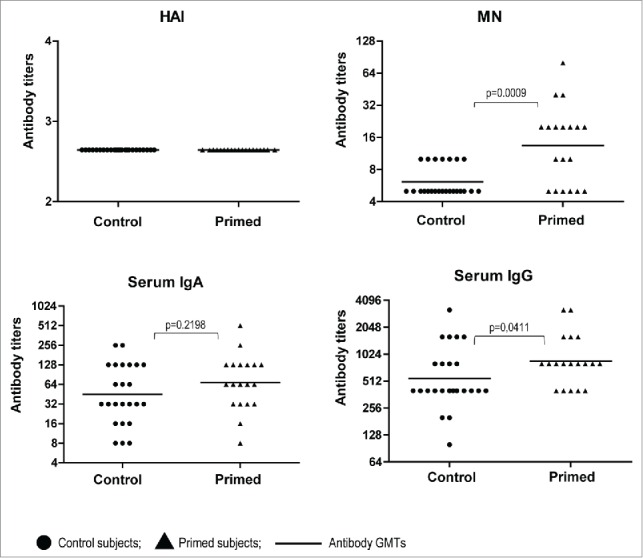
Serum antibody titers to A/17/turkey/Turkey/05/133 (H5N2) in volunteers before vaccination with A(H5N1) IIV (Day 0). Dots– individual data of volunteers vaccinated with H5N2 LAIV in 2012 (n=19), and non-vaccinated with LAIV in 2012 (n = 24); lines– Ab GMTs.P values were calculated using GraphPad Prism 5 software by Mann Whitney U test.
Serum Antibody Responses After One And Two Doses of A(H5N1) IIV
The primary outcome of the study was to establish differences between the LAIV-primed and control groups in term of serum antibody immune responses. The most striking difference between the 2 groups was observed when analyzing the kinetics of HAI and MN antibody titers detected at Days 7, 28 and 56 (Table 2). A significant increase of MN geometric mean titers (GMT) was observed in the LAIV-primed group already at Day 7 after the first A(H5N1) IIV dose; MN antibody against homologous and heterologous H5 antigens reached GMTs of 1:138 and 1:67 at this time point, respectively. In contrast, LAIV-naïve subjects did not develop high titers of MN antibody by Day 7 after the first boost dose, the GMT was 1:21. Although the GMTs for HAI antibody at Day 7 were not significantly different between the 2 groups, a sharp increase of the HAI titers was observed in the LAIV-primed group at Day 28, achieving a GMT of 1:43. High HAI titers were detected when heterologous H5 viruses of different clades (clade 2.1 and clade 0) were used as antigen, while only a slight increase was noted in LAIV-naïve subjects (GMT level not exceeding 1:9). Similarly to HAI, the MN antibody titers in the LAIV-primed group continued to rise over the next 3 weeks and by Day 28 achieved values of 1:413 and 1:166 against homologous and heterologous antigens, respectively. The GMTs of HAI and MN antibodies in the LAIV-primed group were significantly higher than that observed for the control group at Day 28 after the first boost dose (Table 2; Table S3). The second IIV dose further increased the HAI antibody titers in the LAIV-primed group with GMT levels reaching values of 1:36 to 1:62 with various H5 antigens tested. In contrast, the second IIV dose did not increase MN antibody titers in the LAIV-primed subjects when homologous virus was used as antigen., whereas MN GMT against heterologous H5 antigen continued to rise, approaching a value of 1:240 by Day 56. Importantly, the A(H5N2) LAIV-naïve subjects had poor HAI responses and GMT levels did not exceed 1:12 after 2 doses of A(H5N1) IIV, however their MN titers against both homologous and heterologous H5 antigens significantly increased by Day 56, far overreaching seroprotective levels (Table 2).
Table 2.
Serum antibody (Ab) responses on days 7, 28 and 56 following administration of A(H5N1) inactivated influenza vaccine (IIV)
| |
Day 7 |
|
Day 28 |
|
Day 56* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) with ≥1:40 Ab titer2 | Subjects with ≥4-fold Ab rise |
n (%) with ≥1:40 Ab titer | Subjects with ≥4-fold Ab rise |
n (%) with ≥1:40 Ab titer | Subjects with ≥4-fold Ab rise |
|||||||||
| Test antigen | Study group | n | GMT1 | n (%)2 | GMT1 | GMT | n (%) | GMT | GMT | n (%) | GMT | |||
| Hemagglutination inhibition assay | ||||||||||||||
| 17/t/Tur (H5N2) | Primed | 19 | 6.0 | 4 (21.1) | 5 (26.3) | 69.6 | 43.0 | 11 (57.9) | 13 (68.4) | 160.0 | 62.0 | 12 (63.2) | 15 (78.9) | 145.9 |
| Control | 24 | 3.1 | 1 (4.2) | 2 (8.3) | 28.3 | 5.8 | 3 (12.5) | 6 (25.0) | 50.4 | 7.7 | 5 (20.8) | 10 (41.7) | 32.5 | |
| NIBRG-23 (H5N1) | Primed | 19 | 7.5 | 5 (26.3) | 5 (26.3) | 139.3 | 32.1 | 11 (57.9) | 11 (57.9) | 205.9 | 44.6 | 14 (73.7) | 14 (73.7) | 124.9 |
| Control | 24 | 3.1 | 1 (4.2) | 2 (8.3) | 28.3 | 5.9 | 3 (12.5) | 7 (29.2) | 44.2 | 9.2 | 7 (29.2) | 12 (50.0) | 33.6 | |
| Indo (H5N1) | Primed | 19 | 6.5 | 2 (10.5) | 8 (42.1) | 23.8 | 35.9 | 12 (63.2) | 12 (63.2) | 169.5 | 49.8 | 14 (73.7) | 15 (78.9) | 110.6 |
| Control | 24 | 3.4 | 2 (8.3) | 2 (8.3) | 40.0 | 8.7 | 4 (16.7) | 10 (41.7) | 35.3 | 11.9 | 9 (37.5) | 13 (54.2) | 44.5 | |
| d/Pot (H5N2) | Primed | 19 | 7.2 | 5 (26.3) | 7 (36.8) | 36.2 | 32.1 | 11 (57.9) | 14 (73.7) | 80.0 | 35.9 | 14 (73.7) | 14 (73.7) | 92.8 |
| |
Control |
24 |
3.1 |
1 (4.2) |
3 (12.5) |
15.9 |
5.9 |
4 (16.7) |
7 (29.2) |
48.8 |
9.2 |
8 (33.3) |
10 (41.7) |
56.6 |
| Microneutralization assay | ||||||||||||||
| 17/t/Tur (H5N2) | Primed | 19 | 138.3 | 15 (78.9) | 12 (63.2) | 380.5 | 413.1 | 19 (100) | 18 (94.7) | 470.3 | 370.3 | 19 (100) | 18 (94.7) | 387.9 |
| Control | 24 | 20.6 | 7 (29.2) | 11 (45.8) | 58.4 | 49.0 | 14 (58.3) | 18 (75.0) | 80.0 | 142.5 | 22 (91.7) | 23 (95.8) | 155.3 | |
| Indo (H5N1) | Primed | 19 | 66.7 | 12 (63.2) | 11 (57.9) | 219.3 | 165.9 | 17 (89.5) | 18 (94.7) | 194.0 | 239.0 | 19 (100) | 19 (100) | 239.0 |
| Control | 24 | 15.9 | 6 (25.0) | 11 (45.8) | 48.3 | 31.3 | 11 (45.8) | 16 (66.7) | 61.7 | 73.4 | 21 (87.5) | 23 (95.8) | 80.0 | |
17/t/Tur (H5N2): A/17/turkey/Turkey/05/133 (H5N2) LAIV strain; NIBRG-23 (H5N1): A/turkey/Turkey/5/05 (H5N1) PR8-based candidate vaccine virus; Indo (H5N1): A/Indonesia/5/2005 (H5N1) PR8-based candidate vaccine virus; d/Pot (H5N2): A/17/duck/Potsdam/86/92 (H5N2) LAIV strain; Ab, antibody;
Day 56 is counted relative to the first dose of IIV and corresponds to Day 28 after the second dose of the IIV;
statistically significantly higher GMT values in the primed group compared to the control group are shown in bold (Mann-Whitney U test; p < 0.05);
statistically significantly higher proportions of subjects in the primed group compared to the control group are shown in bold (Fisher exact test, 2-tailed; p < 0.05); see Supplementary materials for the exact P values (Table S3).
Seroprotective titers of HAI antibody (≥1:40) were detected in LAIV-primed individuals at much higher frequencies than in naïve subjects at Days 28 and 56 of the study for all antigens tested (Table 2). An even more pronounced effect was observed in the MN assay, with the majority of the primed individuals achieving seroprotective titers at 7 days after the first dose of A(H5N1) IIV. All 19 LAIV-primed volunteers had MN titers ≥1:40 by Day 28 after one dose of IIV, whereas only 14 of 24 subjects from the naïve control group developed similar immune responses at this time point.
Less pronounced differences between the 2 study groups were observed when analyzing HAI and MN seroconversion levels (≥4-fold increases in antibody titers). The only significant difference between the 2 groups at Day 7 was detected by HAI assay using Indo (H5N1) virus as an antigen, where over 42% of LAIV-primed volunteers seroconverted as compared to 8.3% responders in the control group. By Day 28 after the first booster dose, significantly higher seroconversion rates were observed for the primed subjects in HAI assays with homologous and heterologous H5 antigens (Table 2). The second booster dose increased seroconversion rates for both groups and significant differences between them were seen only in HAI assay with homologous antigen. Despite the relatively high proportions of naïve subjects with HAI and MN antibody seroconversion upon IIV vaccination, the GMT levels of the responders (subjects with ≥4-fold antibody rises) were much lower than that of the LAIV-primed individuals (Table 2). These data further indicate that priming with A(H5N2) LAIV resulted in a more vigorous antibody response to subsequent vaccination with A(H5N1) IIV. Similar results were observed by testing serum IgA and IgG antibodies in ELISA using the 2 different vaccine antigens to coat the ELISA plates, A(H5N2) and A(H5N1) (Table 3). Consistent with the HAI and MN results, the IgA and IgG GMTs were much higher in the primed volunteers as compared to the control group for both antigens at all studied time points. In addition, ELISA also demonstrated that the second booster dose did not increase the immune response in the LAIV-primed group (in fact, antibody titers in most of the volunteers decreased by Day 56).
Table 3.
Serum IgA and IgG immune responses in A(H5N2) LAIV-primed and control volunteers immunized with A(H5N1) IIV at indicated time points
| GMT, log2±SD1 |
Subjects with ≥4-fold Ab rise, n (%)2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Assay | Test antigen | Study group | n | Day 0 | Day 7 | Day 28 | Day 56 | Day 7 | Day 28 | Day 56 |
| Serum IgA ELISA | 17/t/Tur (H5N2) | Primed | 19 | 4.5 ± 1.4 | 6.2 ± 1.9 | 6.8 ± 1.5 | 6.7 ± 1.4 | 9 (47.4) | 13 (68.4) | 13 (68.4) |
| Control | 24 | 4.0 ± 1.7 | 5.0 ± 1.4 | 5.3 ± 1.5 | 5.2 ± 1.7 | 8 (33.3) | 9 (37.5) | 9 (37.5) | ||
| NIBRG-23 (H5N1) | Primed | 19 | 2.5 ± 0.8 | 5.3 ± 2.4 | 5.6 ± 2.3 | 5.6 ± 1.9 | 15 (78.9) | 15 (78.9) | 15 (78.9) | |
| Control | 24 | 2.5 ± 1.2 | 4.1 ± 1.5 | 3.9 ± 2.1 | 4.2 ± 2.1 | 12 (50.0) | 11 (45.8) | 13 (54.2) | ||
| Serum IgG ELISA | 17/t/Tur (H5N2) | Primed | 19 | 9.7 ± 0.9 | 10.4 ± 1.1 | 11.2 ± 1.1 | 11.3 ± 0.9 | 3 (15.8) | 9 (47.4) | 10 (52.6) |
| Control | 24 | 9.0 ± 1.1 | 9.7 ± 1.2 | 10.2 ± 1.3 | 10.6 ± 1.0 | 4 (16.7) | 7 (29.2) | 10 (41.7) | ||
| NIBRG-23 (H5N1) | Primed | 19 | 8.3 ± 1.1 | 9.5 ± 1.1 | 10.5 ± 1.1 | 10.3 ± 0.9 | 5 (26.3) | 11 (57.9) | 11 (57.9) | |
| Control | 24 | 7.3 ± 1.3 | 8.1 ± 1.3 | 8.8 ± 1.3 | 9.2 ± 1.3 | 6 (25.0) | 11 (45.8) | 15 (62.5) | ||
17/t/Tur (H5N2): A/17/turkey/Turkey/05/133 (H5N2) LAIV strain; NIBRG-23 (H5N1): A/turkey/Turkey/5/05 (H5N1) PR8-based candidate vaccine virus;
statistically significantly higher GMT values in the primed group compared to the control group are shown in bold (Mann-Whitney U test; p < 0.05);
statistically significantly higher proportions of subjects in the primed group compared to the control group are shown in bold (Fisher exact test, 2-tailed; p < 0.05).
Avidity of Anti-Influenza ELISA IgA and IgG Antibody
We determined avidity indexes of serum IgA and IgG antibodies in subjects with 4-fold or greater increases in the antibody titers after vaccination. Table 4 summarizes the proportion of subjects with ≥15% increases of the IgA and IgG avidity indexes (AI) following one dose of IIV at days 0, 7, and 28. AI increases for both IgA and IgG antibodies were detected at higher frequencies in LAIV-primed subjects as compared to the control group, although statistical significance was recorded only for the difference in AI conversions of influenza A(H5N1)-specific IgA antibody (Table 4). The mean values of the AI at Day 28 were also statistically significantly higher in the primed group.
Table 4.
Avidity of serum IgA and IgG antibodies in A(H5N2) LAIV-primed and control subjects immunized with one dose of A(H5N1) IIV at indicated time points
| n (%) of subjects with ≥15% increase of AI2 |
Mean AI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Test antigen | Ab | Group | n1 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 |
| 17/t/Tur (H5N2) | IgA | Primed | 14 | 6 (42.9) | 5 (35.7) | 75.6 ± 14.6 | 87.7 ± 9.3 | 87.2 ± 8.6 |
| Control | 9 | 2 (22.2) | 1 (11.1) | 75.2 ± 8.6 | 81.5 ± 10.3 | 85.1 ± 7.8 | ||
| IgG | Primed | 11 | 5 (45.5) | 6 (54.5) | 81.6 ± 11.8 | 90.7 ± 5.7 | 92.3 ± 6.4 | |
| Control | 11 | 1 (9.1) | 1 (9.1) | 83.4 ± 9.7 | 86.0 ± 12.0 | 84.2 ± 10.5 | ||
| NIBRG-23 (H5N1) | IgA | Primed | 17 | 9 (52.9) | 7 (41.2) | 72.7 ± 7.5 | 86.4 ± 12.6 | 84.9 ± 8.8 |
| Control | 16 | 3 (18.8) | 0 (0.0) | 77.4 ± 6.4 | 83.3 ± 8.1 | 77.9 ± 8.3 | ||
| IgG | Primed | 11 | 2 (18.2) | 6 (54.5) | 76.2 ± 4.9 | 86.2 ± 10.6 | 90.6 ± 14.2 | |
| Control | 17 | 0 (0.0) | 3 (17.6) | 78.3 ± 8.2 | 78.0 ± 9.1 | 77.9 ± 15.8 | ||
17/t/Tur (H5N2): A/17/turkey/Turkey/05/133 (H5N2) LAIV strain; NIBRG-23 (H5N1): A/turkey/Turkey/5/05 (H5N1) PR8-based candidate vaccine virus; Ab – antibody;
number of subjects with seroconversion.
AI – avidity index (OD450 with urea / OD450 without urea × 100%).
significantly higher rates in one group compared to another are shown in bold (p < 0.05).
Detection of IgA and IgG ALS
To more accurately evaluate B cell-mediated immune responses to the booster vaccination of LAIV-primed or naïve individuals, we cultured PBMCs from immunized volunteers at all studied time points and examined the supernatants by ELISA. This method allows detection of antibody secreted by activated B-cells without interference with pre-existing cross-reactive serum antibodies. The levels of IgA and IgG ALS specific to A(H5N2) LAIV priming virus and to the influenza A(H5N1) virus contained in the booster vaccine are shown in Figure 3. Regardless of the H5 antigen tested, the peak of IgA antibody secretion by PBMC cultures of LAIV-primed individuals was observed at Day 7 after a single dose of subunit A(H5N1) vaccine, with 42.1% of the subjects having 4-fold or greater increases in antibody titers compared to baseline levels (Fig. 2). By Day 28, the IgA ALS titers significantly decreased and were not boosted by an additional dose of the A(H5N1) IIV (Day 56). Naïve individuals did not have a significant increase in the overall IgA ALS titers at any time point and only 3 out of 24 subjects seroconverted in this assay.
Figure 3.
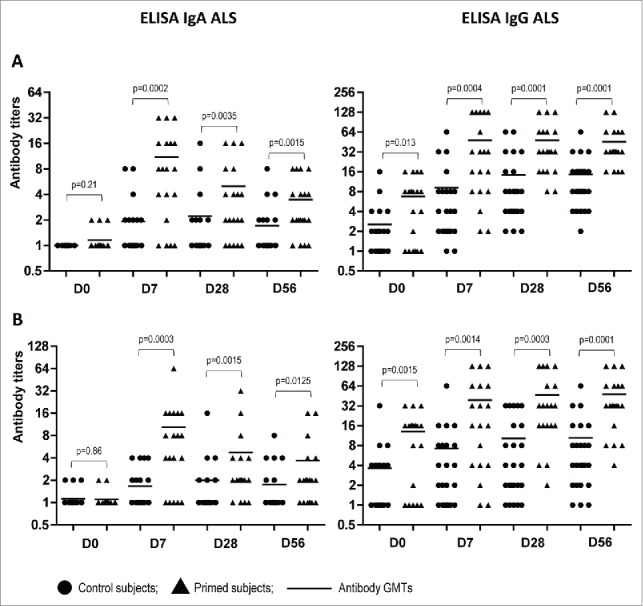
Antibody titers in supernatants of PBMC cultures (ALS) in LAIV-primed (n = 19) and control (n = 24) volunteers at indicated study days after boost immunization with A(H5N1) IIV. (A) NIBRG-23 (H5N1) virus was used as antigen. (B) A/17/turkey/Turkey/05/133 (H5N2) virus was used as antigen. lines– geometric mean titers of antibodies. P values were calculated from log2-transformed titers using GraphPad Prism 5 software by Mann Whitney U test.
Surprisingly, a different pattern was observed while testing the dynamics of IgG secretion by PBMC cultures of vaccinated volunteers from both the LAIV-primed and naïve groups. The IgG titers increased over time for both treatment groups with over 80% seroconversion rates, although the GMTs were 3 to 5 times higher in the primed group as compared to the control group (Fig. 3). These data suggest prolonged circulation in peripheral blood of virus-specific IgG antibody secreting cells (ASC) after vaccination with the adjuvanted A(H5N1) IIV used in our study. Notably, the baseline levels of IgG ALS were significantly higher in the group of volunteers primed with A(H5N2) LAIV as compared to the naïve group, which was in concordance with the data obtained for serum MN and IgG antibody titers at Day 0 of the study (Fig. 2).
Induction of IFN–γ producing CD8+ memory T–cells
We also studied the T-cell immune response to A(H5N1) IIV, which included detecting virus-specific CD8+ T-lymphocytes of central (Tcm) and effector (Tem) immunological memory in peripheral blood. Table 5 presents data on post-vaccination significant increases of CD8+ Tcm and CD8+ Tem at all of the time points studied. In both groups, a relatively low level of such cells was revealed (8 to 17%) and differences between them were considered insignificant.
Table 5.
CD8+ T-cell immune response in A(H5N2) LAIV-primed and control subjects immunized with A(H5N1) IIV at indicated time points
| N (%) of subjects with significant increase of T-cells* |
|||||||
|---|---|---|---|---|---|---|---|
| CD8+Tcm |
CD8+Tem |
||||||
| Group | Number persons | Day 7 | Day 28 | Day 56 | Day 7 | Day 28 | Day 56 |
| Primed | 19 | 3 (16) | 3 (16) | 3 (16) | 2 (10) | 3 (16) | 2 (10) |
| Control | 24 | 2 (8) | 4 (17) | 4 (17) | 3 (12) | 4 (17) | 4 (17) |
≥20 fold change compared to a baseline level (Day 0).
Altogether, our study demonstrated that priming with A(H5N2) LAIV induced a long-lasting B-cell immunological memory in humans against influenza A(H5N1) virus, which was brought on by more prompt and vigorous antibody production to a single dose of A(H5N1) IIV given 1.5 years later.
Discussion
This study was designed to evaluate the ability of 2 doses of A(H5N2) LAIV to prime for prompt and vigorous antibody immune response to a single dose of suboptimal dose of A(H5N1) IIV given 1.5 years later. In addition, a possible effect of the second IIV dose was evaluated. Analysis of serum antibody responses after boosting showed striking differences between the LAIV-primed and the control study groups. Thus, a single dose of A(H5N1) IIV induced robust immune responses in LAIV-primed subjects as early as 7 days after immunization, whereas 2 doses of A(H5N1) IIV were required for naïve volunteers to develop significant immune responses. This difference was more notable when analyzing serum antibody GMTs than seroconversion rates. The majority of the primed volunteers developed seroprotective levels of MN antibody (≥1:40) by Day 7 after IIV administration and by Day 28 all of the subjects had achieved such titers. In addition, the serum antibodies found in the previously primed subjects were characterized by higher avidity indexes than that of the naïve subjects. This was in concordance with a recent study by Talaat et al., which demonstrated that 2 doses of A(H5N1) pandemic LAIV, even in the absence of a primary antibody response, induced a long-lasting immune response, which was unmasked by administration of one dose of subvirion IIV 5 years later.23 These findings support a novel vaccine schedule that would apply different types of vaccines in prime-boost combinations to adequately prepare for the pandemic.5,23
The time interval of 1.5 years can be considered as one possible scenario when population is immunized with fully characterized and clinically tested LAIV candidate once a pandemic is suspected, and then boosted with relevant pandemic vaccine, either live or inactivated, 1-1.5 years later during the second pandemic wave. However, additional studies of the shorter time intervals between prime and boost are required to find the most suitable schedule to protect as many people during the first pandemic wave as possible.
In addition to LAIVs, H5 subtype oil-in-water adjuvanted IIVs have been shown to prime immune system for a prompt, robust and long-lasting antibody immune response to a single dose of IIV given up to 6 years later.7,32 Therefore, the availability of different vaccine modalities with demonstrated good priming effect will allow better vaccination coverage during pandemic, and will largely determine the outcome of the first pandemic waves. Nevertheless, since LAIVs are cheaper and quicker to make and easier to administer (by intranasal spray) than IIV, this vaccine modality might be a preferential platform to use in an emergency situation, such as the first wave of a pandemic. Subsequently, the immune memory response can be boosted with a low dose of IIV prepared from a matched pandemic strain, which will be more cost-effective than the use of 2-dose IIV vaccination regimen recommended so far. Importantly, here in our study, the use of LAIV alone or in combination with IIV was proved to be safe, with no serious adverse events attributable to vaccination recorded during the studies.
Cross-reactivity of the induced HAI and MN antibodies in our study was assessed against clade 0 (d/Pot (H5N2)) and clade 2.1 (Indo (H5N1)) influenza viruses. In contrast to the study by Talaat et al.,23 the HAI and MN antibodies detected in the control group cross-reacted well with the 2 heterologous viruses. This could be due to the better immunogenicity of the adjuvanted A(H5N1) inactivated vaccine used in our study,34 which is supported by the greater percentage of seroconversions detected after 1 and 2 doses of the OrniFlu®, compared to the unadjuvanted subvirion influenza A(H5N1) vaccine used by Talaat et al..23 However the GMTs, seroconversion and seroprotection rates were significantly lower in the control group compared to the LAIV-primed cohort. Altogether, the data presented in this study evidence that A(H5N2) LAIV induced good quality B-cell memory immune responses, which resulted in fast, strong, and cross-reactive antibody immune responses to a single dose of IIV administered 1.5 years later. Importantly, the OrniFlu® vaccine consisted of only 15 µg of influenza A(H5N1) virus HA, in contrast with the 45 µg of subvirion influenza vaccine studied by Talaat et al.23 Surprisingly, administration of the second IIV dose to the LAIV-primed individuals had no benefit over the initial dose, suggesting that a single dose of a booster vaccine may be enough to provide protection on LAIV-primed individuals in the event of a pandemic.
A recent report in which the immune response to a trivalent IIV was examined on a subset of B-cells isolated from culture PBMC suggested that B-cell antibody secretion in vitro (referred to as plasmablast-derived polyclonal antibody or PPAb,) better represents the vaccine-induced B cell repertoire than serum antibodies which are primarily produced by bone marrow B cells, in part due to the exclusion of interfering effect from pre-existing antibodies.30 We performed IgA and IgG ELISA in supernatants of cultured PBMCs (ALS assay) in a format that has been successfully used to study IgA responses after immunization and natural infection with bacterial pathogens.35,36 The results for IgA ALS in A(H5N2) LAIV-primed subjects boosted with A(H5N1) IIV exhibited similar dynamic changes to the IgA PPAb in the volunteers immunized with seasonal IIV noted above. The antibody peaked on Day 7 after IIV immunization and then significantly decreased by Day 28.30 In contrast, IgG ALS titers in both LAIV-primed and naïve groups increased over time, suggesting the continuous circulation of influenza A(H5N1)-specific antibody secreting B cells after administration of A(H5N1) IIV. Several reasons may explain the difference in the IgG response between our study and the report by He et al,30 1) the use of adjuvant, 2) we tested for influenza H5 virus-specific IgG antibody in PBMC supernatants instead of total IgG, 3) we assayed cultures of unfractionated PBMCs, whereas He et al. used PPAbs collected from ex vivo B-cell cultures, and 4) the PPAb response studies were performed on recipients of seasonal influenza vaccines, and therefore most of them must have been previously primed either by infection or vaccination.)37-39 Although we did not measure ALS responses in our initial A(H5N2) LAIV study and made no comparisons between influenza H5 subtype LAIV and IIV, a recent study of the induction of PPAbs by seasonal trivalent LAIV and IIV suggested that IIV was a better inducer of the PPAb.39
We also evaluated the T-cell immune response after boosting primed and naïve individuals, including the detection of virus-specific CD8+ T-lymphocytes of central (Tcm) and effector (Tem) memory in PBMCs. At Day 0, levels of T-lymphocyte CD8+ Tcm and CD8+ Tem specific to influenza A(H5N1) virus appeared to be higher in prior recipients of A(H5N2) LAIV as compared to naïve subjects (data not shown), however, prior priming did not increase the number of significant conversions of T-cell memory CD8+ in response to A(H5N1) IIV. This phenomenon can be explained in at least 2 ways. Either IIV might be a poor inducer of T-cell memory or this process was influenced by higher baseline levels. Analysis showed that baseline T-cell concentrations (%) and the strength of the post-vaccination T-cell immune response revealed a strong negative correlation (−0.71 to −0.84 Spearmen coefficient, data not shown), which was in concordance with our previous studies.15,40 Altogether, the analysis did not reveal any significant impact of previous exposure to LAIV on IIV-immunized subjects on T-cell memory, although the initial immunization with A(H5N2) LAIV did induce this cell-mediated immunity.25
Conclusion
In summary, our study demonstrates that administration of A(H5N2) LAIV primes the immune system and leads to a crisp and strong immune memory response after A(H5N1) IIV boost given ∼1.5 years later. This finding is of special significance since the LAIV technology developed at the Institute of Experimental Medicine in St Petersburg has been licensed to WHO, through whom the vaccine has been provided to vaccine manufacturers in India, China and Thailand — countries particularly vulnerable to a pandemic influenza. The results of our study will be useful to inform the development of vaccination strategies in these countries in the event of a pandemic. Thus, a well-characterized pandemic LAIV candidate can be urgently produced at the beginning of a pandemic to prime population before exact strain-matched vaccine formulations can be produced. In addition, our data on the establishment of immune responses to LAIV, which can be unmasked by subsequent administration of IIV, provide additional information on immune correlates to be potentially used for licensing new LAIVs. Furthermore, these data are likely to be reproduced to the pandemic LAIVs of other subtypes, such as influenza A(H7N3), A(H2N2), and A(H7N9). Finally, it will be of great interest to evaluate the prime-boost responses to seasonal vaccines and to examine how short an interval between prime and boost may be sufficient.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to Tatiana Smolonogina from the Institute of Experimental Medicine and Yuxiao Tang from PATH for statistical analyses of the data; to Oleg Kiselev from the Research Institute of Influenza for the help in organizing this clinical trial; to Vera Krivitskaya from the Research Institute of Influenza for conducting microneutralization assays; to Kathy Neuzil and Kristin Bedell from PATH for their collaboration on this clinical trial; and to the volunteers that participated in the clinical trial. We are also thankful to Microgen for producing the vaccine lot for the clinical trial and to PSI for their competent support in data management, statistical analysis, and other operational aspects of the study.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.WHO, WHO global influenza preparedness plan WHO/CDS/CSR/GIP/2005.5 http://www.who.int/csr/resources/publications/influenza/en/WHO_CDS_CSR_GIP_2005_5.pdf. 2005 [DOI] [PubMed] [Google Scholar]
- 2.Anonymous, Development of a Clinical Trial Plan for Pandemic Influenza Vaccines Department of Health and Human Services. National Institute of Allergy and Infection Diseases; September 22–23, 2003, Bethesda, Maryland Meeting Summary. http://www.niaid.nih.gov/about/organization/dmid/Documents/pansummary.pdf.2003 [Google Scholar]
- 3.WHO, Tables on clinical evaluation of influenza vaccines. http://www.who.int/immunization/diseases/influenza/clinical_evaluation_tables/en/. WHO, Geneva, Switzerland: 2014. [Google Scholar]
- 4.Rudenko L, Isakova-Sivak I. Pandemic Preparedness with Live Attenuated Influenza Vaccines Based on A/Leningrad/134/17/57 (H2N2) Master Donor Virus. Expert Review of Vaccines, 2015. 14(03): p:395-412; PMID:25555687; http://dx.doi.org/ 10.1586/14760584.2015.979159 [DOI] [PubMed] [Google Scholar]
- 5.Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines 2014. 13(7): p:855-71; PMID:24867587; http://dx.doi.org/ 10.1586/14760584.2014.922417 [DOI] [PubMed] [Google Scholar]
- 6.Isakova-Sivak I, Stukova M, Erofeeva M, Naykhin A, Donina S, Petukhova G, Kuznetsova V, Kiseleva I, Smolonogina T, Dubrovina I, et al.. H2N2 live attenuated influenza vaccine is safe and immunogenic for healthy adult volunteers. Hum Vaccin Immunother, 2015. 11(4): p:970-82; PMID:25831405; http://dx.doi.org/ 10.1080/21645515.2015.1010859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res, 2013; 178(1):78-98; PMID:23726847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, Rowe T, Treanor JJ. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis, 2008. 198(5): p:635-41; PMID:18694338; http://dx.doi.org/ 10.1086/590916 [DOI] [PubMed] [Google Scholar]
- 9.Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, Zambon M. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 2003. 21(15): p:1687-93; PMID:12639491; http://dx.doi.org/ 10.1016/S0264-410X(02)00632-1 [DOI] [PubMed] [Google Scholar]
- 10.Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, et al.. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis, 2011. 11(12): p:916-24; PMID:21975270; http://dx.doi.org/ 10.1016/S1473-3099(11)70240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledgerwoo JE, Zephir K, Hu Z, Wei CJ, Chang L, Enama ME, Hendel CS, Sitar S, Bailer RT, Koup RA, et al.. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis 2013. 208(3): p:418-22; PMID:23633407; http://dx.doi.org/ 10.1093/infdis/jit180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO, Global pandemic influenza action plan to increase vaccine supply. Geneva, Belgium. WHO/IVB/06.13. WHO/ODS/EPR/GIP/2006.1 http://whqlibdoc.who.int/hq/2006/WHO_IVB_06.13_eng.pdf, 2006 [Google Scholar]
- 13.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, Swain SL, Dutton RW. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol 2007. 178(2): p:1030-8; PMID:17202366; http://dx.doi.org/ 10.4049/jimmunol.178.2.1030 [DOI] [PubMed] [Google Scholar]
- 14.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis, 2005. 58(4): p:195-207; PMID:16116250 [PubMed] [Google Scholar]
- 15.Chirkova TV, Naykhin AN, Petukhova GD, Korenkov DA, Donina SA, Mironov AN, Rudenko LG. Memory T-cell immune response in healthy young adults vaccinated with live attenuated influenza A (H5N2) vaccine. Clin Vaccine Immunol 2011. 18(10): p:1710-8; PMID:21813657; http://dx.doi.org/ 10.1128/CVI.05116-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 2006. 80(23): p:11756-66; PMID:16971435; http://dx.doi.org/ 10.1128/JVI.01460-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, et al.. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 2011. 204(6): p:845-53; PMID:21846636; http://dx.doi.org/ 10.1093/infdis/jir436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudenko L, van den Bosch H, Kiseleva I, Mironov A, Naikhin A, Larionova N, Bushmenkov D. Live attenuated pandemic influenza vaccine: clinical studies on A/17/California/2009/38 (H1N1) and licensing of the Russian-developed technology to WHO for pandemic influenza preparedness in developing countries. Vaccine, 2011. 29 Suppl 1: p:A40-4; PMID:21684428; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.122 [DOI] [PubMed] [Google Scholar]
- 19.Rudenk LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, Rekstin AR, Beljaev AL, Bragina VE, Cox N, et al.. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis 1993. 168(4): p:881-7; PMID:8376833; http://dx.doi.org/ 10.1093/infdis/168.4.881 [DOI] [PubMed] [Google Scholar]
- 20.Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, Isakova-Sivak I, Chen LM, Voeten JT, Heldens JG, et al.. Comparative immunogenicity and cross-clade protective efficacy of mammalian cell-grown inactivated and live attenuated H5N1 reassortant vaccines in ferrets. J Infect Dis 2011. 204(10): p:1491-9; PMID:21957153; http://dx.doi.org/ 10.1093/infdis/jir596 [DOI] [PubMed] [Google Scholar]
- 21.Barria MI, Garrido JL, Stein C, Scher E, Ge Y, Engel SM, Kraus TA, Banach D, Moran TM. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J Infect Dis 2013. 207(1): p:115-24; PMID:23087433; http://dx.doi.org/ 10.1093/infdis/jis641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petukhova G, Naikhin A, Chirkova T, Donina S, Korenkov D, Rudenko L. Comparative studies of local antibody and cellular immune responses to influenza infection and vaccination with live attenuated reassortant influenza vaccine (LAIV) utilizing a mouse nasal-associated lymphoid tissue (NALT) separation method. Vaccine, 2009. 27(19): p:2580-7; PMID:19428864; http://dx.doi.org/ 10.1016/j.vaccine.2009.02.035 [DOI] [PubMed] [Google Scholar]
- 23.Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KD, Qin J, Follmann DA, et al.. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 2014. 209(12): p:1860-9; PMID:24604819; http://dx.doi.org/ 10.1093/infdis/jiu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babu TM, Levine M, Fitzgerald T, Luke C, Sangster MY, Jin H, Topham D, Katz J, Treanor J, Subbarao K. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine 2014. 32(50): p:6798-804; PMID:25446831; http://dx.doi.org/ 10.1016/j.vaccine.2014.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudenko L, Bloom CE, Kirchenbaum GA, Tsvetnitsky V, Isakova-Sivak I, Rudenko L, Ross TM. Clinical Testing of Pre–pandemic Live Attenuated A/H5N2 Influenza Candidate Vaccine in Adult Volunteers: Results from a Placebo–controlled, Randomized Double–Blind Phase I Study. [DOI] [PubMed] [Google Scholar]
- 26.WHO, WHO Manual on animal influenza diagnosis and surveillance Available from: http://www.bvsde.paho.org/bvsacd/cd52/animal.pdf accessed 12August2013 2002 [Google Scholar]
- 27.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999. 37(4): p:937-43; PMID:10074505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudenko L, Kiseleva I, Naykhin AN, Erofeeva M, Stukova M, Donina S, Petukhova G, Pisareva M, Krivitskaya V, Grudinin M, et al.. Assessment of Human Immune Responses to H7 Avian Influenza Virus of Pandemic Potential: Results from a Placebo–Controlled, Randomized Double–Blind Phase I Study of Live Attenuated H7N3 Influenza Vaccine. PLoS One 2014. 9(2): p:e87962; PMID:24533064; http://dx.doi.org/ 10.1371/journal.pone.0087962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazzarotto T, Spezzacatena P, Pradelli P, Abate DA, Varani S, Landini MP. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin Diagn Lab Immunol 1997. 4(4): p:469-73; PMID:9220166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He XS, Sasaki S, Narvaez CF, Zhang C, Liu H, Woo JC, Kemble GW, Dekker CL, Davis MM, Greenberg HB. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods 2011. 365(1-2): p:67-75; PMID:21182843; http://dx.doi.org/ 10.1016/j.jim.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009. 41(4): p:1149-60; PMID:19897823; http://dx.doi.org/ 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 32.Stephenson I, Nicholson KG, Hoschler K, Zambon MC, Hancock K, DeVos J, Katz JM, Praus M, Banzhoff A. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N Engl J Med 2008. 359(15): p:1631-3; PMID:18843132; http://dx.doi.org/ 10.1056/NEJMc0805274 [DOI] [PubMed] [Google Scholar]
- 33.Rudenko LG, Arden NH, Grigorieva E, Naychin A, Rekstin A, Klimov AI, Donina S, Desheva J, Holman RC, DeGuzman A, et al.. Immunogenicity and efficacy of Russian live attenuated and US inactivated influenza vaccines used alone and in combination in nursing home residents. Vaccine 2000. 19(2-3): p:308-18; PMID:10930686; http://dx.doi.org/ 10.1016/S0264-410X(00)00153-5 [DOI] [PubMed] [Google Scholar]
- 34.Zverev VV, Katlinskiĭ AV, Kostinov MP, Zhirova SN, Erofeeva MK, Stukova MA, Korovkin SA, Mel'nikov SIa, Semchenko AV, Mironov AN. [Comparative clinical trial of vaccines against avian influenza]. Zh Mikrobiol Epidemiol Immunobiol 2007(3): p:10-6; PMID:17672124 [PubMed] [Google Scholar]
- 35.Chang HS, Sack DA, Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin Diagn Lab Immunol 2001. 8(3): p:482-8; PMID:11329444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rekha RS, Kamal SM, Andersen P, Rahim Z, Hoq MI, Ara G, Andersson J, Sack D, Raqib R. Validation of the ALS assay in adult patients with culture confirmed pulmonary tuberculosis. PLoS One 2011. 6(1): p:e16425; PMID:21283655; http://dx.doi.org/ 10.1371/journal.pone.0016425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol 2007; 81(1): p:215-28; PMID:17050593; http://dx.doi.org/ 10.1128/JVI.01957-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 2008. 3(8): p:e2975; PMID:18714352; http://dx.doi.org/ 10.1371/journal.pone.0002975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki S, Holmes TH, Albrecht RA, García-Sastre A, Dekker CL, He XS, Greenberg HB. Distinct cross-reactive B-cell responses to live attenuated and inactivated influenza vaccines. J Infect Dis 2014. 210(6): p:865-74; PMID:24676204; http://dx.doi.org/ 10.1093/infdis/jiu190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naikhin AN, Chirkova TV, Petukhova GD, Koren'kov DA, Donina SA, Rudenko LG. [Stimulation of homo- and heterologic T-cell immunological memory in volunteers inoculated with live influenza A (H5N2) reassortant vaccine]. Vopr Virusol 2012; 57(1): p:38-42; PMID:22624472 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.