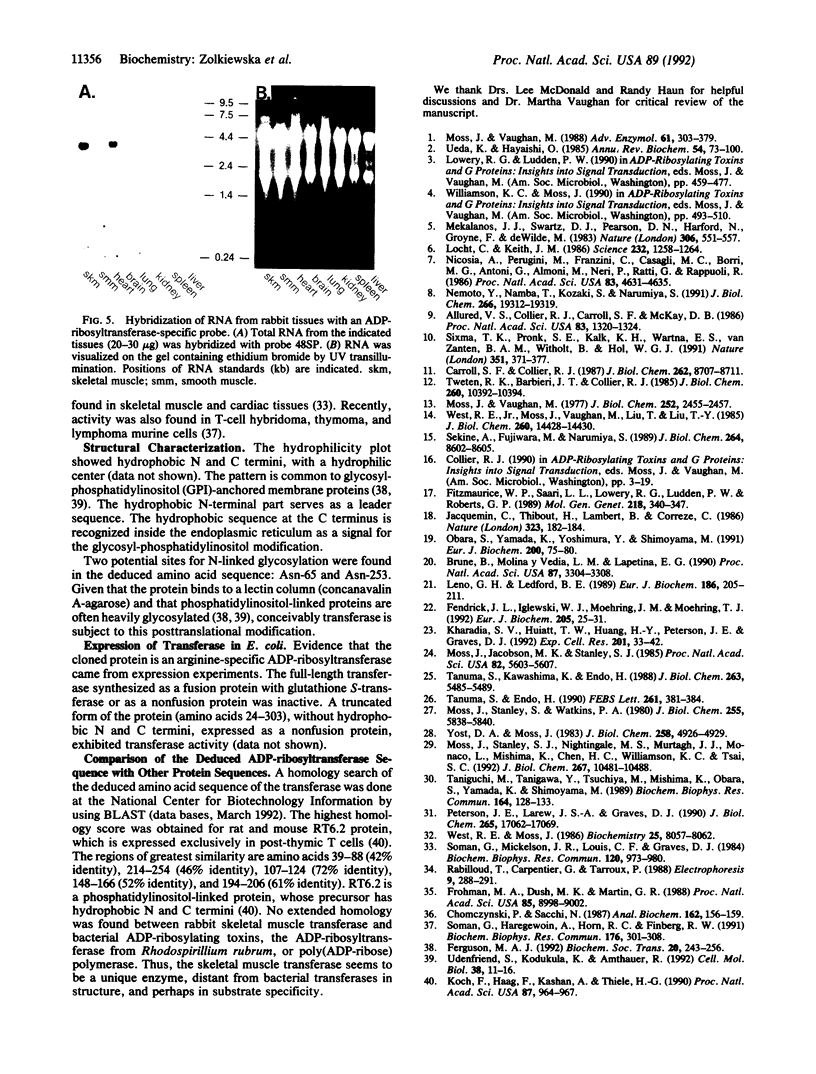
Abstract
Mono-ADP-ribosylation is a reversible modification of proteins, with NAD:arginine ADP-ribosyltransferases (EC 2.4.2.31) and ADP-ribosylarginine hydrolases (EC 3.2.2.19) catalyzing the opposing reactions in an ADP-ribosylation cycle. A membrane-associated arginine-specific (mono)-ADP-ribosyltransferase was purified 215,000-fold from rabbit skeletal muscle. On the basis of the amino acid sequences of HPLC-purified tryptic peptides, degenerate oligonucleotide primers were synthesized and used in a polymerase chain reaction (PCR)-based procedure to generate cDNA. A specific probe, based on PCR-generated sequence, was used to screen a rabbit skeletal muscle cDNA library. A composite cDNA sequence, obtained from library screening and rapid amplification of the 5' end of the cDNA, contained a 981-base-pair open reading frame, encoding a 36,134-Da protein. The deduced amino acid sequence contained the sequences of the tryptic peptides, hydrophobic amino and carboxyl termini, and two potential sites for N-linked glycosylation. Escherichia coli cells transformed with an expression vector containing transferase-specific sequence expressed ADP-ribosyltransferase activity. A transferase-specific oligonucleotide probe recognized a 4-kilobase mRNA expressed primarily in rabbit skeletal and cardiac muscle. There was no extended similarity in deduced amino acid sequences of the muscle transferase and several bacterial ADP-ribosylating toxins. The hydrophobic amino and carboxyl termini may represent a signal peptide and a site for a glycosyl-phosphatidylinositol anchor, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Molina y Vedia L., Lapetina E. G. Agonist-induced ADP-ribosylation of a cytosolic protein in human platelets. Proc Natl Acad Sci U S A. 1990 May;87(9):3304–3308. doi: 10.1073/pnas.87.9.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J Biol Chem. 1987 Jun 25;262(18):8707–8711. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fendrick J. L., Iglewski W. J., Moehring J. M., Moehring T. J. Characterization of the endogenous ADP-ribosylation of wild-type and mutant elongation factor 2 in eukaryotic cells. Eur J Biochem. 1992 Apr 1;205(1):25–31. doi: 10.1111/j.1432-1033.1992.tb16748.x. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A. Colworth Medal Lecture. Glycosyl-phosphatidylinositol membrane anchors: the tale of a tail. Biochem Soc Trans. 1992 May;20(2):243–256. doi: 10.1042/bst0200243. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice W. P., Saari L. L., Lowery R. G., Ludden P. W., Roberts G. P. Genes coding for the reversible ADP-ribosylation system of dinitrogenase reductase from Rhodospirillum rubrum. Mol Gen Genet. 1989 Aug;218(2):340–347. doi: 10.1007/BF00331287. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin C., Thibout H., Lambert B., Correze C. Endogenous ADP-ribosylation of Gs subunit and autonomous regulation of adenylate cyclase. Nature. 1986 Sep 11;323(6084):182–184. doi: 10.1038/323182a0. [DOI] [PubMed] [Google Scholar]
- Kharadia S. V., Huiatt T. W., Huang H. Y., Peterson J. E., Graves D. J. Effect of an arginine-specific ADP-ribosyltransferase inhibitor on differentiation of embryonic chick skeletal muscle cells in culture. Exp Cell Res. 1992 Jul;201(1):33–42. doi: 10.1016/0014-4827(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Koch F., Haag F., Kashan A., Thiele H. G. Primary structure of rat RT6.2, a nonglycosylated phosphatidylinositol-linked surface marker of postthymic T cells. Proc Natl Acad Sci U S A. 1990 Feb;87(3):964–967. doi: 10.1073/pnas.87.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno G. H., Ledford B. E. ADP-ribosylation of the 78-kDa glucose-regulated protein during nutritional stress. Eur J Biochem. 1989 Dec 8;186(1-2):205–211. doi: 10.1111/j.1432-1033.1989.tb15196.x. [DOI] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Jacobson M. K., Stanley S. J. Reversibility of arginine-specific mono(ADP-ribosyl)ation: identification in erythrocytes of an ADP-ribose-L-arginine cleavage enzyme. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5603–5607. doi: 10.1073/pnas.82.17.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Nightingale M. S., Murtagh J. J., Jr, Monaco L., Mishima K., Chen H. C., Williamson K. C., Tsai S. C. Molecular and immunological characterization of ADP-ribosylarginine hydrolases. J Biol Chem. 1992 May 25;267(15):10481–10488. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A. Isolation and properties of an NAD- and guanidine-dependent ADP-ribosyltransferase from turkey erythrocytes. J Biol Chem. 1980 Jun 25;255(12):5838–5840. [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Nemoto Y., Namba T., Kozaki S., Narumiya S. Clostridium botulinum C3 ADP-ribosyltransferase gene. Cloning, sequencing, and expression of a functional protein in Escherichia coli. J Biol Chem. 1991 Oct 15;266(29):19312–19319. [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara S., Yamada K., Yoshimura Y., Shimoyama M. Evidence for the endogenous GTP-dependent ADP-ribosylation of the alpha-subunit of the stimulatory guanyl-nucleotide-binding protein concomitant with an increase in basal adenylyl cyclase activity in chicken spleen cell membrane. Eur J Biochem. 1991 Aug 15;200(1):75–80. doi: 10.1111/j.1432-1033.1991.tb21050.x. [DOI] [PubMed] [Google Scholar]
- Peterson J. E., Larew J. S., Graves D. J. Purification and partial characterization of arginine-specific ADP-ribosyltransferase from skeletal muscle microsomal membranes. J Biol Chem. 1990 Oct 5;265(28):17062–17069. [PubMed] [Google Scholar]
- Rabilloud T., Carpentier G., Tarroux P. Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis. 1988 Jun;9(6):288–291. doi: 10.1002/elps.1150090608. [DOI] [PubMed] [Google Scholar]
- Sekine A., Fujiwara M., Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989 May 25;264(15):8602–8605. [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Soman G., Haregewoin A., Hom R. C., Finberg R. W. Guanidine group specific ADP-ribosyltransferase in murine cells. Biochem Biophys Res Commun. 1991 Apr 15;176(1):301–308. doi: 10.1016/0006-291x(91)90924-v. [DOI] [PubMed] [Google Scholar]
- Soman G., Mickelson J. R., Louis C. F., Graves D. J. NAD: guanidino group specific mono ADP-ribosyltransferase activity in skeletal muscle. Biochem Biophys Res Commun. 1984 May 16;120(3):973–980. doi: 10.1016/s0006-291x(84)80202-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Tanigawa Y., Tsuchiya M., Mishima K., Obara S., Yamada K., Shimoyama M. Arginine-specific ADP-ribosyltransferase from rabbit skeletal muscle sarcoplasmic reticulum is solubilized as the active form with trypsin: partial purification and characterization. Biochem Biophys Res Commun. 1989 Oct 16;164(1):128–133. doi: 10.1016/0006-291x(89)91692-6. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Endo H. Identification in human erythrocytes of mono(ADP-ribosyl) protein hydrolase that cleaves a mono(ADP-ribosyl) Gi linkage. FEBS Lett. 1990 Feb 26;261(2):381–384. doi: 10.1016/0014-5793(90)80597-c. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kawashima K., Endo H. Eukaryotic mono(ADP-ribosyl)transferase that ADP-ribosylates GTP-binding regulatory Gi protein. J Biol Chem. 1988 Apr 15;263(11):5485–5489. [PubMed] [Google Scholar]
- Tweten R. K., Barbieri J. T., Collier R. J. Diphtheria toxin. Effect of substituting aspartic acid for glutamic acid 148 on ADP-ribosyltransferase activity. J Biol Chem. 1985 Sep 5;260(19):10392–10394. [PubMed] [Google Scholar]
- Udenfriend S., Kodukula K., Amthauer R. Cell-free processing of nascent proteins destined to be linked to the plasma membrane by a phosphatidylinositol-glycan anchor. Cell Mol Biol. 1992 Feb;38(1):11–16. [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J. Amino acid specific ADP-ribosylation: specific NAD: arginine mono-ADP-ribosyltransferases associated with turkey erythrocyte nuclei and plasma membranes. Biochemistry. 1986 Dec 2;25(24):8057–8062. doi: 10.1021/bi00372a039. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Yost D. A., Moss J. Amino acid-specific ADP-ribosylation. Evidence for two distinct NAD:arginine ADP-ribosyltransferases in turkey erythrocytes. J Biol Chem. 1983 Apr 25;258(8):4926–4929. [PubMed] [Google Scholar]