Abstract
Integrins comprise a large family of αβ heterodimeric cell adhesion receptors that are expressed on all cells except red blood cells and that play essential roles in the regulation of cell growth and function. The leukocyte integrins, which include members of the β 1, β 2, β 3, and β 7 integrin family, are critical for innate and adaptive immune responses but also can contribute to many inflammatory and autoimmune diseases when dysregulated. This review focuses on the β 2 integrins, the principal integrins expressed on leukocytes. We review their discovery and role in host defense, the structural basis for their ligand recognition and activation, and their potential as therapeutic targets.
Keywords: inflammation, integrins, leukocytes, integrin structure
Introduction
Leukocytes circulate in the blood in a quiescent state before migrating into tissues to defend against invading pathogens or to participate in other immune functions. Improperly activated leukocytes can also be effectors of pathologic inflammation. Most leukocyte functions are dependent on members of the integrin family ( Figure 1). Leukocyte integrins comprise all four β 2 integrins, the two β 7 integrins α 4β 7 and α Eβ 7, in addition to α 4β 1, α 5β 1, α 9β 1, and α vβ 3. Leukocyte integrins play key roles in the innate immune response, which include interaction of phagocytic cells with endothelium and the extracellular matrix, ingestion of complement-opsonized pathogens, degranulation, and cytokine production. They are also involved in lymphocyte proliferation, survival, and differentiation in adaptive immunity. Chemokines, cytokines, lipid signaling molecules, and “cross-talk” from other adhesion molecules regulate the functional state, density, and topography of leukocyte integrins. The leukocyte-specific β 2 integrins are the most abundant leukocyte integrins and the first integrins to be studied functionally and structurally in these cells. In this review, we will focus on β 2 integrins and their role in immunity and their structure and mechanism of their inside-out signaling. Many elements of the integrin outside-in signaling networks have been identified and were the subject of excellent reviews 1– 4 but are outside the scope of this concise review.
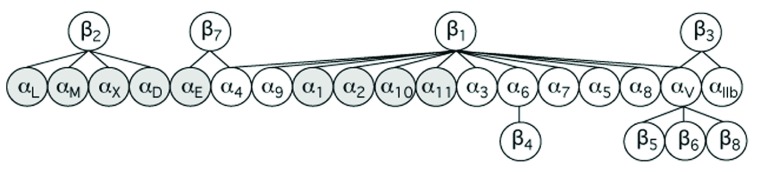
Figure 1. Mammalian integrins.
This protein family consists of 24 α/β heterodimeric receptors assembled from 18 α-subunits and eight β-subunits. Nine α-subunits (shaded) contain an extra von Willebrand factor type A domain (αA or αI). The β 1 integrins are the largest subfamily, with 12 known members.
Discovery of β 2 integrins
The sequential steps leading to an inflammatory response were first documented by Julius Cohnheim in the frog’s tongue 5. He observed that local mechanical irritation induced first an increase in blood flow and then a slowing, at which time white blood cells began to roll and then halt, lining up the wall of venules, whereas red blood cells sped past them. Then some white blood cells began to creep across the wall into the extravascular space 5. Elie Metchnikoff discovered the phagocytic function of certain white blood cells by using the transparent avascular starfish larvae 6. His phagocytosis theory of inflammation complemented Paul Ehrlich’s humoral theory, which attributed bacterial killing to serum-derived “magic bullets”, identified soon after as antibodies and complement proteins. The identity of the molecules involved in leukocyte migration across venules and in phagocytosis remained unknown, however.
In 1979, an experiment of nature led us to the identification of the major surface receptors mediating leukocyte migration and phagocytosis (reviewed in 7). We investigated in a pediatric patient the basis for his life-threatening bacterial infections, impaired wound healing, persistent marked neutrophilia even during infection-free periods, but a paucity of neutrophils within infected tissues. His neutrophils failed to adhere to substrate, migrate across inflamed endothelium, or ingest serum-opsonized particles. We traced these phagocyte defects to a deficiency of a gp150 surface membrane glycoprotein complex 8. Monoclonal antibodies (mAbs) raised by us 9 and by others 10– 15 showed that the gp150 complex comprises four surface glycoproteins now known as CD11a (α L) 16– 18, CD11b (α M) 19, CD11c (α X) 20, and CD11d (α D) 21. Each CD11 glycoprotein non-covalently associates with a common 95 kDa glycoprotein (CD18, β 2) 13– 15, 18 to form what is now known as the β 2 integrin subfamily. Mutations in the CD18 subunit 7, 22– 24 resulted in its partial or complete failure to associate with the synthesized CD11 α-subunits, accounting for the variations in severity of the disease now known as leukocyte adhesion deficiency type I (LAD I) 18, 25.
Tissue distribution of β 2 integrins
β 2 integrins are expressed only on leukocytes, but their expression varies among the leukocyte subpopulations. CD11a is expressed on all leukocytes but predominates on lymphocytes. CD11b predominates on myeloid cells, being the most abundant integrin on neutrophils, and is also expressed on natural killer (NK) cells, fibrocytes, and some mast cells, B cells, CD8 + T cells, and γδ T cells 26– 33. CD11c is most abundant on myeloid dendritic cells, predominating on macrophages and dendritic cells of the splenic white pulp and marginal zone and on pulmonary alveolar macrophages, and has a distribution similar to that of CD11b on NK, B, and T cells 34. CD11d is basally expressed on the majority of circulating human neutrophils and monocytes, on NK cells, and on a small fraction of circulating T cells 35, 36. In mice, CD11d expression is restricted to a small percentage of circulating leukocytes under basal conditions but predominates in splenic red pulp macrophages, lymph node medullary cord and sinus macrophages, and hemosiderin-containing bone marrow macrophages and is upregulated on phagocytes at local inflammatory sites 35– 37 and on differentiated macrophages, which may facilitate their retention at sites of inflammation 38.
β 2 integrin ligands
CD11a binds intercellular adhesion molecules (ICAMs) 1–5, telencephalin, endothelial cell-specific molecule-1 (ESM-1), and junctional adhesion molecule 1 (JAM1) 39– 41. CD11b is the most promiscuous β 2 integrin; it has more than 40 reported ligands, including iC3b, ICAM1, 2, 3 and 4, fibrin(ogen), fibronectin, Factor X, Platelet Ibα, JAM-3, and some proteases (for example, proteinase 3) CD11c binds ICAM1, 4, iC3b, and vascular cell adhesion protein 1 (VCAM-1) 42– 46. Like CD11b, CD11c also binds heparin, various polysaccharides, and negative charges in denatured proteins 26, 47– 49. CD11d binds ICAM-3 and VCAM-1 50 and, like CD11b, also binds several matrix proteins 38.
Functional analysis of the individual β 2 integrins
The defects in leukocyte adhesion demonstrated in patients with LAD I and in mice lacking CD18 51 did not allow an assessment of the relative contribution of each of the four β 2 integrins to the phenotypic abnormalities observed. Generation of mice deficient in the individual CD11 subunits revealed that knockout (KO) of CD11a (but not CD11b) in mice caused neutrophilia, which was not as severe as that found in CD18 KO mice, suggesting additional contributions by the other β 2 integrins. No CD11a−, CD11b−, or CD11d KO mice developed the spontaneous infections observed in CD18 KO mice, suggesting that loss of all CD11/CD18 receptors is necessary to cause spontaneous bacterial infections. Homotypic aggregation and antigen-, mitogen-, and alloantigen-induced lymphoproliferation, which lead to defective host-versus-graft reaction and impaired tumor rejection, were reduced in CD11a −/− but not CD11b −/− or CD11c −/− leukocytes 52, 53. However, cytotoxic T-cell responses to systemic viral infections were normal in CD11a KO mice 54, 55, suggesting molecular redundancy or compensatory changes (or both) by other leukocyte integrins such as α4β1 or α9β1 56, 57. This may explain the rarity of viral infections in patients with LAD I. Defective T-cell proliferation in response to the staphylococcal enterotoxin superantigen was more severe in splenocytes from CD18−, CD11b−, or CD11d KO mice than in CD11a −/− splenocytes but was normal in CD11c −/− splenocytes 58. The defects in CD11b −/− or CD11d −/− lymphocytes have been traced to transient expression of CD11b and CD11d on thymocytes, which appears to be required for normal T-cell development 58.
CD11a–d contributed in variable degrees to the adhesion of phagocytes to inflamed endothelium 21, 42, 59, 60. Transendothelial neutrophil migration in the tumor necrosis factor-induced air pouch inflammation model was reduced in CD11a KO 61, as in CD18 KO, but was surprisingly increased in CD11b KO mice 60. Migration within interstitial matrices was integrin independent 62, 63. Phagocytosis of serum-opsonized particles (with its associated oxygen free radical production, cytokine release, and degranulation) and phagocytosis-induced apoptosis in neutrophils were defective in CD11b −/− null mouse cells 64, confirming an essential role for CD11b in the programmed elimination of neutrophils that have already phagocytosed their target pathogens. Toll receptor-mediated responses were enhanced in CD11b −/− macrophages, rendering mice more susceptible to sepsis and endotoxin shock 65. Thus, whereas neutrophil adhesion to endothelium may require all four β 2 integrins, transendothelial migration appears to be mainly CD11a dependent, while phagocytosis is mediated primarily by CD11b 66. Curiously, CD11b KO mice are obese 67, a phenotype not seen in patients with LAD I, suggesting a role for CD11b in regulating fat metabolism at least in mice. The number of mast cells in the peritoneal cavity is also reduced in CD11b KO mice 27, suggesting an additional role in mast cell development. Mast cells play an important role in the early peritoneal neutrophil response during experimental peritonitis in mice and this may explain the increased mortality of CD11b KO mice after acute septic peritonitis 27.
Integrin structure
The αA domain
Structural studies of integrins began with the identification of a novel metal-ion-dependent adhesion site (MIDAS) in an extracellular von Willebrand factor type A (vWFA) domain (αA or αI domain) present in integrin CD11b 68. The vWFA domain is found in eight additional integrin α-subunits ( Figure 1) as well as in several structurally unrelated proteins 69, 70. αA from CD11b (CD11bA) mediates Mg 2+-dependent binding of the receptor to ligands 68, 71. αA also mediates ligand binding in the other αA-containing integrins. The first crystal structure of recombinant CD11bA showed a compact GTPase-like fold comprising a central, mostly parallel β-sheet surrounded on both sides by seven amphipathic α-helices ( Figure 2a). The catalytic site found at the apex in GTPases is replaced with MIDAS, where an Mg 2+ ion is coordinated by three surface loops ( Figure 2b). A solvent-exposed glutamate (E) or aspartate (D) from ligand completes an octahedral coordination sphere around the Mg 2+ ion 69. This crystal structure first explained why Mg 2+ is required for integrin binding to all physiologic ligands and why a solvent-accessible acidic residue from ligand is essential for binding to any integrin. Ligand-binding specificity in αA domains is imparted by the variable surface-exposed side chains surrounding the MIDAS motif.
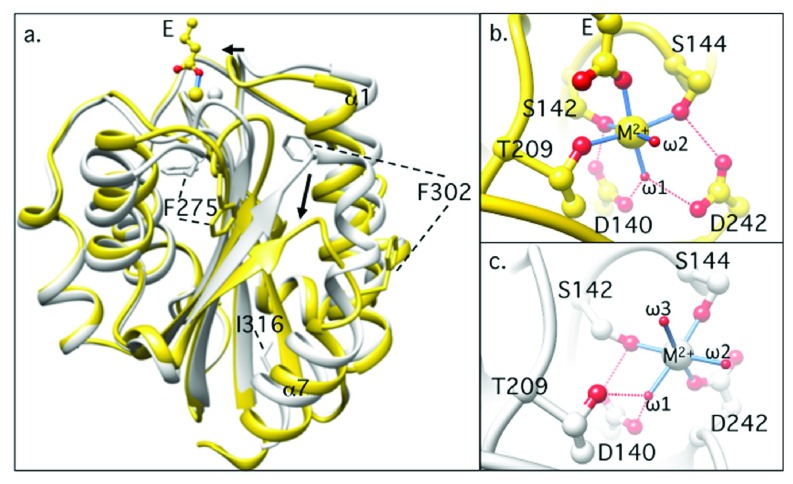
Figure 2. Structural comparisons of inactive and active αA domains.
( a) Ribbon diagrams showing the superposed structures of inactive (gray) and active (yellow) αA domain from the β 2 integrin CD11b/CD18. Major conformational differences are indicated by arrows. The two phenylalanine residues (F275 and F302) buried in the inactive form are solvent exposed in the active state. A glutamate (E) from ligand is shown in the active (ligand-bound) state, ligating the metal-ion-dependent adhesion site (MIDAS) Mg 2+ monodentately. ( b, c) The MIDAS motif in the active ( b) and inactive ( c) states. The metal ion at MIDAS is coordinated by residues from three surface loops, and a carboxyl oxygen from ligand completes the octahedral coordinating sphere ( b). In the inactive state, an oxygen atom from a water molecule replaces the ligand oxygen, and D242 from the third surface loop moves in to coordinate the metal directly ( c). Coordinating oxygen atoms are in red, and hydrogen bonds are shown by dashed red lines. Direct bonds to the metal ion are shown as blue sticks. Water molecules are labeled ω1–ω3.
The αA domain also exists in a second ligand-free “closed” conformation 72, 73, where the ligand coordinating carboxyl oxygen is replaced with a water molecule ( Figure 2c). Superposing the two structures shows the key tertiary changes associated with ligand binding: an inward movement of the N-terminal α1 helix, rearrangements of the metal-coordinating residues at MIDAS, and a 10 Å downward shift of the C-terminal α7 helix at the opposite pole to MIDAS 72, 74 ( Figure 2a). The key residues that stabilize the closed conformation have been identified, and mutations of some of these residues converted the closed into the open conformation 75– 79. Locking the open conformation with a pair of disulfides allowed crystallization of this form in the absence of ligand 80, 81. Crystal structures of αA domains from other integrins (for example, α 2β 1 82), complement factors (for example, factors B and C2 83, 84), certain matrix proteins 85, and microorganisms (for example, anthrax 86) were subsequently determined. These structures displayed the same basic conformational changes observed in CD11bA, underscoring their functional importance. In solution, recombinant wild-type CD11bA exists in an equilibrium where the proportion of the closed to the open state is nearly 9:1 75, 79; the presence of ligand shifts this equilibrium in favor of the open state.
The integrin ectodomain
The modular nature of an integrin was first revealed with the determination of the crystal structure of the ectodomain of the αA-lacking integrin α vβ 3 in its unliganded state 87 and when occupied by a cyclic peptide ligand containing the prototypical Arg-Gly-Asp motif 88. The α v subunit is composed of a seven-bladed propeller domain, followed by a thigh domain and two large Ig-like Calf domains. The β 3 subunit comprises an N-terminal plexin-semaphorin-integrin (PSI) domain, an Ig-like “hybrid” domain in which an αA-like domain (βA) is inserted, four successive epidermal growth factor (EGF)-like domains (IE1–4), and a novel membrane-proximal β-tail domain (βTD) ( Figure 3a, b). In the full-length integrin, Calf2 and βTD each is attached to a transmembrane (TM) domain and a short cytoplasmic tail. An unexpected feature of the α vβ 3 ectodomain is a sharp bending in the structure at the α-genu (between the thigh and calf1 domains) and the β-genu (within IE2) ( Figure 3a). Extension at the knees is expected to produce an extended integrin ( Figure 3b), which resembles the shape seen previously using rotary shadowing electron microscopy 89.
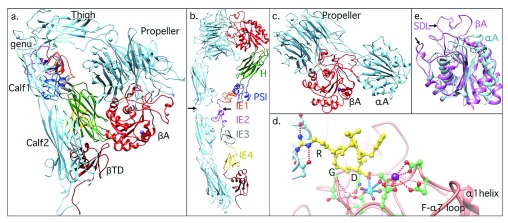
Figure 3. Structure of the integrin ectodomain.
( a) Ribbon drawing of the bent ectodomain from integrin α Vβ 3. α V is in light blue, and the 12 domains of β 3 are shown in different colors for better visualization. The two tails would extend into the plasma membrane in the native integrin. ( b) Model of α Vβ 3 ectodomain linearized by introducing breaks at the α and β genu (arrows). The modular nature of the ectodomain can be readily appreciated. H, hybrid domain; PSI, plexin-semaphorin-integrin. ( c) The integrin head from the αA-containing β 2 integrin CD11c/CD18. ( d) Interactions between arginine-glycine-aspartate (RGD)-containing ligand peptide (yellow) and the α Vβ 3 head. The peptide aspartate (D) completes the metal ion coordination sphere at metal-ion-dependent adhesion site (MIDAS), and ligand arginine forms salt bridges in the propeller pocket. αV and β 3 residues are labeled light blue and orange, respectively. The three metal ions in β 3 at MIDAS, adjacent to MIDAS (ADMIDAS), and ligand-associated metal binding site (LIMBS) are shown in cyan, magenta, and gray, respectively, and their coordinating residues displayed. The upper portion of the α1 helix and the loop between strand-F and α7 helix (F-α7) are also shown. Oxygen and nitrogen atoms are in red and blue, respectively. Hydrogen bonds and salt bridges (distance cutoff, 3.5 Å) are represented with dotted lines. ( e) Superposed structures of αA and βA domains. Shown are the two inserted loops in βA: the specificity determining loop (SDL) and heterodimer-association loop. The hydrophobic phenylalanine residue at the top of α7 helix that contacts α1 helix in αA is replaced in βA with an ionic interaction mediated by ADMIDAS ion.
In αA-lacking integrins, the integrin head is formed of the βA and propeller domains ( Figure 3a, b), which associate non-covalently in a manner that resembles the association of the Gα and Gβ subunits of heterotrimeric G proteins 87. In αA-containing integrins, the head also contains the αA domain, which projects from a surface loop in the propeller ( Figure 3c). The heterodimer-disruptive point mutations found in the β 2 (CD18) and β 3 subunits in patients with LAD I and Glanzmann’s thrombasthenia (a bleeding disorder), respectively, map to the βA domain and commonly involve residues at the βA-propeller interface 87. As in αA domains, an acidic residue from ligand completes the octahedral coordination of Mg 2+ at MIDAS, an interaction stabilized by the arginine residue in the prototypical arginine-glycine-aspartate (RGD) motif, which inserts into a pocket in the propeller domain, making contacts with acidic residues in the pocket ( Figure 3d). Five metal ions (Ca 2+ or Mn 2+) occupy the bases of blades 4–7 of the α v propeller and the α-genu ( Figure 3a, b); these may help rigidify the interfaces the thigh domain makes with the propeller base proximally and the top of Calf1 distally.
The structure of inactive βA is largely superimposable onto that of αA, except for two loop insertions: one forming the core of the interface with the α-subunit’s propeller and the second—the specificity determining loop, SDL—contributing to ligand binding as well as to the βA/propeller interface in some integrins (for example, α IIbβ 3) ( Figure 3e). In addition, a Ca 2+ ion at a site adjacent to MIDAS (ADMIDAS) in βA links the two activation-sensitive α1 and α7 helices, stabilizing this domain in the closed state; in αA, this ionic interaction is replaced by a hydrophobic one ( Figure 3e). In addition to the ADMIDAS ion, ligand-bound βA contains a ligand-associated metal binding site (LIMBS), which is occupied by Ca 2+ in ligand- or pseudoligand-bound integrins 88, 90. The structure of LIMBS in ligand-free integrins is regulated by the α-subunit’s propeller domain 91 and this may explain the variable metal ion occupancy of this site (sometimes also called synergy metal binding site).
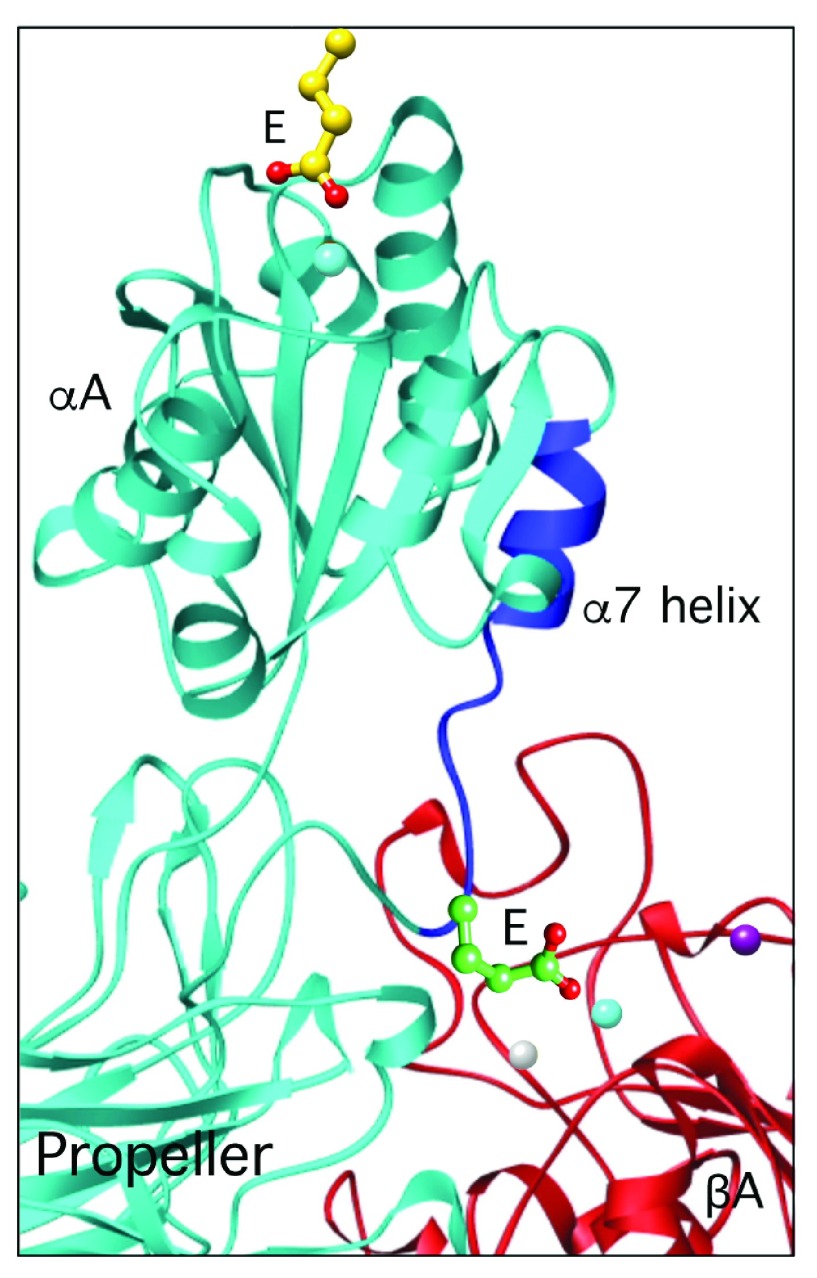
In αA-containing integrins, the ligand-associated downward shift of the C-terminal α7 helix enables an invariant glutamate at the bottom of α7 to ligate the βA MIDAS ion ( Figure 4); mutation of this residue to alanine blocked integrin function 92. This led us to propose that αA serves as an intrinsic ligand for βA in αA-containing integrins. Blocking this coordination by the synthetic molecule XVA143 severs the αA link to βA and blocks integrin signaling 93. Support for this “ligand-relay” model came from the recent crystal structure of the CD11c/CD18 ectodomain 94. Thus, the βA domain transduces outside-in signals that are triggered by either extrinsic (in αA-lacking integrins) or intrinsic (in αA-containing integrins) ligands.
Figure 4. The ligand-relay model.
The downward movement of the c-terminal α7 helix (dark blue) triggered by ligand binding to αA allows an invariant glutamate (E) at the bottom of the α7 helix to reach and ligate the βA metal-ion-dependent adhesion site (MIDAS) ion (cyan), thus relaying the ligand occupancy state of αA to βA.
Integrin transmembrane and cytoplasmic tails
The structure of the lipid-embedded α IIb and β 3 single-pass TM helices was determined by using solution nuclear magnetic resonance (NMR) spectroscopy 95. The structure revealed two dominant integrin TM association motifs or clasps: an outer (membrane-proximal) and an inner (membrane-distal) one that extends to include the adjacent cytoplasmic salt bridge between α IIb and β 3 96. The two clasps maintain the integrin in the inactive state 97. Another structure in hydrophobic organic solvent invokes several differences in the membrane-proximal clasp regions, especially the helical conformation of α IIb in the latter versus a reverse turn in the former structure 98. It is unclear at present whether this difference in the membrane proximal regions in the NMR structures reflects the nature of the lipid-like TM environment in which the TM domains were incorporated or reflects potential changes in response to binding of cytosolic regulators such as filamin 99, 100.
Binding of the N-terminal talin head to the membrane proximal NPxY/F motif in the β cytoplasmic tail destabilizes the α-β TM association 101, 102. Recruitment of talin to the plasma membrane requires ras-related protein 1 (Rap1) and its effector Rap1-GTP-interacting adaptor molecule (RIAM), and the latter is critical in vivo for inside-out signaling of β 2 but not β 1 or β 3 integrins 103, 104. Kindlins have been reported to modulate receptor affinity 105 or avidity 106 or both. Kindlins bind the distal NPxY/F motif and a preceding threonine-containing region of the β cytoplasmic tail 107 but do not appear to destabilize α-β TM association 108. The structural basis for regulation of integrins by kindlins remains to be elucidated. Loss of kindlin 3 causes LAD III, a disease characterized by bleeding diathesis (defective α IIbβ 3 function) and defective leukocyte recruitment to sites of infection (defective β 2 integrin function) 105.
Integrin activation
Integrins are normally expressed in an inactive state on the cell surface. This is critical, as it allows leukocytes and platelets, for example, to freely circulate in blood with minimal aggregation or interaction with blood vessel walls. Binding of an agonist such as a chemokine or a cytokine (for example, granulocyte-macrophage colony-stimulating factor 109) to their respective receptors initiates inside-out signals that rapidly switch the integrin into the active state. Integrins stored in intracellular pools (for example, CD11b/CD18 18, 110, 111 and α IIbβ 3 112) are also recruited to the cell surface in response to agonists, but this process appears to follow the switch of the integrin to the active state 113, 114.
The structural basis for integrin inside-out signaling is debated. Following publication of the bent ectodomain structure 87, a “switchblade” model envisioned that in the bent state, the ligand-binding site in βA (and αA in αA-containing integrin) is inaccessible to soluble ligand because of its proposed proximity to the plasma membrane. It is suggested, therefore, that the integrin linearizes to expose the ligand-binding site 115, which also allows an approximately 80° swingout of the hybrid domain and a switch of βA into high affinity 90 ( Figure 5). An alternate βTD-centric deadbolt model 116 proposed that the ligand-binding site in βA is already accessible to soluble macromolecular ligand in the native integrin 117 and can assume high affinity in the compact structure 118 and that genuextension occurs following binding of ligands or ligand-mimetic drugs to the cellular integrin 119. Movements of the membrane proximal βTD resulting from unpacking of the immediately distal TM segments disrupt βTD contacts with βA and hybrid domains, allowing the central switch of βA into the active state with minimal hybrid domain swingout 118.
Figure 5. Structural changes in the βA domain following ligand binding.
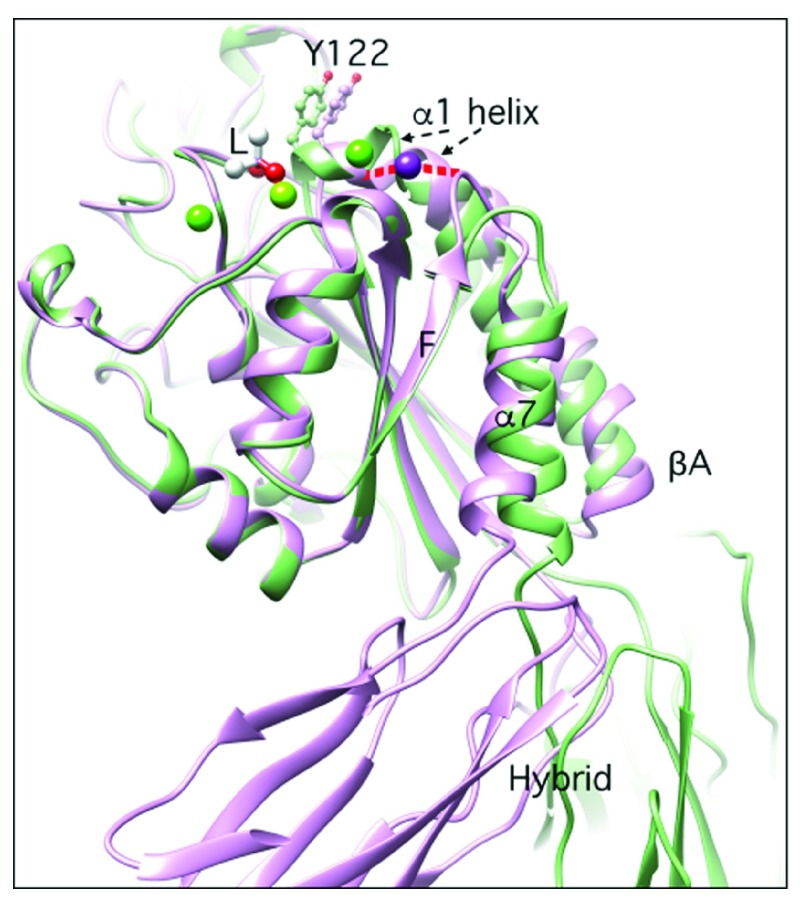
The superposed structures of βA domain of the β3 subunit in its unliganded (pdb 3ije) state and bound to cacodylate (acting as a pseudoligand, L) (pdb 1ty3) are shown in magenta and green, respectively. The main movements involve the α1 and α7 helices, loop F-α7, and the hybrid domain. In the unliganded state, helix α1 and Fα7 loop are connected via the adjacent to MIDAS (ADMIDAS) ion (magenta), and no metal-ion-dependent adhesion site (MIDAS) or ligand-associated metal binding site (LIMBS) atoms are detected. In the liganded state, a ligand oxygen coordinates MIDAS, and the α1 helix moves inwards (reported by tyrosine 122, Y122), bringing the ADMIDAS ion closer to the MIDAS ion and breaking the ionic contact with the F-α7 loop. These changes are coupled with a one-turn descent of the α7 helix and a 135° swingout of the hybrid domain in structures lacking the integrin leg domains.
Both models are supported by experimental data. Two-dimensional imaging using negative-stain electron microscopy (EM) showed a greater proportion of extended integrin ectodomains in the presence of the metal ion Mn 2+ (used as a mimic of inside-out signaling), and hydrodynamic studies showed an increase in the stokes radius of the α Vβ 3 ectodomain in Mn 2+ 115. However, cryoelectron tomography showed that α IIbβ 3 maintained the compact (bent) conformation after Mn 2+ activation in a membrane environment 120. Differences in sample preparation, sampling bias in EM, and differences in ectodomain constructs may explain these discrepancies. A recent EM study of full-length integrin α IIbβ 3 in lipid-embedded nanodiscs showed a small increase in the extended conformation when the integrin was activated by talin 121. More recently, negative-stain EM of membrane-embedded full-length α IIbβ 3 showed that the active ligand-free α IIbβ 3 is mainly bent but that the ligand-bound receptor is predominantly extended 122. High-resolution quantitative dynamic footprinting microscopy combined with homogenous conformation-reporter binding assays showed that a substantial fraction of β 2 integrins on the surface of human neutrophils assumed a high-affinity bent conformation 123. Because of the profound influence of the TM domains on integrin activation by inside-out signaling, settling the ongoing debate regarding the structural basis of integrin activation will likely require a three-dimensional crystal structure determination of a full-length native integrin in its native inactive and high-affinity states.
Ligand-bound integrins cluster, especially when occupied by multivalent ligands, and transduce outside-in signals leading to cell adhesion via new connections established between the integrin cytoplasmic tails and filamentous actin 124. In migrating cells, inward movement of the actin cytoskeleton from the site of assembly at the leading edge toward the cell center generates a pulling force across the nascent-integrin-matrix linkages and this unbends the liganded integrin and strengthens adhesion at these sites by accelerating recruitment of additional cytoskeletal and signaling proteins to the clustered integrins 125. As this pulling force increases in the moving cell, integrin-ligand bonds eventually break and integrins are endocytosed and this allows rear detachment and directional cell movement at the leading edge. Known adaptor proteins involved in integrin uptake and recycling have been recently reviewed 126.
β 2 integrins as therapeutic targets
Although β 2 integrins are critical for innate and adaptive immunity, they can also induce serious pathology if improperly activated. Hyperadherent leukocytes may, for example, bind and injure the blood vessel wall, leukoaggregate intravascularly resulting in blocked capillaries or emboli, or compromise immune surveillance, thus contributing to inflammatory and autoimmune diseases. The finding that CD18 deficiency impaired the inflammatory response suggested that knockout of CD18 or CD11 or inhibiting their functions in leukocytes using antibodies may be beneficial in treating inflammatory or autoimmune diseases 7. A similar logic has been successful in targeting platelet α IIbβ 3 to inhibit pathologic thrombosis and this resulted in two orthosteric inhibitors, eptifibatide and tirofiban, and an allosteric inhibitor Abciximab, all three in clinical use 127.
Genetic deficiency of CD18, CD11a, or CD11b or targeting β 2 integrins with various inhibitory antibodies in rodents ameliorated ischemia-reperfusion injury (IRI) in heart attacks, cerebral stroke, burns, and traumatic shock as well as autoimmune injury of the brain (multiple sclerosis), lung (asthma), and skin (psoriasis) and in native or transplanted kidneys (reviewed in 128). However, humanized forms of these mAbs failed when tested in patients with myocardial infarction, stroke, traumatic shock, multiple sclerosis, asthma, or acute rejection (reviewed in 128). An anti-CD11a mAb that showed promise in treating psoriasis was withdrawn because of fatal brain infections resulting from reactivation of JC virus 129. Inadequate design of some of the trials 128, important differences in immune responses between rodents and humans 130, and the relatively short follow-up period in the preclinical studies may have contributed to these failures. In addition, most clinical studies evaluating IRI syndromes used anti-CD18 antibodies, which might have acted allosterically to switch the integrin into the active proadhesive state. This scenario has precedence in β 3 integrin-targeted mAb or small-molecule drugs, which act as partial agonists, unbending the integrin, thus exposing neoepitopes recognized by natural antibodies and leading to immune thrombocytopenia and bleeding, or inducing proadhesive outside-in signaling leading to paradoxical thrombosis 131, 132. Therefore, recent attempts have been made to solve the problem of partial agonism, making use of the advances made in structural biology of integrins. The central role of the A-domain in integrin activation and signaling made it a main focus of drug development efforts. The non-RGD-containing small molecules RUC-1, RUC-2, and UR-2922 were identified and act by inserting into the arginine-binding pocket in the propeller domain 133, 134, thus interfering with the stable binding of RGD-containing ligands. RUC-2 also binds to the β3 MIDAS residue E220 thus displacing the Mg 2+ at MIDAS 133. In vivo studies of RUC-1 administered intraperitoneally demonstrated anti-thrombotic effects in microvascular injury models in mice 135.
We have approached the problem of partial agonism by identifying orthosteric inhibitors of integrin β 2 (mAb107, 117) and β 3 (a mutant high-affinity form of fibronectin-10, hFN10 136) that do not induce the activating proadhesive changes in the αA or βA domains, respectively. mAb107 stabilized the inhibitory Ca 2+ in place of the proadhesive Mg 2+ at the CD11bA MIDAS, freezing the β 2 integrin CD11b/CD18 in the inactive conformation 117 ( Figure 6a). hFN10 bound the βA MIDAS of integrin α Vβ 3 and blocked the activating inward movement of the α1 helix ( Figure 6b), which is critical for integrin unbending and outside-in signaling 136. In vivo studies in monkeys showed that mAb107 ameliorated leukocyte-mediated inflammation in a severe IRI kidney model, salvaging kidney function from otherwise irreversible failure several months after a single injection of the mAb at the onset of IRI 137.
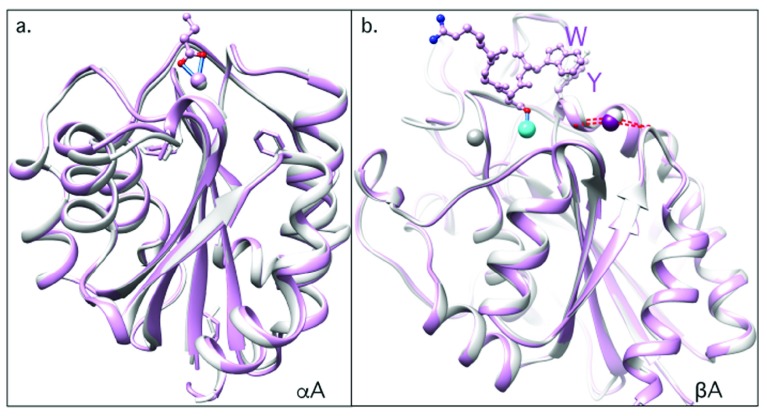
Figure 6. Structural basis of integrin inhibition by “pure” orthosteric inhibitors.
( a) Ribbon drawing showing structure of αA from the β 2 integrin CD11b/CD18 bound to the pure ligand-mimetic antagonist mAb107 (in magenta). For clarity, only the ligand Asp of mAb107 is shown. The unusual symmetric bidentate ligation of the antibody-derived ligand Asp to a hepta-coordinated metal-ion-dependent adhesion site (MIDAS) Ca 2+ (blue sticks) prevents the tertiary changes associated with Mg 2+-dependent ligand binding. The superposed structure in gray is that of unliganded αA from CD11b/CD18. ( b) Structure of unliganded βA from α Vβ 3 (pdb 3ije) (gray), superposed on the structure of βA in complex with a fibronectin-10-derived “pure antagonist” (magenta). Only the RGDW residues (in ball and stick) from ligand are shown (pdb 4mmz). Ligand-associated inward movement of the α1 helix and the resulting activating tertiary changes are prevented by a π–π interaction involving the ligand tryptophan (W) and βA’s tyrosine 122 (Y122). The ionic bridge (dashed red lines) between α1 and α7 helices is unaffected by binding of the pure orthosteric inhibitor. The metal ions at ADMIDAS, MIDAS, and LIMBS are in magenta (or gray), cyan, and dark gray, respectively.
Conclusions
Much has been learned since Cohnheim’s and Metchnikoff’s respective descriptions of leukocyte transendothelial migration and phagocytosis. The receptors involved have been identified, their critical role in innate and adaptive immunity defined, and their structures elucidated, revealing the atomic basis for their Mg 2+ dependency, ligand binding, and activation. Although putting the myriad interactions mediated by integrins into structural and biologic contexts remains a major challenge, the recent advances already made form a basis for structure-based discovery of effective and safer anti-inflammatory and anti-thrombosis therapeutics targeting these dynamic receptors.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Tobias Ulmer, Department of Biochemistry & Molecular Biology and Zilkha Neurogenetic Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Jun Qin, Department of Molecular Cardiology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA
Klaus Ley, Division of Inflammation Biology, La Jolla Institute for Allergy and Immunology; Department of Bioengineering, University of California San Diego, La Jolla, CA, USA
Funding Statement
The author's work presented in this review was supported by National Institutes of Health grants DK088327, DK48549, and DK007540 from the National Institutes of Diabetes, Digestive and Kidney Diseases.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Harburger DS, Calderwood DA: Integrin signalling at a glance. J Cell Sci. 2009;122(Pt 2):159–63. 10.1242/jcs.018093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gahmberg CG, Fagerholm SC, Nurmi SM, et al. : Regulation of integrin activity and signalling. Biochim Biophys Acta. 2009;1790(6):431–44. 10.1016/j.bbagen.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horton ER, Astudillo P, Humphries MJ, et al. : Mechanosensitivity of integrin adhesion complexes: role of the consensus adhesome. Exp Cell Res. 2016;343(1):7–13. 10.1016/j.yexcr.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 4. Mócsai A, Walzog B, Lowell CA: Intracellular signalling during neutrophil recruitment. Cardiovasc Res. 2015;107(3):373–85. 10.1093/cvr/cvv159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohnheim J: Lectures On General Pathology: A Handbook for Practitioners and Students. 2nd edition, (translated from the second German edition) London, The New Sydenham Society.1889; 1 Reference Source [Google Scholar]
- 6. Metchnikoff E: Sur la lutte des cellules de l’organisme contre l’invasion des microbes. Ann Inst Pasteur I. 1887;321 Reference Source [Google Scholar]
- 7. Arnaout MA: Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990;114(1):145–80. 10.1111/j.1600-065X.1990.tb00564.x [DOI] [PubMed] [Google Scholar]
- 8. Arnaout MA, Pitt J, Cohen HJ, et al. : Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982;306(12):693–9. 10.1056/NEJM198203253061201 [DOI] [PubMed] [Google Scholar]
- 9. Dana N, Pitt J, Todd RF, et al. : Deficiency of a monocyte-granulocyte surface glycoprotein (Mo1) in man.[abstract]. Clin Res. 1983;31:489. [Google Scholar]
- 10. Springer T, Galfré G, Secher DS, et al. : Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9(4):301–6. 10.1002/eji.1830090410 [DOI] [PubMed] [Google Scholar]
- 11. Remold-O'Donnell E: Macrophage component gp160, a major trypsin-sensitive surface glycoprotein. J Exp Med. 1980;152(6):1699–708. 10.1084/jem.152.6.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Todd RF, 3rd, Nadler LM, Schlossman SF: Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981;126(4):1435–42. [PubMed] [Google Scholar]
- 13. Trowbridge IS, Omary MB: Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981;154(5):1517–24. 10.1084/jem.154.5.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanchez-Madrid F, Nagy JA, Robbins E, et al. : A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983;158(6):1785–803. 10.1084/jem.158.6.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beatty PG, Ochs HD, Harlan JM, et al. : Absence of monoclonal-antibody-defined protein complex in boy with abnormal leucocyte function. Lancet. 1984;1(8376):535–7. 10.1016/S0140-6736(84)90933-4 [DOI] [PubMed] [Google Scholar]
- 16. LeBien TW, Kersey JH: A monoclonal antibody (TA-1) reactive with human T lymphocytes and monocytes. J Immunol. 1980;125(5):2208–14. [PubMed] [Google Scholar]
- 17. Davignon D, Martz E, Reynolds T, et al. : Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A. 1981;78(7):4535–9. 10.1073/pnas.78.7.4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arnaout MA, Spits H, Terhorst C, et al. : Deficiency of a leukocyte surface glycoprotein (LFA-1) in two patients with Mo1 deficiency. Effects of cell activation on Mo1/LFA-1 surface expression in normal and deficient leukocytes. J Clin Invest. 1984;74(4):1291–300. 10.1172/JCI111539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dana N, Todd RF, 3rd, Pitt J, et al. : Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984;73(1):153–9. 10.1172/JCI111186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lanier LL, Arnaout MA, Schwarting R, et al. : p150/95, Third member of the LFA-1/CR 3 polypeptide family identified by anti-Leu M5 monoclonal antibody. Eur J Immunol. 1985;15(7):713–8. 10.1002/eji.1830150714 [DOI] [PubMed] [Google Scholar]
- 21. Danilenko DM, Rossitto PV, van der Vieren M, et al. : A novel canine leukointegrin, alpha d beta 2, is expressed by specific macrophage subpopulations in tissue and a minor CD8+ lymphocyte subpopulation in peripheral blood. J Immunol. 1995;155(1):35–44. [PubMed] [Google Scholar]
- 22. Springer TA, Thompson WS, Miller LJ, et al. : Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984;160(6):1901–18. 10.1084/jem.160.6.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dana N, Clayton LK, Tennen DG, et al. : Leukocytes from four patients with complete or partial Leu-CAM deficiency contain the common beta-subunit precursor and beta-subunit messenger RNA. J Clin Invest. 1987;79(3):1010–5. 10.1172/JCI112868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson C, Rabb H, Arnaout MA: Genetic cause of leukocyte adhesion molecule deficiency. Abnormal splicing and a missense mutation in a conserved region of CD18 impair cell surface expression of beta 2 integrins. J Biol Chem. 1992;267(5):3351–7. [PubMed] [Google Scholar]
- 25. Anderson DC, Schmalsteig FC, Finegold MJ, et al. : The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152(4):668–89. 10.1093/infdis/152.4.668 [DOI] [PubMed] [Google Scholar]
- 26. Arnaout MA: Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75(5):1037–50. [PubMed] [Google Scholar]
- 27. Rosenkranz AR, Coxon A, Maurer M, et al. : Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J Immunol. 1998;161(12):6463–7. [PubMed] [Google Scholar]
- 28. Wagner C, Hansch GM, Stegmaier S, et al. : The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: activation-dependent up-regulation and regulatory function. Eur J Immunol. 2001;31(4):1173–80. [DOI] [PubMed] [Google Scholar]
- 29. Lahmers KK, Hedges JF, Jutila MA, et al. : Comparative gene expression by WC1 + gammadelta and CD4 + alphabeta T lymphocytes, which respond to Anaplasma marginale, demonstrates higher expression of chemokines and other myeloid cell-associated genes by WC1 + gammadelta T cells. J Leukoc Biol. 2006;80(4):939–52. 10.1189/jlb.0506353 [DOI] [PubMed] [Google Scholar]
- 30. Pilling D, Fan T, Huang D, et al. : Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. 10.1371/journal.pone.0007475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubtsova K, Rubtsov AV, van Dyk LF, et al. : T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110(34):E3216–24. 10.1073/pnas.1312348110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clements M, Gershenovich M, Chaber C, et al. : Differential Ly6C Expression after Renal Ischemia-Reperfusion Identifies Unique Macrophage Populations. J Am Soc Nephrol. 2016;27(1):159–70. 10.1681/ASN.2014111138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghosn EE, Yang Y, Tung J, et al. : CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proc Natl Acad Sci U S A. 2008;105(13):5195–200. 10.1073/pnas.0712350105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keizer GD, Borst J, Visser W, et al. : Membrane glycoprotein p150,95 of human cytotoxic T cell clone is involved in conjugate formation with target cells. J Immunol. 1987;138(10):3130–6. [PubMed] [Google Scholar]
- 35. van der Vieren M, Le Trong H, Wood CL, et al. : A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995;3(6):683–90. 10.1016/1074-7613(95)90058-6 [DOI] [PubMed] [Google Scholar]
- 36. Miyazaki Y, Vieira-de-Abreu A, Harris ES, et al. : Integrin α Dβ 2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling. PLoS One. 2014;9(11):e112770. 10.1371/journal.pone.0112770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noti JD: Expression of the myeloid-specific leukocyte integrin gene CD11d during macrophage foam cell differentiation and exposure to lipoproteins. Int J Mol Med. 2002;10(6):721–7. 10.3892/ijmm.10.6.721 [DOI] [PubMed] [Google Scholar]
- 38. Yakubenko VP, Belevych N, Mishchuk D, et al. : The role of integrin α Dβ 2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res. 2008;314(14):2569–78. 10.1016/j.yexcr.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tian L, Yoshihara Y, Mizuno T, et al. : The neuronal glycoprotein telencephalin is a cellular ligand for the CD11a/CD18 leukocyte integrin. J Immunol. 1997;158(2):928–36. [PubMed] [Google Scholar]
- 40. Béchard D, Scherpereel A, Hammad H, et al. : Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167(6):3099–106. 10.4049/jimmunol.167.6.3099 [DOI] [PubMed] [Google Scholar]
- 41. Ostermann G, Weber KS, Zernecke A, et al. : JAM-1 is a ligand of the β 2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3(2):151–8. 10.1038/ni755 [DOI] [PubMed] [Google Scholar]
- 42. Sadhu C, Ting HJ, Lipsky B, et al. : CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J Leukoc Biol. 2007;81(6):1395–403. 10.1189/jlb.1106680 [DOI] [PubMed] [Google Scholar]
- 43. Bilsland CA, Diamond MS, Springer TA: The leukocyte integrin p150,95 (CD11c/CD18) as a receptor for iC3b. Activation by a heterologous beta subunit and localization of a ligand recognition site to the I domain. J Immunol. 1994;152(9):4582–9. [PubMed] [Google Scholar]
- 44. Malhotra V, Hogg N, Sim RB: Ligand binding by the p150,95 antigen of U937 monocytic cells: properties in common with complement receptor type 3 (CR3). Eur J Immunol. 1986;16(9):1117–23. 10.1002/eji.1830160915 [DOI] [PubMed] [Google Scholar]
- 45. Blackford J, Reid HW, Pappin DJ, et al. : A monoclonal antibody, 3/22, to rabbit CD11c which induces homotypic T cell aggregation: evidence that ICAM-1 is a ligand for CD11c/CD18. Eur J Immunol. 1996;26(3):525–31. 10.1002/eji.1830260304 [DOI] [PubMed] [Google Scholar]
- 46. Ihanus E, Uotila LM, Toivanen A, et al. : Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: characterization of the binding sites on ICAM-4. Blood. 2007;109(2):802–10. 10.1182/blood-2006-04-014878 [DOI] [PubMed] [Google Scholar]
- 47. Xia Y, Vetvicka V, Yan J, et al. : The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162(4):2281–90. [PubMed] [Google Scholar]
- 48. Yakubenko VP, Solovjov DA, Zhang L, et al. : Identification of the binding site for fibrinogen recognition peptide γ383-395 within the α MI-domain of integrin α Mβ 2. J Biol Chem. 2001;276(17):13995–4003. 10.1074/jbc.M010174200 [DOI] [PubMed] [Google Scholar]
- 49. Humphries JD, Byron A, Humphries MJ: Integrin ligands at a glance. J Cell Sci. 2006;119(pt 19):3901–3. 10.1242/jcs.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Vieren M, Crowe DT, Hoekstra D, et al. : The leukocyte integrin alpha D beta 2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163(4):1984–90. [PubMed] [Google Scholar]
- 51. Scharffetter-Kochanek K, Lu H, Norman K, et al. : Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188(1):119–31. 10.1084/jem.188.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shier P, Ngo K, Fung-Leung WP: Defective CD8+ T cell activation and cytolytic function in the absence of LFA-1 cannot be restored by increased TCR signaling. J Immunol. 1999;163(9):4826–32. [PubMed] [Google Scholar]
- 53. Shier P, Otulakowski G, Ngo K, et al. : Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the beta2 integrin leukocyte function-associated antigen-1. J Immunol. 1996;157(12):5375–86. [PubMed] [Google Scholar]
- 54. Bose TO, Pham QM, Jellison ER, et al. : CD11a regulates effector CD8 T cell differentiation and central memory development in response to infection with Listeria monocytogenes. Infect Immun. 2013;81(4):1140–51. 10.1128/IAI.00749-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmits R, Kündig TM, Baker DM, et al. : LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183(4):1415–26. 10.1084/jem.183.4.1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnston B, Kubes P: The alpha4-integrin: an alternative pathway for neutrophil recruitment? Immunol Today. 1999;20(12):545–50. 10.1016/S0167-5699(99)01544-3 [DOI] [PubMed] [Google Scholar]
- 57. Taooka Y, Chen J, Yednock T, et al. : The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145(2):413–20. 10.1083/jcb.145.2.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu H, Rodgers JR, Perrard XY, et al. : Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173(1):297–306. 10.4049/jimmunol.173.1.297 [DOI] [PubMed] [Google Scholar]
- 59. Arnaout MA, Lanier LL, Faller DV: Relative contribution of the leukocyte molecules Mo1, LFA-1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue- and stimulus-specific. J Cell Physiol. 1988;137(2):305–9. 10.1002/jcp.1041370214 [DOI] [PubMed] [Google Scholar]
- 60. Ding ZM, Babensee JE, Simon SI, et al. : Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163(9):5029–38. [PubMed] [Google Scholar]
- 61. Lu H, Smith CW, Perrard J, et al. : LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99(6):1340–50. 10.1172/JCI119293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Luo D, McGettrick HM, Stone PC, et al. : The roles of integrins in function of human neutrophils after their migration through endothelium into interstitial matrix. PLoS One. 2015;10(2):e0118593. 10.1371/journal.pone.0118593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lämmermann T, Bader BL, Monkley SJ, et al. : Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–5. 10.1038/nature06887 [DOI] [PubMed] [Google Scholar]
- 64. Coxon A, Rieu P, Barkalow FJ, et al. : A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5(6):653–66. 10.1016/S1074-7613(00)80278-2 [DOI] [PubMed] [Google Scholar]
- 65. Han C, Jin J, Xu S, et al. : Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11(8):734–42. 10.1038/ni.1908 [DOI] [PubMed] [Google Scholar]
- 66. Milde R, Ritter J, Tennent GA, et al. : Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Rep. 2015;13(9):1937–48. 10.1016/j.celrep.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dong ZM, Gutierrez-Ramos JC, Coxon A, et al. : A new class of obesity genes encodes leukocyte adhesion receptors. Proc Natl Acad Sci U S A. 1997;94(14):7526–30. 10.1073/pnas.94.14.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Michishita M, Videm V, Arnaout MA: A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72(6):857–67. 10.1016/0092-8674(93)90575-B [DOI] [PubMed] [Google Scholar]
- 69. Lee JO, Rieu P, Arnaout MA, et al. : Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18). Cell. 1995;80(4):631–8. 10.1016/0092-8674(95)90517-0 [DOI] [PubMed] [Google Scholar]
- 70. Whittaker CA, Hynes RO: Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13(10):3369–87. 10.1091/mbc.E02-05-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Diamond MS, Garcia-Aguilar J, Bickford JK, et al. : The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120(4):1031–43. 10.1083/jcb.120.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee JO, Bankston LA, Arnaout MA, et al. : Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3(12):1333–40. 10.1016/S0969-2126(01)00271-4 [DOI] [PubMed] [Google Scholar]
- 73. Qu A, Leahy DJ: Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, alpha L beta 2) integrin. Proc Natl Acad Sci U S A. 1995;92(22):10277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bajic G, Yatime L, Sim RB, et al. : Structural insight on the recognition of surface-bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci U S A. 2013;110(41):16426–31. 10.1073/pnas.1311261110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li R, Rieu P, Griffith DL, et al. : Two functional states of the CD11b A-domain: correlations with key features of two Mn 2+-complexed crystal structures. J Cell Biol. 1998;143(6):1523–34. 10.1083/jcb.143.6.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xiong JP, Li R, Essafi M, et al. : An isoleucine-based allosteric switch controls affinity and shape shifting in integrin CD11b A-domain. J Biol Chem. 2000;275(49):38762–7. 10.1074/jbc.C000563200 [DOI] [PubMed] [Google Scholar]
- 77. Shimaoka M, Shifman JM, Jing H, et al. : Computational design of an integrin I domain stabilized in the open high affinity conformation. Nat Struct Biol. 2000;7(8):674–8. 10.1038/77978 [DOI] [PubMed] [Google Scholar]
- 78. Aquilina A, Korda M, Bergelson JM, et al. : A novel gain-of-function mutation of the integrin alpha2 VWFA domain. Eur J Biochem. 2002;269(4):1136–44. 10.1046/j.0014-2956.2001.02740.x [DOI] [PubMed] [Google Scholar]
- 79. McCleverty CJ, Liddington RC: Engineered allosteric mutants of the integrin alphaMbeta2 I domain: structural and functional studies. Biochem J. 2003;372(Pt 1):121–7. 10.1042/BJ20021273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ponnuraj K, Xu Y, Macon K, et al. : Structural analysis of engineered Bb fragment of complement factor B: insights into the activation mechanism of the alternative pathway C3-convertase. Mol Cell. 2004;14(1):17–28. 10.1016/S1097-2765(04)00160-1 [DOI] [PubMed] [Google Scholar]
- 81. Shimaoka M, Xiao T, Liu JH, et al. : Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112(1):99–111. 10.1016/S0092-8674(02)01257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Emsley J, King SL, Bergelson JM, et al. : Crystal structure of the I domain from integrin alpha2beta1. J Biol Chem. 1997;272(45):28512–7. 10.1074/jbc.272.45.28512 [DOI] [PubMed] [Google Scholar]
- 83. Bhattacharya AA, Lupher ML, Jr, Staunton DE, et al. : Crystal structure of the A domain from complement factor B reveals an integrin-like open conformation. Structure. 2004;12(3):371–8. 10.1016/j.str.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 84. Krishnan V, Xu Y, Macon K, et al. : The crystal structure of C2a, the catalytic fragment of classical pathway C3 and C5 convertase of human complement. J Mol Biol. 2007;367(1):224–33. 10.1016/j.jmb.2006.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Emsley J, Cruz M, Handin R, et al. : Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J Biol Chem. 1998;273(17):10396–401. 10.1074/jbc.273.17.10396 [DOI] [PubMed] [Google Scholar]
- 86. Santelli E, Bankston LA, Leppla SH, et al. : Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature. 2004;430(7002):905–8. 10.1038/nature02763 [DOI] [PubMed] [Google Scholar]
- 87. Xiong JP, Stehle T, Diefenbach B, et al. : Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294(5541):339–45. 10.1126/science.1064535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xiong JP, Stehle T, Zhang R, et al. : Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296(5565):151–5. 10.1126/science.1069040 [DOI] [PubMed] [Google Scholar]
- 89. Nermut MV, Green NM, Eason P, et al. : Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7(13):4093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xiao T, Takagi J, Coller BS, et al. : Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432(7013):59–67. 10.1038/nature02976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rui X, Mehrbod M, van Agthoven JF, et al. : The α-subunit regulates stability of the metal ion at the ligand-associated metal ion-binding site in β 3 integrins. J Biol Chem. 2014;289(33):23256–63. 10.1074/jbc.M114.581470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alonso JL, Essafi M, Xiong JP, et al. : Does the integrin alphaA domain act as a ligand for its betaA domain? Curr Biol. 2002;12(10):R340–2. 10.1016/S0960-9822(02)00852-7 [DOI] [PubMed] [Google Scholar]
- 93. Shimaoka M, Salas A, Yang W, et al. : Small molecule integrin antagonists that bind to the β 2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity. 2003;19(3):391–402. 10.1016/S1074-7613(03)00238-3 [DOI] [PubMed] [Google Scholar]
- 94. Sen M, Yuki K, Springer TA: An internal ligand-bound, metastable state of a leukocyte integrin, α Xβ 2. J Cell Biol. 2013;203(4):629–42. 10.1083/jcb.201308083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lau T, Kim C, Ginsberg MH, et al. : The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 2009;28(9):1351–61. 10.1038/emboj.2009.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hughes PE, Diaz-Gonzalez F, Leong L, et al. : Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem. 1996;271(12):6571–4. 10.1074/jbc.271.12.6571 [DOI] [PubMed] [Google Scholar]
- 97. Ginsberg MH: Integrin activation. BMB Rep. 2014;47(12):655–9. 10.5483/BMBRep.2014.47.12.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang J, Ma YQ, Page RC, et al. : Structure of an integrin αIIbβ3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proc Natl Acad Sci U S A. 2009;106(42):17729–34. 10.1073/pnas.0909589106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sharma CP, Ezzell RM, Arnaout MA: Direct interaction of filamin (ABP-280) with the beta 2-integrin subunit CD18. J Immunol. 1995;154(7):3461–70. [PubMed] [Google Scholar]
- 100. Liu J, Das M, Yang J, et al. : Structural mechanism of integrin inactivation by filamin. Nat Struct Mol Biol. 2015;22(7):383–9. 10.1038/nsmb.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Calderwood DA, Campbell ID, Critchley DR: Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14(8):503–17. 10.1038/nrm3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Anthis NJ, Wegener KL, Ye F, et al. : The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009;28(22):3623–32. 10.1038/emboj.2009.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Klapproth S, Sperandio M, Pinheiro EM, et al. : Loss of the Rap1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice. Blood. 2015;126(25):2704–12. 10.1182/blood-2015-05-647453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Su W, Wynne J, Pinheiro EM, et al. : Rap1 and its effector RIAM are required for lymphocyte trafficking. Blood. 2015;126(25):2695–703. 10.1182/blood-2015-05-644104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Moser M, Nieswandt B, Ussar S, et al. : Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–30. 10.1038/nm1722 [DOI] [PubMed] [Google Scholar]
- 106. Ye F, Petrich BG, Anekal P, et al. : The mechanism of kindlin-mediated activation of integrin αIIbβ3. Curr Biol. 2013;23(22):2288–95. 10.1016/j.cub.2013.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Harburger DS, Bouaouina M, Calderwood DA: Kindlin-1 and -2 directly bind the C-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284(17):11485–97. 10.1074/jbc.M809233200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bledzka K, Liu J, Xu Z, et al. : Spatial coordination of kindlin-2 with talin head domain in interaction with integrin β cytoplasmic tails. J Biol Chem. 2012;287(29):24585–94. 10.1074/jbc.M111.336743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Metelitsa LS, Gillies SD, Super M, et al. : Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcγRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–73. 10.1182/blood.V99.11.4166 [DOI] [PubMed] [Google Scholar]
- 110. Todd RF 3rd, Arnaout MA, Rosin RE, et al. : Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984;74(4):1280–90. 10.1172/JCI111538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Arnaout MA, Wang EA, Clark SC, et al. : Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J Clin Invest. 1986;78(2):597–601. 10.1172/JCI112615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shattil SJ, Kashiwagi H, Pampori N: Integrin signaling: the platelet paradigm. Blood. 1998;91(8):2645–57. [PubMed] [Google Scholar]
- 113. Vedder NB, Harlan JM: Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988;81(3):676–82. 10.1172/JCI113372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim M, Carman CV, Yang W, et al. : The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha} L{beta} 2. J Cell Biol. 2004;167(6):1241–53. 10.1083/jcb.200404160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Takagi J, Petre BM, Walz T, et al. : Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110(5):599–11. 10.1016/S0092-8674(02)00935-2 [DOI] [PubMed] [Google Scholar]
- 116. Xiong JP, Stehle T, Goodman SL, et al. : New insights into the structural basis of integrin activation. Blood. 2003;102(4):1155–9. 10.1182/blood-2003-01-0334 [DOI] [PubMed] [Google Scholar]
- 117. Mahalingam B, Ajroud K, Alonso JL, et al. : Stable coordination of the inhibitory Ca 2+ ion at the metal ion-dependent adhesion site in integrin CD11b/CD18 by an antibody-derived ligand aspartate: implications for integrin regulation and structure-based drug design. J Immunol. 2011;187(12):6393–401. 10.4049/jimmunol.1102394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Adair BD, Xiong JP, Maddock C, et al. : Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J Cell Biol. 2005;168(7):1109–18. 10.1083/jcb.200410068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cox D, Smith R, Quinn M, et al. : Evidence of platelet activation during treatment with a GPIIb/IIIa antagonist in patients presenting with acute coronary syndromes. J Am Coll Cardiol. 2000;36(5):1514–9. 10.1016/S0735-1097(00)00919-0 [DOI] [PubMed] [Google Scholar]
- 120. Ye F, Liu J, Winkler H, et al. : Integrin α IIbβ 3 in a Membrane Environment Remains the Same Height after Mn 2+ Activation when Observed by Cryoelectron Tomography. J Mol Biol. 2008;378(5):976–86. 10.1016/j.jmb.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ye F, Hu G, Taylor D, et al. : Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188(1):157–73. 10.1083/jcb.200908045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dai A, Ye F, Taylor DW, et al. : The Structure of a Full-length Membrane-embedded Integrin Bound to a Physiological Ligand. J Biol Chem. 2015;290(45):27168–75. 10.1074/jbc.M115.682377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fan Z, McArdle S, Marki A, et al. : Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis. Nat Commun. 2016;7:12658. 10.1038/ncomms12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Friedland JC, Lee MH, Boettiger D: Mechanically activated integrin switch controls alpha 5beta 1 function. Science. 2009;323(5914):642–4. 10.1126/science.1168441 [DOI] [PubMed] [Google Scholar]
- 125. Puklin-Faucher E, Sheetz MP: The mechanical integrin cycle. J Cell Sci. 2009;122(Pt 2):179–86. 10.1242/jcs.042127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Morse EM, Brahme NN, Calderwood DA: Integrin cytoplasmic tail interactions. Biochemistry. 2014;53(5):810–20. 10.1021/bi401596q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Coller BS: Translating from the rivers of Babylon to the coronary bloodstream. J Clin Invest. 2012;122(11):4293–9. 10.1172/JCI66867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yonekawa K, Harlan JM: Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77(2):129–40. 10.1189/jlb.0804460 [DOI] [PubMed] [Google Scholar]
- 129. Major EO: Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. 10.1146/annurev.med.080708.082655 [DOI] [PubMed] [Google Scholar]
- 130. Mestas J, Hughes CC: Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8. 10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- 131. Aster RH, Curtis BR, McFarland JG, et al. : Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost. 2009;7(6):911–8. 10.1111/j.1538-7836.2009.03360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cox D: Oral GPIIb/IIIa antagonists: what went wrong? Curr Pharm Des. 2004;10(14):1587–96. 10.2174/1381612043384673 [DOI] [PubMed] [Google Scholar]
- 133. Zhu J, Choi WS, McCoy JG, et al. : Structure-guided design of a high-affinity platelet integrin αIIbβ3 receptor antagonist that disrupts Mg 2+ binding to the MIDAS. Sci Transl Med. 2012;4(125):125ra32. 10.1126/scitranslmed.3003576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cox D, Brennan M, Moran N: Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–20. 10.1038/nrd3266 [DOI] [PubMed] [Google Scholar]
- 135. Blue R, Kowalska MA, Hirsch J, et al. : Structural and therapeutic insights from the species specificity and in vivo antithrombotic activity of a novel alphaIIb-specific alphaIIbbeta3 antagonist. Blood. 2009;114(1):195–201. 10.1182/blood-2008-08-169243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van Agthoven JF, Xiong JP, Alonso JL, et al. : Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin. Nat Struct Mol Biol. 2014;21(4):383–8. 10.1038/nsmb.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cosimi A, Dehnadi A, Smith R, et al. : Targeting Leukocyte Integrin CD11b/CD18 With a Novel mAb Salvages Renal Function Following an Otherwise Irreversible Ischemic Insult in Cynomolgus Monkeys.[abstract]. Am J Transplant. 2015;15(suppl 3). Reference Source [Google Scholar]