ABSTRACT
BACKGROUND AND PURPOSE
Two definitions of T1 hypointense (T1H) lesions can be derived from pre‐contrast images: those that may or may not have a corresponding gadolinium‐enhancing correlate on post‐contrast images (T1H total), and those that are simultaneously non‐gadolinium‐enhancing on post‐contrast scans (T1H non‐enhancing). To determine the differences in lesion evolution between these two T1H definitions, we examined the effect of glatiramer acetate 40 mg/mL three times weekly subcutaneous injection (GA40) on the number of new or enlarging T1H total and T1H non‐enhancing lesions in patients with relapsing‐remitting multiple sclerosis (RRMS).
METHODS
The Phase III GALA study randomized 1404 RRMS subjects 2:1 to receive GA40 or placebo for 12 months. MRI scans were obtained at baseline and at months 6 and 12. Cumulative numbers of T1H total and of T1H non‐enhancing lesions were analyzed using an adjusted negative binomial regression model. A total of 1,357 patients had MRI data collected at either the month 6 or month 12 visit.
RESULTS
Among the 1,357 patients with MRI scans performed at either the month 6 or month 12 visit, 883 treated with GA40 developed an adjusted cumulative mean of 1.72 T1H total lesions versus 2.62 in 440 placebo controls (risk ratio, .66; 95% CI, .54‐.80; P < .0001). On T1H non‐enhanced scans, GA40‐treated patients developed an adjusted cumulative mean of 1.35 T1H non‐enhancing lesions versus 1.91 in placebo controls (risk ratio, .71; CI, .58‐.87; P = .0009).
CONCLUSIONS
GA40 significantly reduced the number of new or enlarging T1H total lesions and T1H non‐enhancing lesions compared with placebo. Although the treatment effect magnitude was comparable with both definitions, the use of T1H non‐enhancing lesions may be more relevant for more uniform standardization in future clinical trials.
Keywords: Glatiramer acetate, relapsing‐remitting multiple sclerosis, T1 hypointense lesions, gadolinium‐enhancing lesions
Introduction
Conventional MRI techniques, such as T2‐weighted and gadolinium‐enhanced T1‐weighted sequences, are sensitive indicators of disease activity in subjects with multiple sclerosis (MS).1, 2 However, these methods are limited in their ability to depict underlying disease pathology. T1‐weighted post‐contrast, gadolinium‐enhanced images are capable of identifying newly forming active lesions associated with increased vascular permeability and inflammation.1, 2 On T1‐weighted pre‐contrast scans, focal areas appearing hypointense compared with surrounding, normal‐appearing white matter are termed T1 hypointense (T1H) lesions.3 T1H lesions appearing on pre‐contrast T1‐weighted images (WI) may capture various pathologic hallmarks of lesion evolution, including demyelination, axonal loss, and tissue loss, and have been termed “black holes.”3 In subjects with relapsing‐remitting MS (RRMS), the formation of new T1H black holes can yield important information with regard to areas of matrix destruction, and their presence has been associated with MS‐related disability.4, 5
Therefore, monitoring the conversion of new active lesions into permanent T1H black holes has been used for more than a decade as a complementary approach for evaluating the clinical efficacy of disease‐modifying therapies.3, 6, 7 The assessment of new active lesions converting into permanent T1H black holes allows for detection of more advanced brain tissue deterioration in subjects with MS.8 Placebo‐controlled studies have shown that, over a 6‐12 month period, approximately 25‐40% of active MS lesions will evolve into T1H lesions.6, 7 , 9, 10, 11, 12
In addition to monitoring conversion of new active lesions into permanent T1H black holes, counting of new or enlarging T1H lesions represents a relatively novel MRI outcome to determine efficacy of disease‐modifying treatment in MS clinical trials.13, 14, 15, 16, 17 Two definitions of T1H lesions can be applied on pre‐contrast T1‐WI: (i) T1H lesions that may or may not have corresponding gadolinium‐enhancing correlates on post‐contrast T1‐WI (T1H total), and (ii) T1H lesions that are non‐gadolinium‐enhancing on post‐contrast scans (T1H non‐enhancing). When evaluating MRI measures of disease activity in clinical trials, specifically the effects of treatment on the formation of new T1H lesions, there are no defined guidelines indicating whether T1H total or T1H non‐enhancing lesion counts should be assessed.
Glatiramer acetate (GA) is an immunomodulating drug approved for the treatment of RRMS.18 The Glatiramer Acetate Low‐frequency Administration (GALA) study was a Phase III trial conducted to investigate the efficacy and safety of a GA 40 mg/mL subcutaneous injection administered three times weekly (GA40) over 12 months to patients with RRMS. The GALA study showed that GA40 significantly reduced the number of confirmed relapses and the number of cumulative gadolinium‐enhancing T1 and T2 lesions compared with placebo in patients with RRMS.19 Compared with placebo, GA40 also significantly reduced the mean number (.31 versus .45; P = .0258) and proportion (15.8% versus 19.6%; P = .006) of new lesions converting to T1H lesions at month 12.12
The purpose of this study was to compare outcomes of using the two definitions for determining accumulation of new or enlarging lesions T1H lesions most commonly used in clinical trials,13, 14, 15, 16 using MRI data from a Phase III trial. This issue is of great importance, as variable new or enlarging lesion T1H outcomes in different clinical trials have been reported,13, 14, 15, 16, 17 and it is difficult to compare the findings of these studies without the definitions being standardized. By performing a post‐hoc analysis of the MRI data collected in the GALA study to examine the effect of GA40 treatment versus placebo on the development of cumulative new or enlarging T1H lesions as assessed by T1H total and T1H non‐enhancing lesion counts, we aimed to provide evidence that will support uniform standardization of new or enlarging T1H lesion definition outcomes in future clinical trials.
Methods
Study Design and Patients
GALA was a randomized, placebo‐controlled, parallel‐group, Phase III study conducted at 142 sites in 17 countries (Protocol MS‐GA‐301; EudraCT No: 2009‐018084‐27).19 All institutional review boards or ethics committees of the participating centers approved the protocol, and all patients gave written informed consent before any study‐related procedures were performed. GALA was conducted in accordance with good clinical practice and International Conference on Harmonization guidelines. Study progress was overseen by an independent data‐monitoring committee.
Eligible subjects (n = 1,404) were randomized in a 2:1 ratio to receive GA40 (n = 943) or matched placebo (n = 461) injections for 12 months (Fig 1). Subjects were eligible for study participation if they were 18‐55 years of age and had a confirmed diagnosis of RRMS (revised McDonald criteria),20 an Expanded Disability Status Scale score ≤5.5, and were relapse‐free for at least 30 days prior to screening. Subjects with progressive forms of MS were excluded, as were those previously treated with GA or any other glatiramoid.
Figure 1.
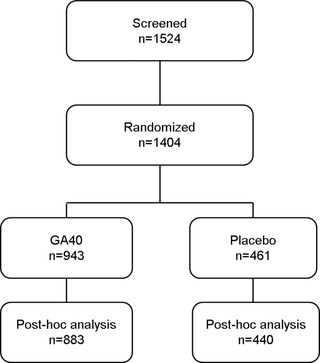
Study design. GA40 = glatiramer acetate 40 mg/mL three times weekly.
T1‐Weighted MRI Assessments
MRI scans were obtained at baseline and at months 6 and 12 according to a standard protocol provided by the MRI reading center (Buffalo Neuroimaging Analysis Center, Buffalo, NY, USA). Before scanning study participants, MRI facilities underwent a qualification procedure to ensure that appropriate images were produced for measuring the endpoints specified by the study protocol. The following MRI scans were obtained, with and without gadolinium contrast, according to a standard protocol: 2D T2/PD‐WI, 3D T1‐WI, 2D fluid attenuation inversion recovery (FLAIR), and 2D spin‐echo (SE) T1‐WI. All MRI scans were transferred to the MRI reading center, where they were evaluated for the purpose of performing quantitative measurements. All brain slices were 3‐mm thick, except for the 3D T1‐WI, in which slices were 1.5‐mm thick. Additional geometry and other sequence parameters were standardized between the sites to the greatest extent possible. For this post‐hoc study, we used 2D SE T1‐WI with and without gadolinium contrast to obtain the new or enlarging T1H lesion count. Follow‐up images were aligned to baseline using FSL's Linear Image Registration Tool (FLIRT)21 with a rigid body (six degrees of freedom) transformation model and correlation ratio cost function. Analysis involved the synthesis of data across three time points (Fig 2). First, T1‐WI image changes between baseline and 6 months and 6 and 12 months were calculated to create T1‐WI subtraction maps, on which new T1H lesions were manually identified by trained analysts. On these images, areas becoming more hypointense (ie, forming new T1H lesions) appear darker, whereas areas with partial or complete resolution appear lighter.
Figure 2.
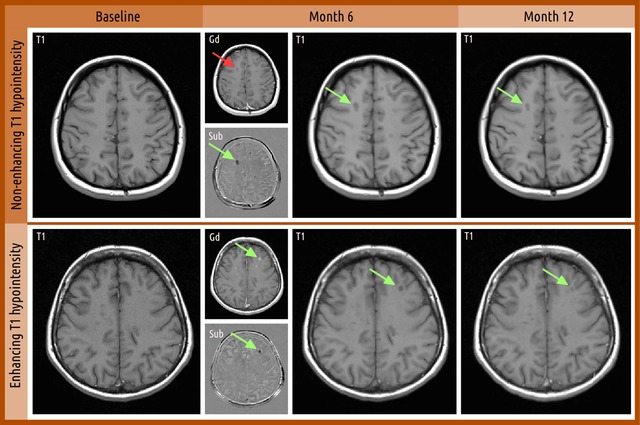
Examples of T1H lesions that may or may not present corresponding gadolinium‐enhancing correlates on post‐contrast T1‐weighted images. Identification of new simultaneously non‐gadolinium‐enhancing T1H lesion at months 6 and 12 versus baseline. For month 6, smaller panels show T1 gadolinium‐enhanced images of the same slice (Gd) and T1 non‐enhancing subtraction images from baseline (Sub). All images were co‐registered to baseline prior to analysis. Green arrows indicate new T1H lesion, and red arrows indicate the lesion location but with negative findings on that particular image. T1H, T1 hypointense.
The cumulative number of new or enlarging lesions on T1‐weighted images taken at months 6 and 12 was calculated as a secondary endpoint in the GALA study. New or enlarging T1H total lesions were quantified regardless of whether they were gadolinium‐enhancing or non‐gadolinium‐enhancing on post‐contrast T1‐weighted scans. New or enlarging T1H total lesions were defined as those that may or may not have corresponding gadolinium‐enhancing correlates on post‐contrast T1‐WI, whereas T1H non‐enhancing lesions were defined as T1H lesions that are non‐gadolinium‐enhancing on post‐contrast scans. Representative MRI images are shown in Figure 2 to outline the definitions of these T1H lesions on T1‐WI.
Statistical Analyses
Cumulative lesion counts were analyzed by an adjusted negative binomial regression model using the number of MRI scans acquired as an offset to adjust for missing data. The model included number of gadolinium‐enhancing T1 lesions on post‐contrast T1‐WI at baseline and country or geographic region as covariates. Lesion counts at each scan were analyzed by an adjusted repeated‐measures negative binomial regression model. The model included the same covariates as the cumulative model as well as terms for visit number and the treatment by visit interaction. An unstructured covariance structure was used to model within‐subject covariance between visits. All analyses were performed using SAS statistical software package version 9.3.
Results
Baseline Patient and Disease Characteristics
A total of 1,357 patients (GA40, n = 907; placebo, n = 450) had an evaluable MRI scan at either the month 6 or month 12 visit. The baseline characteristics for these patients were generally similar between treatment groups (Table 1).
Table 1.
Baseline Patient Characteristics by Treatment Assignment.
| GA40 (n = 907) | Placebo (n = 450) | |
|---|---|---|
| Age, mean years ± SD | 37.3±9.4 | 38.±9.3 |
| Female, n (%) | 615 (67.8) | 305 (67.8) |
| EDSS, mean score ± SD | 2.8±1.2 | 2.8±1.2 |
| Years from first MS symptoms, mean ± SD | 7.7±6.8 | 7.6±6.4 |
| Years from MS diagnosis, mean ± SD | 3.7±5.0 | 3.8±4.7 |
| Patients with ≥1 relapse in 1 year prior to screening, n (%) | 879 (96.9) | 442 (98.2) |
| Number of relapses 1 year prior to screening, mean ± SD | 1.3±0.6 | 1.3±0.6 |
| Patients with ≥1 T1 gadolinium‐enhancing lesion(s) at baseline, n (%) | 326 (35.9) | 152 (33.8) |
| Number of T1 gadolinium‐enhancing lesion(s), mean ± SD | 1.7±4.8 | 1.4±3.7 |
| Volume (cc) of T2 lesion(s), mean ± SD | 19.8±20.8 | 17.5±17.1 |
EDSS = Expanded Disability Status Scale; GA40 = glatiramer acetate 40 mg/mL three times weekly; SD = standard deviation.
Cumulative Number of New or Enlarging T1H Total Lesions
Patients treated with GA40 had a significantly lower adjusted mean cumulative number of new or enlarging T1H total lesions at months 6 and 12 (1.72; 95% CI, 1.48‐2.00) compared with placebo controls (2.62; 95% CI, 2.18‐3.16; risk ratio, .66; 95% CI, .54‐.80; P < .0001) (Fig 3).
Figure 3.
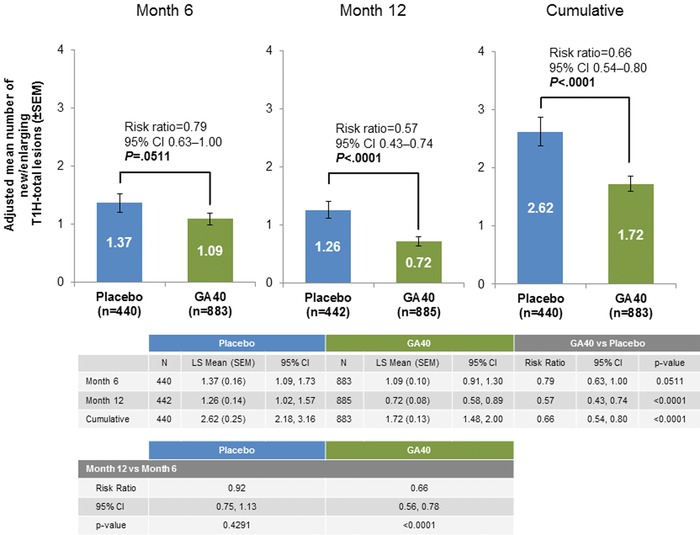
Adjusted mean cumulative number of new or enlarging T1H total lesions at months 6 and 12. GA40 = glatiramer acetate 40 mg/mL three times weekly; SEM = standard error of the mean; T1H, T1 hypointense.
At month 6, there was a trend toward a lower adjusted mean number of new or enlarging T1H total lesions for patients treated with GA40 versus placebo (risk ratio, .79; 95% CI, .63‐1.00; P = .0511). From baseline to month 12, GA40‐treated patients had a significantly lower adjusted mean number of new or enlarging T1H total lesions compared with those treated with placebo (risk ratio, .57; 95% CI, .43‐.74; P <. 0001). Further analyses showed that there was a significant decrease in the adjusted mean number of new or enlarging T1H total lesions for patients treated with GA40 at month 12 versus month 6 (risk ratio, .66; 95% CI, .56‐.78; P < .0001), whereas placebo‐treated patients did not demonstrate such changes (risk ratio, .92; 95% CI, .75‐1.13; P = .4291) (Fig 4).
Figure 4.
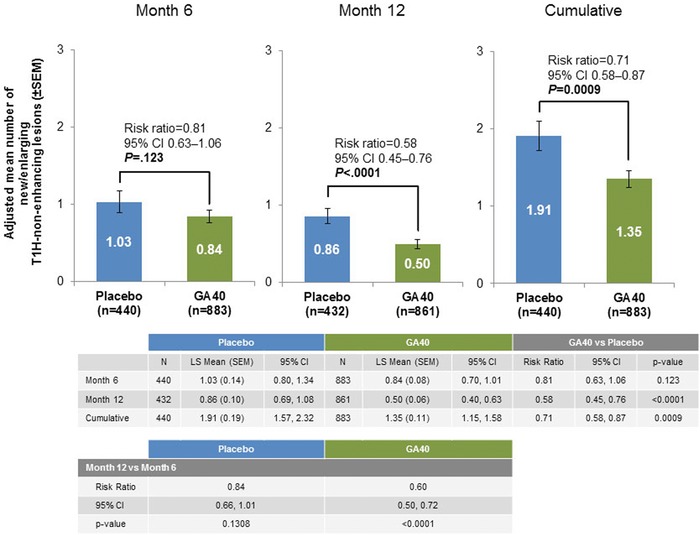
Adjusted mean cumulative number of new or enlarging T1H non‐enhancing lesions at months 6 and 12. GA40 = glatiramer acetate 40 mg/mL three times weekly; SEM = standard error of the mean; T1H, T1 hypointense.
Cumulative Number of New or Enlarging T1H Non‐Enhancing Lesions
Patients treated with GA40 had a significantly lower adjusted mean cumulative number of active, new or enlarging T1H non‐enhancing lesions at months 6 and 12 (1.35; 95% CI, 1.15‐1.58) compared with placebo‐treated patients (1.91; 95% CI, 1.57‐2.32; risk ratio, .71; 95% CI, .58‐.87; P = .0009) (Fig 4).
Patients treated with GA40 had a significantly lower adjusted mean number of new or enlarging T1H non‐enhancing lesions compared with those treated with placebo when evaluated at month 12 (risk ratio, .58; 95% CI, .45‐.76; P < .0001) but not at month 6. As seen with analyses of T1H total lesions, there was a significant decrease in the adjusted mean number of new or enlarging T1H non‐enhancing lesions for patients treated with GA40 at month 12 versus month 6 (risk ratio, .60; 95% CI, .50‐.72; P < .0001), without such changes in the placebo‐treated patients (risk ratio, .84; 95% CI, .66‐1.01; P = .1308) (Fig 4).
Discussion
Evidence from placebo‐controlled studies correlating the cumulative number of new, gadolinium‐enhancing lesions to the number of relapses suggests that accurate analysis and interpretation of MRI outcomes may be informative in predicting MS clinical course.22 Clinical trials of potential neuroreparative therapies are becoming more important in the spectrum of MS research. Imaging techniques used in modern MS clinical trials are required to be feasible from a time and practicality point of view, sensitive and specific for detection of demyelination and axonal loss, reproducible, and clinically meaningful.23 Although the use of non‐conventional MRI techniques, such as magnetization transfer, diffusion tensor, and position emission tomography imaging, have great potential to identify specific pathological substrate changes over time, their use in clinical MS trials has been limited in the past.24
In the last decade, the assessment of conversion of new active lesions into permanent T1H black holes has become frequently used for evaluating the clinical efficacy of disease‐modifying therapies.3, 6, 7 Measurement of T1H black holes may capture various aspects of lesion evolution, such as demyelination and axonal loss.4, 5, 8
Nevertheless, less is known about the pathobiologic correlates of counting new or enlarging T1H lesions, which is becoming more commonly used as a secondary MRI outcome in clinical MS trials.13, 14, 15, 16, 17 Although this outcome may have lower sensitivity and specificity than the conversion of new active inflammatory lesions into permanent T1H black holes, it is simple to obtain and may add additional value versus conventional counting of new or enlarging gadolinium‐enhancing lesions and T2 lesions.
In this study, treatment with GA40 significantly reduced both the number of new or enlarging T1H total lesions and new or enlarging T1H non‐enhancing lesions compared with placebo. The treatment effects of GA40 on T1H total and T1H non‐enhancing lesion counts were comparable numerically; nonetheless, these two lesion definitions may represent distinct type of lesions. Therefore, distinguishing between T1H total and T1 non‐enhancing lesions may provide important insight on discrete disease processes and how they relate to disability progression and disease stage in MS, as well to the treatment effect. This hypothesis is supported by previous research examining the evolution of MS lesions as assessed by signal intensity on T1‐WI over time and the respective changes in magnetization transfer ratio (MTR).5, 25 The recent application of MTR in the assessment of the stage and extent of MS has suggested an inverse correlation between demyelination and the degree of MTR within the tissue. Thus, correlations between MTR values and lesion signal intensities on T1‐WI may elucidate the subtle differences between T1H total and T1H non‐enhancing lesions.
A study by Hiehle and colleagues found no difference in MTR as a function of T1H lesion enhancement in 133 white matter lesions from 17 consecutive patients.26 However, the MTRs of T1H non‐gadolinium‐enhancing lesions were significantly lower than the MTRs of T1‐weighted non‐gadolinium‐enhancing lesions that appeared isointense to the surrounding white matter. A study by Rovira et al also found that the MTRs of T1H lesions were significantly lower than those in isointense non‐enhancing lesions.27 Over a 12‐month follow‐up, the MTRs of isointense non‐enhancing lesions showed no significant variation from baseline values, indicating moderate but persistent demyelination with little matrix destruction. The MTRs of T1H non‐enhancing lesions showed nominal but significant increases in MTR from baseline, suggesting a demyelination and axonal loss with progressive remyelination. Interestingly, Rovira et al found a marked reduction in the size or complete disappearance of T1H ring‐enhancing lesions over 12 months that corresponded with significantly increased MTRs, suggesting a dynamic and reversible process of expansion of the extracellular space surrounding the lesion because of fluid accumulation in response to a severe inflammatory reaction.
Taken together, the observations of Rovira and Hiehle support the hypothesis that the presence (eg, T1H ring‐enhancing lesions) or absence (eg, T1H and T1 isointense lesions) of contrast uptake in T1H lesions may be an indicator of different lesion evolution in MS. Whereas T1H lesions with areas of gadolinium enhancement may represent an inflammatory and potentially reversible disease process with less significant demyelination, T1H non‐enhancing lesions (including the central hypointense portion of ring‐enhancing lesions) may reflect severe considerable demyelination and axonal loss.
Compared with conventional MRI measures, changes in MTR over time can indicate lesion repair and axonal remyelination and, therefore, may correlate better with the evolution of pathologic changes and clinical disability. While we did not use non‐conventional MRI measures such as MTR to define pathobiologic substrates of new or enlarging T1H total lesions and new or enlarging T1H non‐enhancing lesions in the current study, previous work has demonstrated significantly increased MTR values in T1H non‐enhancing lesions in RRMS patients over 12 months with GA (20 mg/mL once daily) treatment. These findings indicate that treatment with GA may aid in the recovery of lesional tissue and provide a greater potential for remyelination of T1H lesions and can highlight important outcome measures that may be used to determine the efficacy of treatments in future trials.25
The results of the current study are in line with the recent findings from the GALA study demonstrating that GA40 favorably influenced MRI outcomes indicative of tissue destruction in patients with RRMS.12 Compared with placebo, GA40 significantly reduced the number of T1H black holes that evolved from new active inflammatory lesions at month 6 and suppressed the evolution of new active inflammatory lesions into T1H black holes at month 12.
This study is not without limitation. This was a post‐hoc analysis in which the predefined study protocol only explored the accumulation of new or enlarging T1H lesions between the two study treatment arms. The analysis of new T1H lesions at months 6 and 12 in the present study does not definitely distinguish between persisting (chronic) black holes or temporary (subacute) black holes, as some lesions could have become T1H lesions over longer period of follow‐up. Therefore, the results of this study should be interpreted with some caution and confirmed over longer term follow‐up. In addition, no clinical associations of the two T1H lesion measures were explored, because the duration of the study was too short to detect any relevant changes in disability outcomes over 12 months. Therefore, clinical trials of longer study duration are needed to determine clinical relevance of counting T1H total and T1H non‐enhancing lesions on development of disability and progression of brain atrophy.
Overall, the findings of this analysis demonstrate that GA40 favorably influences MRI outcomes, as indicated by a reduction in the number of T1H lesions indicative of tissue destruction and axonal loss in patients with RRMS. Although the magnitude of treatment effect was comparable with both definitions, the study suggests that use of T1H non‐enhancing lesions may be more relevant for more uniform standardization in future clinical trials.
Acknowledgements
This study was funded by Teva Pharmaceutical Industries, Petach Tikva, Israel. We thank the patients and site personnel involved with this study; Nicholas Gross and Svetlana Rubinchick (Teva Pharmaceuticals) for assistance with study conduct and statistical analyses; and Peter Feldman, PhD (Teva Pharmaceuticals) and Lisa Grauer, MSc (Chameleon Communications International with funding from Teva Pharmaceutical Industries) for editorial assistance in the preparation of this report.
Acknowledgments and Disclosures: Dr. Zivadinov has received compensation for speaking and consulting from Teva Pharmaceutical Industries, Biogen Idec, EMD Serono, Inc, Novartis, Claret, and Sanofi‐Genzyme, and has received research support from Biogen Idec, Teva Pharmaceutical Industries, Claret, Sanofi‐Genzyme, Novartis, and EMD Serono, Inc.
Dr. Dwyer has received compensation for consulting from Claret Medical and for serving on a scientific advisory board from EMD Serono, and has received research support from Novartis International.
Mr. Ramasamy has no disclosures to report.
Dr. Davis and Dr. Steinerman are employees of Teva Pharmaceutical Industries.
Dr. Khan has received compensation for consulting from Biogen Idec, Genzyme, and Novartis, and for serving on speaker bureaus from Teva, Novartis, and Biogen Idec, and has received research support from NIH, NINDS, NMSS, Teva, Biogen Idec, Genzyme, Roche, and Novartis.
References
- 1. Rovira A, Auger C, Alonso J. Magnetic resonance monitoring of lesion evolution in multiple sclerosis. Ther Adv Neurol Disord 2013;6:298‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zivadinov R, Bakshi R. Role of MRI in multiple sclerosis II: brain and spinal cord atrophy. Front Biosci 2004;9:647‐64. [DOI] [PubMed] [Google Scholar]
- 3. Sahraian MA, Radue EW, Haller S, et al. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 2010;122:1‐8. [DOI] [PubMed] [Google Scholar]
- 4. van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 1999;46:747‐54. [DOI] [PubMed] [Google Scholar]
- 5. van Waesberghe JH, van Walderveen MA, Castelijns JA, et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1‐weighted spin‐echo and magnetization transfer MR. AJNR Am J Neuroradiol 1998;19:675‐83. [PMC free article] [PubMed] [Google Scholar]
- 6. Filippi M, Rovaris M, Rocca MA, et al. Glatiramer acetate reduces the proportion of new MS lesions evolving into "black holes". Neurology 2001;57:731‐3. [DOI] [PubMed] [Google Scholar]
- 7. Cadavid D, Cheriyan J, Skurnick J, et al. New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta‐1b or glatiramer acetate. J Neurol Neurosurg Psychiatry 2009;80:1337‐43. [DOI] [PubMed] [Google Scholar]
- 8. van Walderveen MA, Barkhof F, Pouwels PJ, et al. Neuronal damage in T1‐hypointense multiple sclerosis lesions demonstrated in vivo using proton magnetic resonance spectroscopy. Ann Neurol 1999;46:79‐87. [DOI] [PubMed] [Google Scholar]
- 9. Filippi M, Rocca MA, Camesasca F, et al. Interferon ‐1b and glatiramer acetate effects on permanent black hole evolution. Neurology 2011;76:1222‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filippi M, Rocca MA, Pagani E, et al. Placebo‐controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry 2014;85:851‐8. [DOI] [PubMed] [Google Scholar]
- 11. Nagtegaal GJ, Pohl C, Wattjes MP, et al. Interferon beta‐1b reduces black holes in a randomised trial of clinically isolated syndrome. Mult Scler 2014;20:234‐42. [DOI] [PubMed] [Google Scholar]
- 12. Zivadinov R, Dwyer M, Barkay H, et al. Effect of glatiramer acetate three‐times weekly on the evolution of new, active multiple sclerosis lesions into T1‐hypointense "black holes": a post hoc magnetic resonance imaging analysis. J Neurol 2015;62:648‐53. [DOI] [PubMed] [Google Scholar]
- 13. Arnold DL, Gold R, Kappos L, et al. Effects of delayed‐release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol 2014;261:1794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barkhof F, Calabresin PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009;5:256‐66. [DOI] [PubMed] [Google Scholar]
- 15. Radue EW, O'Connor P, Polman CH, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol 2012;69:1259‐69. [DOI] [PubMed] [Google Scholar]
- 16. Wolinsky JS, Narayana PA, Nelson F, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler 2013;19:1310‐9. [DOI] [PubMed] [Google Scholar]
- 17. Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol 2014;13:977‐86. [DOI] [PubMed] [Google Scholar]
- 18.Teva Neuroscience. Copaxone® (glatiramer acetate) solution for subcutaneous injection: full prescribing information, FDA‐approved labeling. North Wales, PA 2014.
- 19. Khan O, Rieckmann P, Boyko A, et al. Three times weekly glatiramer acetate in relapsing‐remitting multiple sclerosis. Ann Neurol 2013;73:705‐13. [DOI] [PubMed] [Google Scholar]
- 20. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 2005;58:840‐6. [DOI] [PubMed] [Google Scholar]
- 21. Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825‐41. [DOI] [PubMed] [Google Scholar]
- 22. Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double‐blind, randomized, placebo‐controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol 2001;49:290‐7. [PubMed] [Google Scholar]
- 23. Mallik S, Samson RS, Wheeler‐Kingshott CA, et al. Imaging outcomes for trials of remyelination in multiple sclerosis. J Neurol Neurosurg Psychiatry 2014;85:1396‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zivadinov R, Stosic M, Cox JL, et al. The place of conventional MRI and newly emerging MRI techniques in monitoring different aspects of treatment outcome. J Neurol 2008;255 Suppl 1:61‐74. [DOI] [PubMed] [Google Scholar]
- 25. Zivadinov R, Hussein S, Bergsland N, et al. Magnetization transfer imaging of acute black holes in patients on glatiramer acetate. Front Biosci (Elite Ed) 2012;4:1496‐504. [DOI] [PubMed] [Google Scholar]
- 26. Hiehle JF Jr, Grossman RI, Ramer KN, et al. Magnetization transfer effects in MR‐detected multiple sclerosis lesions: comparison with gadolinium‐enhanced spin‐echo images and nonenhanced T1‐weighted images. AJNR Am J Neuroradiol 1995;16:69‐77. [PMC free article] [PubMed] [Google Scholar]
- 27. Rovira A, Alonso J, Cucurella G, et al. Evolution of multiple sclerosis lesions on serial contrast‐enhanced T1‐weighted and magnetization‐transfer MR images. AJNR Am J Neuroradiol 1999;20:1939‐45. [PMC free article] [PubMed] [Google Scholar]


