Abstract
Background
Patients with “refractory angina” (RA) unsuitable for coronary revascularization experience high levels of hospitalization and poor health‐related quality of life. Randomized trials have shown spinal cord stimulation (SCS) to be a promising treatment for chronic stable angina and RA; however, none has compared SCS with usual care (UC). The aim of this pilot study was to address the key uncertainties of conducting a definitive multicenter trial to assess the clinical and cost‐effectiveness of SCS in RA patients, i.e., recruitment and retention of patients, burden of outcome measures, our ability to standardize UC in a UK NHS setting.
Methods
RA patients deemed suitable were randomized in a 1:1 ratio to SCS plus UC (SCS group) or UC alone (UC group). We sought to assess: recruitment, uptake, and retention of patients; feasibility and acceptability of SCS treatment; the feasibility and acceptability of standardizing UC; and the feasibility and acceptability of the proposed trial outcome measures. Patient outcomes were assessed at baseline (prerandomization) and three and six months postrandomization.
Results
We failed to meet our planned recruitment target (45 patients) and randomized 29 patients (15 SCS group, 14 UC group) over a 42‐month period across four sites. None of the study participants chose to withdraw following consent and randomization. With exception of two deaths, all completed evaluation at baseline and follow‐up. Although the study was not formally powered to compare outcomes between groups, we saw a trend toward larger improvements in both primary and secondary outcomes in the SCS group.
Conclusions
While patient recruitment was found to be challenging, levels of participant retention, outcome completion, and acceptability of SCS therapy were high. A number of lessons are presented in order to take forward a future definitive pragmatic randomized trial.
Keywords: Randomized controlled trial, refractory angina, spinal cord stimulation
Introduction
Refractory angina (RA) has been defined as “a chronic condition characterized by the presence of angina caused by coronary insufficiency in the presence of coronary artery disease which cannot be controlled by a combination of medical therapy, angioplasty and coronary bypass surgery. The presence of reversible myocardial ischaemia should be clinically established to be the cause of the symptoms. Chronic is defined as a duration of more than 3 months” 1. The commonest causes of unsuitability for revascularization are unfavorable coronary anatomy, unsuccessful response to receiving one or more previous coronary bypass procedures or percutaneous angioplasties, lack of suitable grafting material, significant extra cardiac comorbidities, or advanced age, often in combination of the above 1, 2, 3, 4. Both increasing success and innovation in conventional approaches to treat angina and better survival rates following primary and subsequent coronary events have led to significant proportions of patients presenting with angina refractory to conventional treatment 4. The most recent published estimate of the incidence of RA is 25,000 to 75,000 new cases per year and an estimated prevalence of 300,000 to 900,000 patients in the United States 5. The wide variability in prevalence and incidence figures may be due to a lack of a standardized definition, as the diagnosis of refractory angina depends on the lack of revascularization alternatives, which vary in different contexts according to the availability of revascularization technology, appropriateness of medical treatment, and other interventions targeted to control anginal pain 6. RA patients experience severe chest pain leading to a high rate of hospitalization, resource use, and poor levels of health‐related quality of life (HRQoL) 1, 7.
RA patients can be offered a number of therapies including anti‐angina drugs, sympathectomy, analgesics, angina management cognitive behavioral therapy, stimulation‐induced analgesia, stellate ganglion block, epidural blocks, spinal cord stimulation (SCS), enhanced external counterpulsation, and percutaneous laser myocardial revascularization 3, 8, 9, 10.
It has been suggested that SCS produces its effects in refractory angina via an interaction of the following mechanisms: 1) pain reduction; 2) a reduction of sympathetic tone; 3) reduced myocardial oxygen demand; and 4) improved coronary microcirculatory blood flow, resulting in a lessening of myocardial ischemia 11. Attenuation of pain is accomplished by stimulation of the dorsal columns and an accompanying decrease in the transmission of nociceptive impulses via the spinothalamic tract. This may occur via an increase in γ‐amino butyric acid (GABA) release from dorsal horn interneurons and also by release of beta‐endorphins, which is associated with decreased myocardial oxygen consumption. Human studies of norepinephrine spillover as well as other animal models have shown a clear effect of SCS on the sympathetic system with consequent reduction in oxygen demand 12, 13, 14. Further improvement of myocardial ischemia occurs due to improved blood flow at the coronary microvascular level, which is supported by studies showing increased homogenization of myocardial blood flow 15, 16. Animal models have shown that SCS modifies the capacity of intrinsic cardiac neurons to generate activity 17. SCS also acts to suppress the excitatory effects that local myocardial ischemia exerts on such neurons.
Two systematic reviews and meta‐analyses have indicated that SCS can improve anginal symptoms, functional status, and HRQoL of individuals with RA with no negative effects on mortality 18, 19. However, trials to date have been small, range considerably in their methodological quality, and have included variable and nonpragmatic comparators. In 2008, the National Institute for Health and Care Excellence (NICE) in the United Kingdom concluded that there was inadequate evidence to recommend the routine use of SCS and called for a pragmatic randomized controlled trial (RCT) that compares SCS against current usual care management for RA 20, 21.
The overarching aim of this pilot study was to assess the feasibility of undertaking a definitive multicenter trial to assess the clinical effectiveness and cost‐effectiveness of adding SCS to usual care in patients with RA. Before undertaking such a definitive study, a number of uncertainties exist. The specific objectives of this study were to address these key uncertainties: 1) assess recruitment, uptake, and retention of patients; 2) assess the feasibility and acceptability of SCS treatment from the point of view of patients and referring physicians; 3) assess the feasibility and acceptability of standardizing usual care; and 4) test the feasibility and acceptability of the proposed trial outcome measures in both groups.
Methods
The study design and methods have been described in the published study protocol 22 and are summarized here.
Study Design
The Refractory Angina Spinal Cord stimulation and usuAL care study (RASCAL) study is a pragmatic multicenter external pilot RCT that allocated RA patients to receive SCS plus usual care (“SCS group”) or usual care alone (“UC group”). Recruitment took place in four English centers (The James Cook University Hospital, South Tees Hospitals NHS Foundation Trust; Russells Hall Hospital, Dudley Group of Hospitals NHS Foundation Trust; Basildon University Hospital, Basildon and Thurrock University Hospitals NHS Foundation Trust; and Derriford Hospital, Plymouth Hospitals NHS Trust) between January 2011 and June 2014.
Given the paresthesia associated with SCS, it was not possible to blind patients, clinicians, or researchers to group allocation. However, given the potential observer bias associated with assessing exercise capacity, we sought to blind those conducting this outcome assessment. The research nurses conducting the remainder of the data collection were independent of the delivery of patient care in the trial.
The study was reviewed by the Ethics committee NRES committee UK North East Northern and Yorkshire and received a favorable opinion in July 2011 reference: 11/NE/0175. The study was registered with ISRCTN65254102. All patients provided written informed consent prior to randomization.
Study Population
Patients were eligible for inclusion if they were 18 years of age or older with limiting angina despite optimal anti‐angina therapy, had a Canadian Cardiovascular Society functional classification of angina of Class III or IV, and had angiographically documented Ccoronary artery disease that was deemed not suitable for revascularization by the referring cardiologist or cardiothoracic surgeon. In addition, all eligible patients had to show demonstrable ischemia on functional testing and satisfactory multidisciplinary assessment in accordance with the British Pain Society guidelines for SCS 23.
Exclusions included a pacemaker or implanted defibrillator incompatible with SCS, a comorbidity considered by the assessing clinician to overshadow the effect of the angina or render a patient an unsuitable candidate for neuromodulation (e.g., advanced spinal disease or deformity), poor cognitive ability, ongoing anticoagulation therapy (where anticoagulants cannot be safely discontinued without jeopardizing patient safety), or refusal to participate in the study.
Patients fulfilling these criteria were consented and offered entry to the study.
Study Procedure
Eligible patients were randomized sequentially to either the SCS or the UC group in a 1:1 ratio. The randomization sequence was computer generated and stratified by hospital, age, gender, and Canadian Cardiovascular Society functional classification of angina. The randomization sequence was prepared by the trial statistician (RT) and allocation concealment maintained by only revealing allocation of each participant by email correspondence to the study coordinator following completion of baseline outcomes.
Interventions
SCS
For the patients allocated to the SCS group, the intervention was started within six weeks from the date of randomization with an acute on‐table trial of SCS followed by full implantation if the trial was successful. In case of failure to obtain >80% paresthesia coverage of the painful area or if painful sensations were generated when the temporary stimulator was switched on, the procedure was aborted and the leads removed with no full implantation (“failed trial”). Participants who had a failed trial of SCS continued to receive treatment and follow‐up as per the study protocol (both groups were to receive UC).
The SCS implant was performed by an experienced health professional (i.e., ≥15 SCS implants in the previous 12 months) at the Pain Departments across the four study sites. The procedure consisted of inserting one or two epidural leads of SCS through a needle into a thoracic epidural interspace under local anesthetic with patients either in prone, lateral, or sitting position according to patient and operator preference. The leads were positioned at C7/T1 (N = 7), T11/T12 (N = 1), T1/T2 (N = 5), and C2 (N = 1). Once in position, the leads were connected to an external stimulator and the position adjusted to obtain optimum coverage of the painful area. In cases where the paresthesia covered 80% or more of the painful area, the leads were anchored to the spine via a small incision and connected via tunneled subcutaneous extensions, where required, to an implanted pulse generator placed in the anterior abdominal wall or the buttock. Implanted participants were instructed on how to adjust their SCS device to generate a comfortable paresthesia level. They were instructed to do this regularly for two hours, three times per day to terminate any angina attack for as long as is necessary or before any exertion known or anticipated to generate angina pain.
UC
In order to standardize the delivery of UC, it was agreed by the site investigators that both groups of participants across the four research sites be offered the following sequence of UC therapies: an education session with a pain consultant; trial of a transcutaneous electrical nerve stimulation (TENS); serial thoracic sympathectomy (where no medical contraindications existed); and oral or systemic analgesics and adjuvant analgesia. UC was based on a survey of current RA management in the UK reported in the study protocol 22. It should be noted that thoracic sympathectomy, although common practice in the UK, may not be routinely used in other countries and is not recommended in the European Society of Cardiology 24 and American College of Cardiology/American Heart Association guidelines 25. These therapies were started, if possible, on the day of randomization and were received sequentially where this was felt to be clinically appropriate by the investigator. Participants who had already tried and failed to obtain relief from any of the sequence of therapies above, were moved onto the next therapy. Following completion of the above sequence, the treating physician was able to apply any therapy deemed appropriate, with the exception of repeat coronary artery bypass graft, percutaneous revascularization (or stenting), percutaneous myocardial laser revascularization, or enhanced external counterpulsation. Any patient with a significant and sudden downturn in their symptoms could be reevaluated by their cardiologists, including repeat angiography and a reappraisal of revascularization options if the angiographic findings had changed. Given the pragmatic nature of this pilot trial, we did not seek to control the pattern of accepted UC treatments received by participants in either SCS or UC groups over the period of study. However, UC treatments received by both groups were documented.
Outcome Measures
We collected the following pilot study outcomes:
Recruitment and retention: We documented procedures for recruiting patients in both groups and any problems that have arisen during this process. Retention was assessed by documenting the number of dropouts and lost to follow‐up in both groups.
Feasibility and acceptability of design: We quantified the time for attainment of recruitment numbers against preplanned targets, the proportion of suitable patients who failed to provide consent, and the ratio of participants screened vs. randomized.
Feasibility and acceptability of treatment: Patients were asked to assess their willingness to recommend SCS or UC to other patients. This was assessed by three questions based on a Likert response scale: Are you satisfied with the pain relief provided by your treatment? Very Satisfied/Satisfied/Unsatisfied/Very Unsatisfied; Based on your experience so far, would you have agreed to this treatment? Definitely Yes/Yes/No/Definitely No; Can you tell us how acceptable these treatments were to you? [list of treatments provided] Extremely acceptable/Acceptable/Not acceptable/Extremely unacceptable /Not Applicable.
Feasibility and acceptability of proposed patient outcome measures: quantified as the number of returned and complete outcome assessments available for assessment.
Outcome variance: Mean and standard deviation for all outcomes were calculated for each group at all assessment visits (to inform power calculations for a definitive trial).
Patient outcome measures were collected at baseline (prerandomization), and three and six months postrandomization by a clinic visit undertaken by the research nurse. The study's primary outcome parameter was disease‐specific HRQoL assessed using the UK version of the Seattle Angina Questionnaire (SAQ) 26. The SAQ quantifies patients' physical limitations caused by angina, the frequency of and recent changes in their symptoms, their satisfaction with treatment, and the degree to which they perceive their disease to affect their quality of life. Each scale is transformed to a score of 0 to 100, where higher scores indicate better function (e.g., less physical limitation, less angina, and better quality of life). Secondary outcomes included: angina attacks recorded by diary for a week; exercise capacity assessed by a symptom‐limited treadmill; and generic HRQoL assessed using the EuroQol (EQ‐5D‐3L) converted into single (utility) indices using the UK tariff 27 and Short Form‐36 (SF‐36) questionnaires 28, 29. Exercise capacity was assessed at baseline and six months follow‐up only. Where patients were unable or unwilling to undertake a symptom‐limited treadmill test, a six‐minute walk test was instead undertaken. To allow comparison over time, each participant performed the same exercise tests at baseline and follow‐up. The nature and frequency of device‐specific and nondevice complications and adverse events (serious and nonserious) were collected for both groups at each follow‐up visit and in‐between visits.
Statistical Analysis
Study sample size was chosen to inform the feasibility objectives of this study, i.e., provide sufficiently precise estimates of the likely rates of recruitment and retention, and to yield estimates of the variability of the primary and secondary outcomes to inform power calculations for a subsequent full‐scale RCT. We sought to recruit and randomize a total of 45 RA patients (15 per center) over a 20‐month period.
Given the pilot nature of this trial, we did not seek to formally inferentially test and report p values for the comparison of outcomes either between or within SCS and UC groups. Instead, we report mean and standard deviation (or relevant summary statistics) for both groups for all assessments at each follow‐up point based on the intention‐to‐treat principle (i.e., according to the original randomization). We also report the mean (and 95% CIs) between group difference in outcomes at three‐ and six‐month follow‐up adjusting for baseline outcome score and stratification variables. The between‐group difference in angina frequency is also reported as a rate ratio. All analyses were conducted using STATA (Release 13.1; College Station, TX, USA: StataCorp LP).
Results
Recruitment, Retention, and Feasibility of Design
Study enrollment, allocation to the SCS and UC groups, and follow‐up of study participants are summarized in the CONSORT flow diagram shown in Figure 1. Sixty‐seven patients were screened and a total of 29 RA patients were randomized (SCS group: 15; UC group: 14), a screening to randomization ratio of 2.3:1. The original forecast was a recruitment rate of 1.5 patients per month. However, the actual recruitment rate across the initial three sites was 0.7 patients per month. Given this lower recruitment rate, the period of recruitment had to extend by 22 months and took place over a total of 42 months (January 2011 to June 2014) and a fourth center was opened (on month 24).
Figure 1.
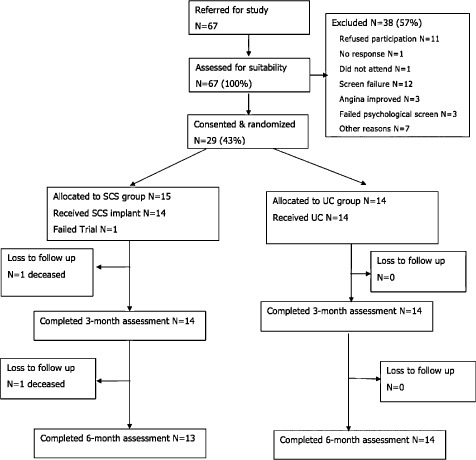
RASCAL study CONSORT diagram.
None of the patients recruited into the study declined his/her treatment allocation and none of the referring physicians counseled patients against their treatment allocation. No participants chose to withdraw from the study. No patients required repeat angiography during the study period.
Baseline Characteristics and Assessments
There appeared to be good balance between SCS and UC group participants in terms of their baseline demographic characteristics (Table 1). However, there was evidence of imbalance in some of the primary and secondary assessment scores as summarized in Table 1. Other cardiovascular morbidities included hypercholesterolemia (N = 23), family history of coronary artery disease (N = 22), hypertension (N = 19), insulin‐dependent diabetes mellitus (N = 5), body mass index > 30 (N = 14), and smoking (current smoker N = 3; previous smoker N = 19). Previous treatments include coronary artery bypass graft (N = 24), TENS (N = 7), stents (N = 14), percutaneous coronary intervention (N = 6), stellate ganglion blocks (N = 3), and anti‐angina medications (N = 29). Seventy‐six percent (22/29) of the patients were taking eight or more different cardiovascular medications, including anticoagulants, antiplatelets, beta blockers, ACE‐inhibitors or angiotensin receptor blockers, aldosterone antagonists, and the full range of available antianginal agents.
Table 1.
Baseline Demographic Characteristics
| UC group, N = 14 | SCS group, N = 15 | |
|---|---|---|
| N (%) or mean (SD) | N (%) or mean (SD) | |
| Participant characteristics | ||
| Gender (number of men) | 10 (71%) | 11 (73%) |
| Age (years) | 66.5 (2.1) | 65.4 (3.0) |
| BMI (kg/m2) | 30.9 (6.9) | 32.5 (5.8) |
| Ethnicity | ||
| Caucasian | 14 (100%) | 15 (100%) |
| CCS Classification | ||
| Class III | 9 (64.3%) | 9 (60%) |
| Class IV | 5 (35.7%) | 6 (40%) |
BMI, body mass index; CCS, Canadian Cardiovascular Society functional classification of angina; SCS, spinal cord stimulation; UC, usual care.
Acceptability of Treatment Received
Participants in both SCS and UC groups generally rated their acceptability with treatment as good (Table 2). The number of patients receiving UC therapies was equal to the number of responders to the patient's satisfaction/acceptability questionnaire. In the SCS group, one patient received thoracic sympathectomy after randomization but before SCS implantation. Eight patients used TENS following randomization and until the SCS implantation. Patients in the SCS group did not use TENS following SCS implant. Median satisfaction with pain relief and agreement to receipt of treatment were 4 (i.e., rating of “very satisfied” and “definitely yes,” respectively) in the SCS group and 3 (i.e. rating of “satisfied” and “yes”) in the UC group. There was some evidence of higher scores for SCS than UC. Acceptability of specific UC therapies was variable.
Table 2.
Patient Satisfaction at Six‐Month Follow‐Up
| SCS group | UC group | |
|---|---|---|
| N median (range) | N median (range) | |
| Satisfied with the pain relief *? | 13 4 (2 to 4) | 14 3 (2 to 4) |
| Based on experience so far, would you have agreed to this treatment † ? | 13 4 (2 to 4) | 14 3 (2 to 4) |
| How acceptable was each treatment ‡ ? | ||
| Education session with consultant | 12 4 (3 to 4) | 13 4 (3 to 4) |
| TENS machine | 8 2 (1 to 4) | 12 3 (1 to 4) |
| Thoracic sympathectomy | 1 1 (1 to 1) | 4 3.5 (2 to 4) |
| Medications | 11 3 (1 to 4) | 14 3 (2 to 4) |
*4: very satisfied; 3: satisfied; 2: not satisfied; 1: very unsatisfied.†4: definitely yes; 3: yes; 2: no; 1: definitely no.‡4: extremely acceptable; 3: acceptable; 2: not acceptable; 1: extremely unacceptable.SCS, spinal cord stimulation; TENS, transcutaneous electrical nerve stimulation; UC, usual care.
Feasibility of Outcome Collection and Outcome Results at Follow‐Up
Primary and secondary outcome measures were completed by all 29 randomized patients at baseline. Allowing for two deaths, outcomes were completed at three and six‐month follow‐up in the 27 remaining participants. While the vast majority of patients undertook the six‐minute walk test, only two patients were able to perform a symptom‐limited treadmill test. Primary and secondary outcome results at three months and six months follow‐up are shown in Table 3. There was a consistent pattern of a positive change in both SCS and UC groups in outcomes at three‐ and six‐month follow‐up compared to baseline, i.e., increases in SAQ scores, exercise capacity, EQ‐5D tariff, and SF‐36 scores, and a reduction in the frequency of angina attacks. The magnitude of these outcome improvements appeared to be higher for the SCS than the UC group (Figs. 2, 3, 4, 5, 6).
Table 3.
Primary and Secondary Outcome Scores at Baseline and Follow‐Up
| Baseline | Three‐month follow‐up | Six‐month follow‐up | ||||||
|---|---|---|---|---|---|---|---|---|
| SCS group | UC group | SCS group | UC group | SCS‐UC difference* | SCS group | UC group | SCS‐UC difference* | |
| N Mean (SD) | N Mean (SD) | N Mean (SD) | N Mean (SD) | Mean (95% CI) | N Mean (SD) | N Mean (SD) | Mean (95% CI) | |
| Primary outcome | ||||||||
| Seattle Angina Questionnaire | ||||||||
| Physical limitations | 15 33.3 (15.4) | 14 32.1 (15.3) | 14 41.1 (18.6) | 13 33.9 (15.8) | −7.3 (−21.5 to 6.7) | 14 38.5 (19.4) | 14 33.9 (15.8) | −3.5 (−14.6 to 7.7) |
| Angina stability | 15 36.7 (18.5) | 14 26.8 (6.7) | 14 44.6 (20.0) | 14 32.1 (15.3) | −8.2 (−23.7 to 7.2) | 13 46.2 (26.9) | 14 37.5 (19.0) | 0.5 (−16.9 to 17.9) |
| Angina frequency | 15 29.3 (12.8) | 14 27.1 (12.7) | 14 32.8 (23.2) | 14 32.8 (16.8) | −17.8 (−33.1 to −2.5) | 13 46.2 (26.3) | 14 31.4 (21.7) | −12.8 (−29.1 to 3.5) |
| Treatment status | 15 80.0 (23.5) | 14 73.2 (20.7) | 14 85.7 (18.9) | 14 83.9 (18.6) | 4.1 (−9.3 to 17.5) | 13 90.4 (16.2) | 13 76.9 (21.6) | −9.1 (−24.6 to 6.3) |
| Quality of life | 15 36.7 (16.0) | 14 30.4 (10.6) | 14 55.4 (20.0) | 14 35.7 (16.1) | −19 (−32.6 to −5.3) | 13 63.5 (21.9) | 14 42.8 (22.8) | −22.3 (−39.2 to −5.3) |
| Secondary outcomes | ||||||||
| Frequency of angina attacks (per week) | 15 22.0 (17.1) | 14 14.6 (13.6) | 12 11.2 (18.1) | 14 16.1 (15.1) |
−8 (−22 to 6) Rate ratio: 0.48 (0.37 to 0.62) |
13 13.1 (19.8) | 14 19.5 (20.9) |
−11 (−27 to 5) Rate ratio: 0.41 (0.33 to 0.52) |
| Exercise capacity | ||||||||
| Six‐minute walking distance (m) | 13 121 (98) | 12 175 (100) | Not assessed | Not assessed | — | 10 191 (81) | 9 140 (78) | 60 (−4 to 123) |
| EQ‐5D tariff | 15 0.27 (0.34) | 14 0.50 (0.26) | 14 0.53 (0.24) | 14 0.52 (0.22) | −0.1 (−0.3 to 0.1) | 13 0.55 (0.29) | 14 0.48 (0.24) | −0.2 (−0.4 to 0) |
| Short‐Form 36 | ||||||||
| Physical functioning | 15 26.7 (23.4) | 14 22.5 (17.4) | 14 32.5 (16.9) | 14 25.0 (21.6) | 4.5 (−7.9 to 16.8) | 13 31.1 (20.2) | 14 28.6 (28.0) | 0.1 (−18.8 to 18.9) |
| Role limitations caused by physical problems | 15 3.3 (8.8) | 14 1.8 (6.7) | 14 16.1 (23.2) | 14 5.4 (14.5) | 10.6 (−5.8 to 27.1) | 13 13.4 (21.9) | 14 5.4 (10.6) | 5.9 (−6.4 to 18.2) |
| Role limitations caused by emotional problems | 15 33.3 (43.6) | 14 33.3 (43.6) | 14 69.0 (46.2) | 14 47.6 (42.8) | 26.6 (−.6.6 to 59.4) | 13 71.7 (40.5) | 14 40.4 (47.2) | 28.8 (−2.6 to 60.3) |
| Energy/vitality | 15 28.7 (21.2) | 14 27.5 (17.6) | 13 38.8 (16.1) | 14 26.8 (15.9) | 9.6 (−1.5 to 20.7) | 13 38.8 (18.5) | 14 26.8 (18.5) | 16.4 (4.6 to 28.2) |
| Mental health | 15 53.3 (23.5) | 14 58.8 (19.7) | 13 67.1 (23.4) | 14 62.3 (20.9) | 8.5 (−5.2 to 22.2) | 13 72.6 (16.6) | 14 63.7 (17.8) | 11.5 (1.3 to 21.7) |
| Social functioning | 15 30.8 (24.5) | 14 41.1 (13.4) | 15 49.1 (34.8) | 14 45.5 (18.7) | 6.8 (−11.4 to 25.1) | 13 52.9 (24.6) | 14 47.3 (23.1) | 9.8 (−7.1 to 26.6) |
| Bodily pain | 15 22.2 (14.5) | 14 29.6 (10.4) | 13 36.9 (18.3) | 14 35.4 (17.7) | 4.0 (−11.6 to 19.6) | 13 42.3 (16.9) | 14 35.4 (11.3) | 8.8 (−3.7 to 21.4) |
| General health | 15 56.0 (10.6) | 14 53.2 (8.4) | 13 57.7 (11.6) | 14 50.7 (9.2) | 3.3 (−5.1 to 11.7) | 13 54.2 (7.9) | 14 52.1 (12.0) | −0.1 (−7.4 to 7.2) |
*Between‐group difference adjusted for baseline score and stratification variables (i.e., center, CCS class, age <65 vs. ≥65 years.CCS, Canadian Cardiovascular Society functional classification of angina; SCS, spinal cord stimulation; UC, usual care.
Figure 2.
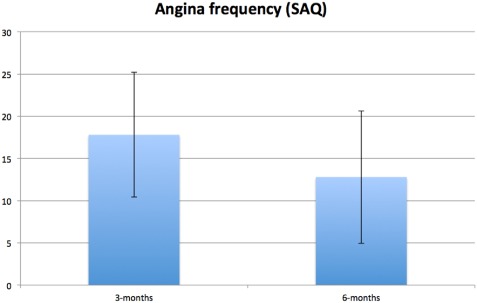
Between‐group mean difference at three and six months for angina frequency (SAQ) adjusted for baseline score and stratification variables (i.e., center, CCS class, age <65 vs. ≥65 years). Error bars represent standard error of difference. Positive between‐group difference in SAQ indicates superior scores for SCS group compared with UC group. CCS, Canadian Cardiovascular Society functional classification of angina; SAC, Seattle Angina Questionnaire; SCS, spinal cord stimulation; UC, usual care.
Figure 3.
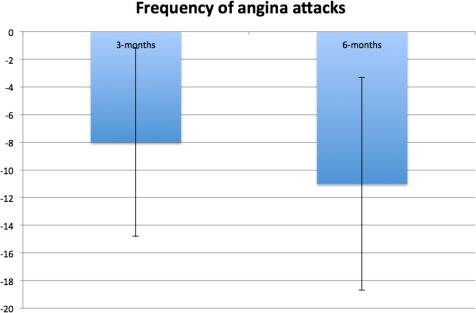
Between‐group mean difference at three and six months for frequency of angina attacks adjusted for baseline score and stratification variables (i.e., center, CCS class, age <65 vs. ≥65 years). Error bars represent standard error of difference. Negative between‐group difference indicates larger reduction in angina frequency in SCS group compared with UC group. CCS, Canadian Cardiovascular Society functional classification of angina; SCS, spinal cord stimulation; UC, usual care.
Figure 4.
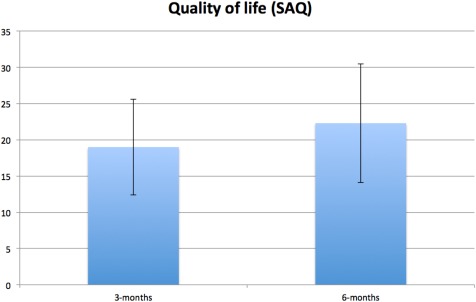
Between‐group mean difference at three and six months for SAQ angina quality of life score adjusted for baseline score and stratification variables (i.e., center, CCS class, age <65 vs. ≥65 years). Error bars represent standard error of difference. Positive between‐group difference in SAQ indicates superior scores for SCS group compared with UC group. CCS, Canadian Cardiovascular Society functional classification of angina; SAC, Seattle Angina Questionnaire; SCS, spinal cord stimulation; UC, usual care.
Figure 5.
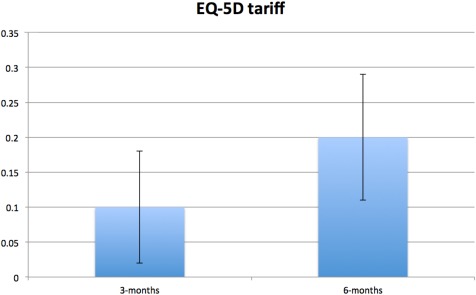
Between‐group mean difference at three and six months for EQ‐5D tariff adjusted for baseline score and stratification variables (i.e., center, CCS class, age <65 vs. ≥65 years). Error bars represent standard error of difference. Positive between‐group difference in EQ‐5D tariff indicates superior scores for SCS group compared with UC group. CCS, Canadian Cardiovascular Society functional classification of angina; SCS, spinal cord stimulation; UC, usual care.
Figure 6.
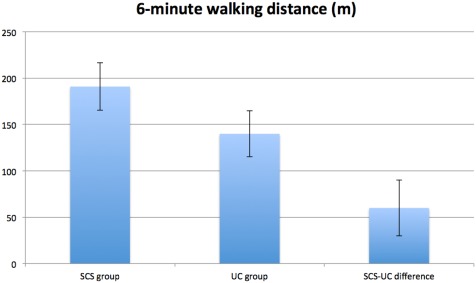
Exercise capacity at six months for SCS and UC group and between‐group mean difference for six‐minute walking distance adjusted for baseline score and stratification variables (i.e., center, CCS class, age <65 vs. ≥65 years). Error bars represent standard error of difference. Positive between‐group difference in six‐minute walking distance indicates superior scores for SCS group compared with UC group. CCS, Canadian Cardiovascular Society functional classification of angina; SCS, spinal cord stimulation; UC, usual care.
Adverse Events
Two participants died of causes related to their RA (i.e., cardiogenic shock and cardiac arrest) but were judged to be unrelated to study procedures. Both were randomized to the SCS group; one receiving a permanent implant and the other failing the SCS screening trial.
A total of 25 adverse events were recorded over the six‐month study follow‐up (see Table 4). Of these, four were related to SCS therapy: two cases of superficial infection, one case of pain over the implant, and one case of inadequate paresthesia coverage. Five adverse events were related to antiangina therapy and included three cases of headache, one case of diplopia, and one case of dizziness. Twelve participants required hospitalization (six SCS group, six UC group), five for the treatment of chest pain, one for intravenous antibiotic treatment of SCS‐related infection. Five admissions were unrelated to angina or study procedures (hemiplegic migraine and epilepsy, vestibular pathology, pulmonary edema and septic shock, migraine, cellulitis left leg). The patient admitted with pulmonary edema and septic shock made a full recovery.
Table 4.
Serious Adverse Events Observed During the Study Period
| Site | Randomized to: | Serious adverse event | Category | Device related |
|---|---|---|---|---|
| 01 | UC | Hemiplegic migraine and epilepsy | Hospitalization | No |
| UC | Central chest pain (unstable angina) | Hospitalization | No | |
| UC | Migraine and nonepileptic seizures, right occipital nerve neuralgia and chest pain | Hospitalization | No | |
| SCS | Ongoing microvascular angina | Hospitalization | No | |
| 02 | SCS | Exacerbation of angina—unrelieved by GTN spray | Hospitalization | No |
| SCS | Cardiogenic shock | Death | No | |
| 03 | UC | Chest pains | Hospitalization | No |
| UC | Chest pains | Hospitalization | No | |
| SCS | Cardiac arrest | Death | No | |
| SCS | Vestibular pathology | Hospitalization | No | |
| SCS | SCS infection | Hospitalization | Yes | |
| UC | Pulmonary edema and septic shock | Hospitalization | No | |
| SCS | Migraine | Hospitalization | No | |
| SCS | Cellulitis left hand | Hospitalization | No | |
| 04 | No SAEs reported |
GTN, glyceryl trinitrate; SAE, serious adverse event; SCS, spinal cord stimulation; UC, usual care.
Discussion
A pilot study was undertaken to inform progression to a fully powered randomized trial to determine the clinical effectiveness and cost‐effectiveness of the addition of SCS to UC compared with UC alone in patients with RA. Patient recruitment was found to be challenging in this pilot; we failed to achieve the target study sample size, recruiting and randomizing 29 patients out of the original target of 45. Nevertheless, once patients were randomized, we found participant retention and outcome completion to be very high; except for two patients who died due to their disease during the study, there was no loss to follow‐up and all participants provided outcomes at three‐ and six‐month follow‐up.
Although not formally powered to compare outcomes either within or between groups, we did see evidence of an improvement in HRQoL demonstrated by the disease‐specific SAQ as well as generic quality of life questionnaires, exercise capacity, and reduction in angina frequency at follow‐up in both the SCS and UC groups, with a trend towards larger improvements in the SCS group. This trend can be partly explained by baseline differences and without a sham comparator we cannot rule out the possibility of a placebo effect contributing to improvements with SCS. In addition, SCS appeared to be a safe therapy in RA; there was one device‐related serious adverse event over the six‐month follow‐up period of the trial. One patient randomized to SCS had a failed test trial, a success rate of 93%. These findings are in agreement with those of previous clinical studies and systematic reviews of SCS in RA 18, 19, 30, 31, 32. RA is a condition affecting an elderly population with numerous comorbidities. We did not find this to be a barrier to SCS implant or usage in this population.
The main strengths of this study were its randomized trial design and the ability to retain patients once recruited. However, the pilot did have limitations. Although study logs recorded 67 patients to be screened, it was apparent from discussions with the study sites that many more patients were deemed to not fulfill the study entry criteria from the referral letter such as: no clear demonstrable ischemia, severe spinal pain, and the presence of other implanted devices. Slow patient recruitment, resulting in study termination, has been previously reported in an evaluation of SCS for RA; however, the possible reasons for slow recruitment were not discussed 32. Causes for the slow recruitment in our study include higher than expected screening failures; the majority of screening failures were subjects referred with symptoms typical of RA but no demonstrable reversible ischemia on functional testing. The requirement to demonstrate reversible ischemia on functional testing was agreed by the study steering committee at its initial meeting to be important to provide a homogenous population suffering from chest pain related to ischemia rather than other potential causes such as atypical and functional chest pain. We believe that this single inclusion criterion had the most profound effect on our ability to recruit to this study. The requirement for demonstration of ischemia as the etiology of RA is explicit in the definition of RA coined by the European Task Force 33. By contrast, the UK RA group defined the condition as chronic stable angina that persists despite optimal medication and when revascularization is unfeasible or where the risks are unjustified 34. It has been recently proposed that the definition of RA should be more precise by specifying the reason for revascularization unsuitability, such as microvascular dysfunction, epicardial stenosis, or generalized lesions 35. Finally, the study encountered protracted negotiations with the Primary Care Trusts relating to excess treatment costs (ETC) associated with the devices and the implant procedure, which resulted in significant delays.
Implications for Planning a Future Trial
The study demonstrates the importance of a pilot and feasibility research prior to conducting a fully funded powered RCT 36. The key finding from the pilot trial is the need for a multifaceted approach to ensure the recruitment of patients in order to meet the needs of a future definitive trial. Based on a minimally important difference of 10 37 on the SAQ and the conservative estimates of both standard deviation of 20 and attrition rate of 20% (except for two deaths, we saw no loss to follow‐up in this pilot), a definitive trial will need to recruit 110 patients per group at 90% power and 5% alpha. Future studies exploring SCS use in RA may wish to adopt the UK group definition, which does not stipulate the demonstration of reversible ischemia as a cause for the angina but takes a more patient‐centered view of the condition. We observed a variation in the point of diagnosing RA between the sites. The definition of RA was seen to differ in some centers where some of the research sites required an attempt of all potential medication choices before diagnosing patients as suffering from RA and referring to the study. Recruitment rates may be increased with greater education regarding the treatment options for these patients and the involvement of general practitioners and patient groups. SCS implantation by cardiologists should also be considered. We however would like to point out that we have during the recruitment period of this study contacted GP practices and invited them to refer patients with no success. A number of elderly subjects required some assistance with outcome measure completion from the study nurse. The six‐minute walk test was more acceptable to an RA population than the treadmill test. A future trial should include use of the six‐minute walk test in preference to the treadmill as well as provision of a study‐independent professional to conduct exercise tests in a blinded manner. The patients randomized to UC considered the treatment options available to be acceptable and based on their experience would have agreed to receive these treatments. Subgroup analysis should include analysis of patients allocated to the SCS group with respect to prior treatments with therapies affecting the nervous system, such as TENS, stellate ganglion blockade, or sympathectomy vs. naive patients. We conclude that the study outcome measures are acceptable and appropriate for a future RCT. Collection of some of the interim data by post with telephone guidance where required should be considered by a future RCT. This will reduce the patient burden in an elderly population with multiple comorbidities.
Conclusion
In summary, there were several difficulties associated with recruitment of patients for this study, including a higher than expected screening failure, difficulty in confirming whether individual patients met the entry criteria of reversible ischemia, a lack of consensus on the point at which a diagnosis of RA is reached, and protracted negotiations with local hospitals and primary care trusts relating to agreement of device and procedure costs for the trial. By contrast, we managed to retain and collect three‐ and six‐month outcomes from all surviving participants. We found SCS therapy and trial outcome measures to be acceptable and appropriate for future RCTs. Although we were not formally powered to investigate within‐ or between‐group treatment differences, compared with baseline, there were trends across a number of the outcomes (including exercise capacity and HRQoL) of larger improvements in the SCS compared with UC group at three and six months. In addition, SCS appeared to be a safe therapy; there was one device‐related serious adverse event over the six‐month follow‐up period of the trial.
Acknowledgements
We wish to thank Dr. Duncan McNab for chairing the study steering committee. We acknowledge the unrestricted grant of some SCS device materials from Medtronic and Boston Scientific.
Authorship Statements
Rui Duarte, Sam Eldabe, Rod Taylor, and Morag Brookes authored the final manuscript; all authors commented on the manuscript and approved the final manuscript. Sam Eldabe, Simon Thomson, Jon Raphael, Mark deBelder, and Ed Davies recruited patients into the study on the four sites. Mark deBelder acted as study cardiology advisor, Morag Brookes was study coordinator, and Rod Taylor was study statistician.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to
Funding sources: This article presents independent research funded by the National Institute of Health Research (NIHR) under its Research for Patient Benefit (RfPB) program (grant reference: PB‐PG‐1208‐18031). Rod Taylor is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLARHC) at the Royal Devon and Exeter NHS Foundation Trust. The views expressed here are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The NIHR had no role in study design, writing of the manuscript or the decision to submit for publication.
Conflict of Interest: The authors wish to disclose the following competing interests: Sam Eldabe and Rod Taylor have received consultancy fees from Medtronic. Rod Taylor is currently an independent member of Trial Steering Committees for PROCESS and SubQStim, two ongoing Medtronic‐funded studies. Simon Thomson has received consultancy fees from Boston Scientific. The other authors have no conflicts of interest.
References
- 1. Mannheimer C, Camici P, Chester MR et al. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 2002;23:355–370. [DOI] [PubMed] [Google Scholar]
- 2. Henry TD, Satran D, Hodges JS et al. Long‐term survival in patients with refractory angina. Eur Heart J 2013;34:2683–2688. [DOI] [PubMed] [Google Scholar]
- 3. Henry TD, Satran D, Jolicoeur EM. Treatment of refractory angina in patients not suitable for revascularization. Nat Rev Cardiol 2014;11:78–95. [DOI] [PubMed] [Google Scholar]
- 4. DeJongste MJ, Tio RA, Foreman RD. Chronic therapeutically refractory angina pectoris. Heart 2004;90:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manchanda A, Aggarwal A, Aggarwal N, Soran O. Management of refractory angina pectoris. Cardiol J 2011;18:343–351. [PubMed] [Google Scholar]
- 6. Briones E, Lacalle JR, Marin‐Leon I, Rueda JR. Transmyocardial laser revascularization versus medical therapy for refractory angina. Cochrane Database Syst Rev 2015;(2):CD003712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Povsic TJ, Broderick S, Anstrom KJ et al. Predictors of long‐term clinical endpoints in patients with refractory angina. J Am Heart Assoc 2015;4:e001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis–how many patients might be eligible? Am J Cardiol 1999;84:598–600. [DOI] [PubMed] [Google Scholar]
- 9. Chester M, Hammond C, Leach A. Long‐term benefits of stellate ganglion block in severe chronic refractory angina. Pain 2000;87:103–105. [DOI] [PubMed] [Google Scholar]
- 10. van Kleef M, Staats P, Mekhail N, Huygen F. Chronic refractory angina pectoris. Pain Pract 2011;11:476–482. [DOI] [PubMed] [Google Scholar]
- 11. Latif OA, Nedeljkovic SS, Stevenson LW. Spinal cord stimulation for chronic intractable angina pectoris: a unified theory on its mechanism. Clin Cardiol 2001;24:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naver H, Augustinsson LE, EIam M. The vasodilating effect of spinal cord stimulation is mediated by sympathetic nerves. Clin Auton Res 1992;2:41–45. [DOI] [PubMed] [Google Scholar]
- 13. Norrsell H, Eliasson T, Mannheimer C et al. Effects of pacing‐induced myocardial stress and spinal cord stimulation on whole body and cardiac norepinephrine spillover. Eur Heart J 1997;18:1890–1896. [DOI] [PubMed] [Google Scholar]
- 14. Sanderson JE, Tomlinson B, Lau MS et al. The effect of transcutaneous electrical nerve stimulation (TENS) on autonomic cardiovascular reflexes. Clin Auton Res 1995;5:81–84. [DOI] [PubMed] [Google Scholar]
- 15. Hautvast RW, Blanksma PK, DeJongste MJ et al. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol 1996;77:462–467. [DOI] [PubMed] [Google Scholar]
- 16. Jessurun GA, Tio RA, De Jongste MJ, Hautvast RW, Den Heijer P, Crijns HJ. Coronary blood flow dynamics during transcutaneous electrical nerve stimulation for stable angina pectoris associated with severe narrowing of one major coronary artery. Am J Cardiol 1998;82:921–926. [DOI] [PubMed] [Google Scholar]
- 17. Foreman RD, Linderoth B, Ardell JL et al. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 2000;47:367–375. [DOI] [PubMed] [Google Scholar]
- 18. Börjesson M, Andrell P, Lundberg D, Mannheimer C. Spinal cord stimulation in severe angina pectoris–a systematic review based on the Swedish Council on Technology assessment in health care report on long‐standing pain. Pain 2008;140:501–508. [DOI] [PubMed] [Google Scholar]
- 19. Taylor RS, De Vries J, Buchser E, Dejongste MJ. Spinal cord stimulation in the treatment of refractory angina: systematic review and meta‐analysis of randomised controlled trials. BMC Cardiovasc Disord 2009;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NICE . Technology Appraisal Guidance 159: spinal cord stimulation for chronic pain of neuropathic or ischaemic origin. London: National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
- 21. Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess 2009;13:iii,ix–x,1–154. [DOI] [PubMed] [Google Scholar]
- 22. Eldabe S, Raphael J, Thomson S et al. The effectiveness and cost‐effectiveness of spinal cord stimulation for refractory angina (RASCAL study): study protocol for a pilot randomized controlled trial. Trials 2013;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simpson K, Stannard C, Raphael J. Spinal cord stimulation for the management of pain: recommendations for best clinical practice. London: The British Pain Society, 2009. [Google Scholar]
- 24. Montalescot G, Sechtem U, Achenbach S et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 25. Amsterdam EA, Wenger NK, Brindis RG et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 26. Garratt AM, Hutchinson A, Russell I. Network for evidence‐based practice in Northern and Yorkshire (NEBPINY). The UK version of the Seattle Angina Questionnaire (SAQ‐UK): reliability, validity and responsiveness. J Clin Epidemiol 2001;54:907–915. [DOI] [PubMed] [Google Scholar]
- 27. Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. York: University of York, 1995. [Google Scholar]
- 28. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 29. Brazier J. The Short‐Form 36 (SF‐36) Health Survey and its use in pharmacoeconomic evaluation. Pharmacoeconomics 1995;7:403–415. [DOI] [PubMed] [Google Scholar]
- 30. Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J 1998;136:1114–1120. [DOI] [PubMed] [Google Scholar]
- 31. Eddicks S, Maier‐Hauff K, Schenk M, Müller A, Baumann G, Theres H. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo‐controlled randomised study. Heart 2007;93:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zipes DP, Svorkdal N, Berman D et al. Spinal cord stimulation therapy for patients with refractory angina who are not candidates for revascularization. Neuromodulation 2012;15:550–558. [DOI] [PubMed] [Google Scholar]
- 33. Fox K, Garcia MA, Ardissino D et al. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341–1381. [DOI] [PubMed] [Google Scholar]
- 34. Angina.org, 2015. http://www.angina.org/?page_id=18
- 35. Jolicoeur EM, Cartier R, Henry TD et al. Patients with coronary artery disease unsuitable for revascularization: definition, general principles, and a classification. Can J Cardiol 2012;28:S50–S59. [DOI] [PubMed] [Google Scholar]
- 36. Craig P, Dieppe P, Macintyre S et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spertus JA, Winder JA, Dewhurst TA et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]


