Abstract
Human‐derived placental tissues have been shown in randomized clinical trials to be effective for healing chronic wounds, and have also demonstrated the ability to recruit stem cells to the wound site in vitro and in vivo. In this study, PURION® Processed dehydrated human amnion/chorion membrane allografts (dHACM, EpiFix®, MiMedx Group, Marietta, GA) were evaluated for their ability to alter stem cell activity in vitro. Human bone marrow mesenchymal stem cells (BM‐MSCs), adipose derived stem cells (ADSCs), and hematopoietic stem cells (HSCs) were treated with soluble extracts of dHACM tissue, and were evaluated for cellular proliferation, migration, and cytokine secretion. Stem cells were analyzed for cell number by DNA assay after 24 h, closure of an acellular zone using microscopy over 3 days, and soluble cytokine production in the medium of treated stem cells was analyzed after 3 days using a multiplex ELISA array. Treatment with soluble extracts of dHACM tissue stimulated BM‐MSCs, ADSCs, and HSCs to proliferate with a significant increase in cell number after 24 h. dHACM treatment accelerated closure of an acellular zone by ADSCs and BM‐MSCs after 3 days, compared to basal medium. BM‐MSCs, ADSCs, and HSCs also modulated endogenous production of a number of various soluble signals, including regulators of inflammation, mitogenesis, and wound healing. dHACM treatment promoted increased proliferation and migration of ADSCs, BM‐MSCs, and HSCs, along with modulation of secreted proteins from those cells. Therefore, dHACM may impact wound healing by amplifying host stem cell populations and modulating their responses in treated wound tissues. © 2015 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 104B: 1495–1503, 2016.
Keywords: wound healing, dermal wound dressing, stem cells, amniotic membrane, dHACM
INTRODUCTION
Chronic wound management, including diabetic foot ulcers and venous ulcers, remains a significant clinical challenge, affecting approximately 6.5 million patients in the United States with cost of care exceeding $25 billion annually.1 Expediting wound closure and preventing them from progressing to the point of chronicity is the aim of the clinician treating the patient. When the wound first presents, the initial treatment consists mainly of nonsurgical techniques including: debridement, off‐loading, appropriate dressings, and/or multi‐layer compression therapy. The majority (60–80%) of foot ulcers will heal with this treatment;2 however, of the remainder that progress to the chronic state, tissue damage in these wounds may eventually require amputation of the affected limb. The lack of progression toward healing in chronic wounds is hampered by an impaired response in one or more critical phases of healing, including persistent necrotic inflammation, growth factor deficiency, as well as impaired vascularity and neoangiogenesis, fibroblast and stem cell migration and proliferation in the wound bed, and re‐epithelialization to close the wound.
Adult stem cells are important for the normal maintenance and repair of wounded tissues through their ability to differentiate, remodel extracellular matrix (ECM), modulate the immune response, and secrete growth factors and cytokines that stimulate cell migration and neovascularization.3, 4 Stem cells including mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) are recruited to a healing wound, where they support healing in a variety of ways.3, 4, 5, 6 The healing potential of stem cells has resulted in the development of stem cell therapies for the treatment of chronic wounds. These therapies mainly consist of stem cell transplants which are assumed to be promoting repair through differentiation and paracrine activity. A clinical study comprised of 24 patients presenting with nonhealing wounds were treated with autologous cultured bone‐marrow MSCs (BM‐MSCs) and resulted in a decrease in ulcer size, pain levels, and biochemical parameters.7 The data on transplanted stem cells for treating diabetic wounds is limited; however, these results indicate the therapeutic potential of harnessing the healing power of stem cells.
Mesenchymal stem cells originate in bone‐marrow and can also be derived from adipose tissue (ADSCs). Mesenchymal stem cells are known to differentiate down several cell lineage pathways to form, as examples, cartilage, fat, muscle, and connective tissue; however, they are also actively involved in regulation of wound healing.4, 8 MSCs have been demonstrated to regulate the immune response and inflammation in wounds through the secretion of immunomodulatory cytokines, while also causing enhanced proliferation, migration, and secretion of biologically active molecules by paracrine signaling.4, 8, 9 Studies suggest that paracrine activity of MSCs significantly enhances responsiveness and migration of macrophages, epithelial, and endothelial cells.4, 10 As a result, autologous and allogeneic stem cell therapies have been considered as a form of treatment to stimulate healing of chronic wounds.
An elevated population of HSCs may also impact the presence of other immune related cell types in a wound bed. Multipotent HSCs, found in bone marrow and peripheral blood, proliferate and differentiate into the myeloid or lymphoid cell lineages of blood cells, including lymphocytes, neutrophils, monocytes, and macrophages.11, 12 These immune cells are critical in wound healing where they infiltrate a wound and are responsible for disposing of pathogens and apoptotic cells in blood and tissue, thereby preventing inflammation and eliminating damaged tissue.13 Specifically, macrophages aid in wound healing by accelerating the proliferation of fibroblasts and promoting angiogenesis through the release of basic fibroblast growth factor (bFGF) and platelet‐derived growth factor (PDGF) in the wound and the recruitment of endothelial cells.14
Human amniotic membrane has been applied historically to enhance healing due to its immune privileged and antibacterial properties, along with its ability to modulate inflammation, reduce pain and scarring, and provide a matrix supporting cellular migration and proliferation.15, 16, 17, 18, 19, 20 Recently, multipotent MSCs have also been isolated in placental tissues, including the amnion and chorion layers of the amniotic membrane,21, 22, 23, 24 and these amniotic membrane derived stem cells have demonstrated the ability to regulate immune cells though secretion of various immunomodulatory cytokines.25, 26, 27, 28
Dehydrated human amnion/chorion membrane (dHACM) is a human allograft comprised of laminated amnion and chorion membranes derived from the placenta. The dHACM allografts have been utilized in the treatment of chronic wounds, such as diabetic foot ulcers and venous ulcers.29, 30, 31 Over 50 growth factors, cytokines, chemokines, and tissue inhibitors of metalloproteinases (TIMPs) have been previously identified in dHACM tissues,32, 33 many of which are known to stimulate paracrine responses in cells involved in healing and tissue repair, such as human dermal fibroblasts, microvascular endothelial cells, and stem cells. Previously published animal dermal wound models have shown that dHACM promotes the recruitment of both hematopoietic and mesenchymal stem cells to the treated site of injury.3 Studies measured a significant increase in BM‐MSC recruitment by day 7 of dHACM treatment,33 as well as a ten‐fold increase of migrated HSCs to the wound site by day 14 in a mouse subcutaneous implantation model, accompanied by an increase in SDF‐1α expression.3 These in vivo results suggest that the combined impact of dHACM cytokine protein elution and regulation of stem cell activity at a wound site may be involved in enhancing tissue repair.
Therefore, we hypothesized that a potential mechanism for dHACM's clinical efficacy is the release of regulatory cytokines from dHACM into the wound bed resulting in modulation of various types of stem cells, by promoting their proliferation, migration, and altering their cytokine and chemokine secretion profile. Therefore, the impact of dHACM on various reparative stem cells was evaluated, including BM‐MSCs, ADSCs, and HSCs, using in vitro cell closure and proliferation models, and the measurement of changes in the growth factor/cytokine secretion profile for each cell type to further elucidate the potential mechanisms by which dHACM may enhance healing.
MATERIALS AND METHODS
Cells and cell culture media
Table 1 summarizes sources of cells and cell culture media and media formulations used in experiments.
Table 1.
Sources of ADSC, BM‐MSC, and HSC Cells and Cell Culture Media
| Cell Source | Basal Media (Negative Control) | Complete Media (Positive Control) | |
|---|---|---|---|
| Adipose‐derived stem cells (ADSCs) | Lonza, PT5006, Basel, Switzerland | ADSC media: Lonza, PT3273 plus gentamicin sulfate and l‐glutamine from ADSC kit: Lonza, PT4503 | Basal media plus serum supplement from ADSC kit: Lonza, PT4503 |
| Bone marrow mesenchymal stem cells (BM‐MSCs) | Lonza, PT2501 |
BM‐MSC media: Lonza, PT3238 plus gentamicin sulfate and l‐glutamine from BM‐MSC kit: Lonza, PT4105 |
Basal media plus serum supplement from BM‐MSC kit: Lonza, PT4105 |
| Hematopoietic stem cells (HSCs) | Life Technologies Corp. (Life Tech), A14059, Carlsbad, CA |
Stem Pro 34 media: Life Tech, 10640‐019 plus 1% Penicillin/Streptomycin (Pen/Strep: GE Healthcare, SV30010, Waukesha, WI), 1% 200 mM l‐Glutamine: (GE Healthcare, SH3003401), 100 ng/mL of stem cell factor (SCF: Life Tech, PHC2116), 50 ng/mL of interleukin‐3 (IL‐3: Life Tech, PHC0035), and 25 ng/mL of granulocyte–macrophage colony‐stimulating factor (GM‐CSF: Life Tech, PHC2015) |
Basal media plus 10% StemPro®‐34 Nutrient Supplement: Life Tech, 10641‐025 |
Supplements from media kits were used per manufacturers' instructions.
Processing of dehydrated human amnion/chorion membrane (dHACM)
Dehydrated human amnion/chorion membrane (dHACM) (EpiFix®, MiMedx Group, Marietta, GA) is a dehydrated human tissue allograft comprised of laminated amnion and chorion membranes derived from human placenta. Human placentas were donated under informed consent following Caesarean sections, in compliance with the Food and Drug Administration's (FDA) Good Tissue Practice and American Association of Tissue Banks (AATB) standards. No additional ethical approval was required for these studies. All donors were tested and confirmed free of infectious diseases, including human immunodeficiency virus (HIV), human T‐lymphotropic virus (HTLV), hepatitis B and C, and syphilis.
Amnion and chorion tissue layers were isolated from placenta, processed using the proprietary PURION® Process that involves gentle cleansing of the layers with water‐based solutions, and then laminated to form the graft, which was dehydrated under controlled drying conditions.34, 35, 36 A specific configuration of dHACM (EpiFix®, MiMedx Group) was used as the test material in this study.
Preparation of dHACM extracts
To prepare extracts of dHACM for cell culture experiments, sterilized grafts from a single donor were minced, yielding approximately 1 mm × 1 mm pieces of tissue, which were extracted at 5 mg tissue/mL in basal media appropriate for the cell type to be evaluated. After overnight extraction at 4°C, the tissue residue was removed by centrifugation at 3000 RCF for 5 min at room temperature, and the extract was sterile filtered using a 0.22 µm filter unit (EMD Millipore, SLMP025SS, Billerica, MA). Previous studies have established that a significant amount of the growth factors and cytokines in dHACM elute from the tissue under these conditions.33
ADSC, BM‐MSC, and HSC proliferation
Proliferation following exposure to dHACM extract was tested in ADSCs, BM‐MSCs, and HSCs. ADSCs were plated at 2500 cells/well in a 96‐well plate in ADSC complete media and were allowed to adhere to tissue culture treated plastic overnight. The media was then removed and the wells were washed with sterile phosphate buffered saline (PBS) (GE Healthcare, SH30028.01) to remove unattached cells and serum components. ADSCs were treated with dHACM extract at 5, 1, and 0.5 mg tissue per mL of basal media, which were prepared by making serial dilutions of the original 5 mg/mL extract using ADSC basal media. The positive and negative control wells were treated with complete and basal ADSC media, respectively. After 24 h, the plate was washed with PBS to remove unattached cells and phenol red residuals. A CyQuant assay (Molecular Probes, Life Technologies Corp., C7026, Carlsbad, CA) was performed according to manufacturer's instructions, to quantify DNA content (n = 4 replicates of samples per treatment) as a measure of cellular proliferation. DNA content was translated to cell number, using a standard curve of known quantities of cells as determined by counting on a hemocytometer.
BM‐MSCs were plated at 2500 cells/well in a 96‐well plate in BM‐MSC complete media and allowed to adhere overnight. From this point forward the BM‐MSCs were treated and analyzed using the same methods as the ADSCs, using BM‐MSC basal and complete media.
HSCs were plated at 10,000 cells/well in 200 µL of treatment media in a 96‐well plate. HSCs do not attach to the well, but rather are cultured in suspension. The treatment groups included HSC complete media (positive control) and HSC basal media (negative control), along with dHACM extract at 5, 1, and 0.5 mg/mL diluted in HSC basal media. The cells were incubated for 24 h before pelleting the cells via centrifugation of the 96‐well plate at 100 RPM for 7.5 min. The media was gently removed and cells were rinsed with 100 µL of PBS to remove residual media. The cells were then pelleted once more and the PBS was removed from each well. HSC proliferation was evaluated using cell permeant Calcein AM (Life Tech, C3099) to detect live cells (n = 5 replicates of samples per treatment). The fluorescence reading was translated to cell number using a standard curve of known cellular quantities determined at the time of treatment.
BM‐MSC and ADSC closure
Individual cell closure assays were performed for both adherent ADSCs and BM‐MSCs. ADSCs were plated at 15,000 cells/well and BM‐MSCs were plated at 10,000 cells/well in a 96‐well Oris™ Pro plate (Oris™ PROCMA5, Platypus Technologies, Madison, WI) in the appropriate complete medium for each cell type. The cells were allowed to adhere to plastic for 4 h at 37°C and 5% CO2 to form a confluent layer surrounding a central gel stopper. This nontoxic, biocompatible gel stopper produces a 2 mm diameter circular, cell‐free zone in the center of the well. The stopper dissolves after 20 min in media, allowing the population of cells to move toward the center of the cell‐free zone. Following dissolution of the gel stopper, the media was removed and the wells were rinsed with PBS before treatment with dHACM extract. Both ADSCs and BM‐MSCs were treated with dHACM extract diluted in the corresponding basal media at 5, 1, and 0.5 mg/mL (n = 5 replicates of samples per treatment). Each cell type was also treated using the appropriate media with and without supplement to act as positive and negative controls, respectively.
Digital images were captured every 24 h; the t = 0 image was taken immediately following treatment and used to determine the initial area of the cell‐free zone in each well using ImageJ (National Institutes of Health, Bethesda, MD) and calculating the area of the cell‐free zone. After 24 (t = 1 day), 48 (t = 2 days), and 72 h (t = 3 days), ImageJ analysis software was used to automatically trace and measure the margin of the migrating cell front from time course phase contrast images (t = 0, 1, 2, 3 days) and to calculate the remaining cell‐free area after each time point. The software was used in a consistent and optimized format for each image analysis. Percent closure of the cell‐free zone was calculated as follows:
Stem cell cytokine, chemokine, and growth factor modulation
Protein secretions from ADSCs, BM‐MSCs, and HSCs were quantified following 72 h of dHACM extract treatment at 5 mg/mL in comparison to the appropriate basal media treatments. After 72‐h treatment, the supernatant from five wells per sample group was recovered, pooled, and tested for the presence of 80 growth factors, cytokines, and soluble growth factor receptors. Cell numbers were measured in each well using a CyQuant assay (Life Technologies Corp.) after the culture media was collected, and treatment media was analyzed for elution content using Quantibody® Human Growth Factor and Inflammation Arrays (RayBiotech, QAH‐CAA‐1000, Norcross, GA). Growth factor values were normalized per cell number. The amounts of each growth factor in the extracts were also measured and subtracted from that in the culture media to determine the amount of additional growth factor produced by the cells themselves, calculated as follows:
Statistical analysis
Data from multiple test groups were analyzed using the analysis of variance (ANOVA) method with pairwise comparisons performed using the Tukey's post hoc test. Significant differences were assigned when p ≤ 0.05.
RESULTS
Effects of dHACM on stem cell proliferation
Extracts of dHACM increased proliferation of BM‐MSCs, ADSCs, and HSCs in culture over 24 h [Figure 1(A–C)]. At all concentrations of dHACM extract evaluated, all stem cell types responded by proliferating to a significantly greater extent than their respective negative controls without growth supplements, in which the extracts were prepared (p ≤ 0.05). In addition, the presence of dHACM extracts in basal medium caused cell numbers to approach or exceed those of positive controls containing growth supplements, representing the maximum growth rate for these cell types under the conditions utilized for this study. Although there was no dose response observed among the three extract concentrations, these data indicate that the maximum response was achieved with the lowest concentration of extract (0.5 mg/mL). The results suggest that dHACM extracts contain biologically active mitogens that promote cell division and increased cellular proliferation of ADSCs, BM‐MSCs, and HSCs.
Figure 1.
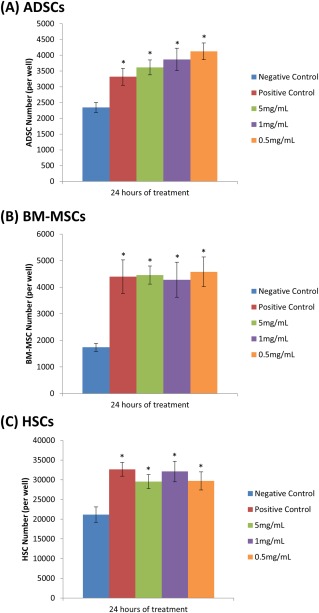
Cellular proliferation of ADSCs (A), BM‐MSCs (B), and HSCs (C) over 24 h in response to treatment with soluble extracts of dHACM, as well as negative and positive controls. The negative control wells contained cell‐specific basal medium, while the positive control wells contained basal medium supplemented with serum. Each bar represents the average number of cells per well (ADSC and BM‐MSC n = 4, HSC n = 5). Error bars indicate standard deviation from mean values. *p ≤ 0.05 where indicated versus negative controls.
Effects of dHACM on stem cell closure in vitro
Extracts of dHACM contain protein factors that have been shown to stimulate cellular chemotaxis in vitro and in vivo;3, 33 therefore, this study evaluated whether different stem cell types would respond to treatment with dHACM extract by populating a cell‐free zone using an in vitro cell closure model. Closure assays of ADSCs and BM‐MSCs, conducted by treating the cells with different concentrations of dHACM extract over 72 h, were evaluated based on percent closure of an initially acellular zone in each well. As cells migrate inward, the rate of closure is depicted by percent closure per time point. Using this in vitro closure model with BM‐MSCs and ADSCs, treatment with dHACM extract resulted in significantly accelerated closure of a circular cell‐free gap (BM‐MSCs shown in Figure 2(A).
Figure 2.
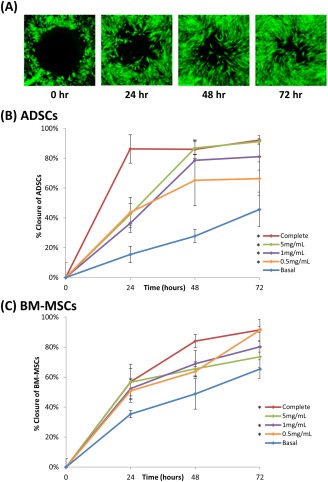
In vitro cellular closure responses by ADSCs and BM‐MSCs following treatment with dHACM extract over 72 h. (A) Representative calcein AM stained images of BM‐MSCs (green) in complete media at each time point evaluated in the closure assay. (B) ADSC migration expressed as percent closure of the cell‐free zone in response to treatment with basal and complete media, acting as the negative and positive controls respectively, and 5, 1, and 0.5 mg/mL of dHACM extract. (C) BM‐MSC migration expressed as percent closure of the cell‐free zone in response to treatment with dHACM extracts of varying concentration. Each time point represents the mean percent closure of five wells (n = 5 per treatment), *p ≤ 0.05 where indicated versus basal control after 72 h.
After 24 h, ADSCs [Figure 2(B)] began populating the acellular zone resulting in an average of 41% closure in dHACM extract‐treated ADSCs, significantly greater than only 15% closure in basal controls (p ≤ 0.05). Closure continued to progress over 72 h. After 3 days, treatment of ADSCs with 5 mg/mL dHACM extract resulted in 91% closure, significantly greater than the 46% closure observed in basal medium (p ≤ 0.05) after the same period of time. At 48 and 72 h, 1 and 5 mg/mL dHACM treated ADSCs were not statistically different from the positive, serum‐enhanced control (p > 0.05), demonstrating near maximal closure rate, while the ADSCs treated with 0.5 mg/mL dHACM extracts responded less compared to the other treatments, but exceeded basal medium‐treated negative controls.
Similarly, dHACM treated BM‐MSCs [Figure 2(C)] demonstrated an average of 52% closure after 24 h, compared to only 34% closure in basal medium, and at 48 h a statistically greater outcome was observed for BM‐MSC closure in dHACM treated wells with an average of 66% closure. MSC closure in 1 and 0.5 mg/mL dHACM extract at 48 and 72 h was significantly greater than MSC closure in basal media (p ≤ 0.05). After 72 h, BM‐MSCs treated with 0.5 mg/mL dHACM extract resulted in 91% closure, significantly greater than 65% in basal medium (p ≤ 0.05). The results indicate that treatment with dHACM extracts significantly accelerated closure of an acellular zone in both ADSCs (2B) and BM‐MSCs (2C).
Effects of dHACM on cytokine, chemokine, and growth factor modulation in stem cells
The responses of ADSCs, BM‐MSCs, and HSCs to dHACM were further investigated by measuring growth factor and cytokine levels secreted by the cells using Quantibody® analysis of conditioned media following the treatment of cells with dHACM extract, in vitro. This model represented healthy stem cell responses to dHACM under favorable cell culture conditions. Despite the favorable conditions of this in vitro cell culture model, a significant change was observed in the secretion profile of several immunomodulatory proteins for each cell type after a 72 h treatment of dHACM, as shown in Figure 3. Protein values were calculated on a per cell basis, minus the protein value from the extracts themselves, and displayed as values normalized over the basal media control for each cell type. The complete data is presented as a heat map with upregulation represented in varying intensities of green coloration and downregulation represented in varying intensity of red coloration [Figure 3(A)]. Because a large number of cytokines altered their expression to varying degrees in response to dHACM treatment, only factors that experienced at least a tenfold change in modulation, representing a substantial change by at least one order of magnitude, over the basal control treatment value were analyzed for further interpretation [Figure 3(B–E)]. In response to dHACM extracts ADSCs were found to upregulate immunoregulatory proteins: growth differentiation factor 15 (GDF‐15), chemokine ligand 1 (I‐309), intercellular adhesion molecule 1 (ICAM‐1), interleukins 6, 8, and 16 (IL‐6, IL‐8, and IL‐16), monocyte chemotactic protein 1 (MCP‐1), and stem cell factor receptor (SCF R); mitogenesis‐related proteins: epidermal growth factor receptor (EGF R), and insulin‐like growth factor binding proteins 1 and 2 (IGFBP‐1 and IGFBP −2); and the matrix metalloproteinase inhibitor: tissue inhibitor of metalloproteinases 1 (TIMP‐1). Macrophage colony‐stimulating factor (MCSF), eotaxin, interleukin 1β (IL‐1β), and macrophage inflammatory protein 1β (MIP‐1β) were downregulated. BM‐MSCs upregulated immunoregulatory proteins: IL‐6, ICAM‐1, interleukin 1 receptor antagonist (IL‐1ra), and stem cell factor (SCF), as well as mitogenesis‐related proteins: fibroblast growth factor 4 (FGF‐4) and growth hormone (GH), while eotaxin‐2 was downregulated. Finally, in HSCs, immunoregulatory proteins: macrophage inflammatory protein 1α and 1β (MIP‐1α and MIP‐1β), and ICAM‐1 were upregulated, as was mitogenesis‐related: IGFBP‐1, and the protease inhibitor: TIMP‐1.
Figure 3.
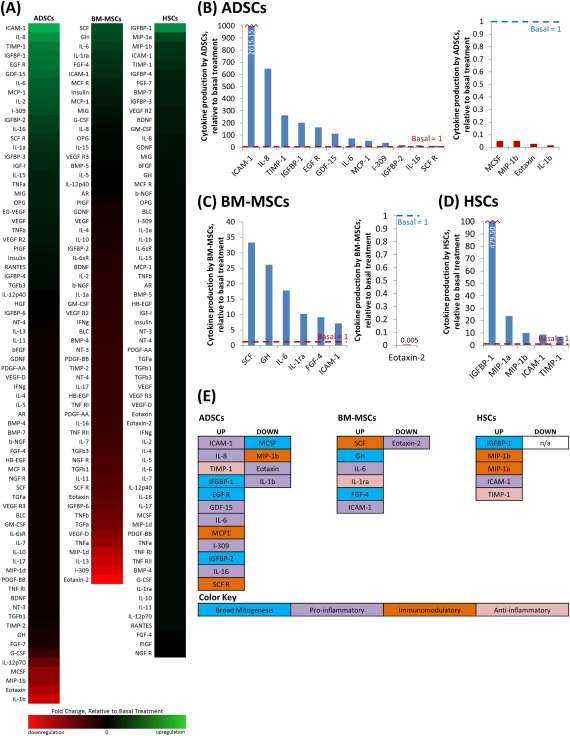
Alterations of protein secretion from ADSCs, BM‐MSCs, and HSCs in response to 72 h of dHACM treatment. The complete data (5 pooled wells per sample group) is presented as a heat map with upregulation represented in varying intensities of green coloration and downregulation represented in varying intensity of red coloration (A). For further analysis, all reported factors were either up or down regulated by greater than tenfold over the basal treatment group for each cell type (B–E). (B–D) The values of secreted growth factors/cytokines per cell substantially regulated by dHACM treatment. The displayed values are normalized to the associated basal control for ADSCs (B), BM‐MSCs (C), and HSCs (D). (E) The growth factors and cytokines grouped into their general functional category as a broad mitogenesis, proinflammatory, immunomodulatory, stem cell maintenance, or anti‐inflammatory factor to identify the processes being up or downregulated with treatment.
DISCUSSION
This study demonstrated that dHACM extracts stimulate cellular proliferation and migration responses in a variety of adult stem cells, including BM‐MSCs, ADSCs, and HSCs. BM‐MSCs, ADSCS, and HSCs responded to dHACM treatment with significant increases in cell proliferation after 24 h. Additionally, treatment of ADSCs and BM‐MSCs with dHACM extracts accelerated closure of predefined cell‐free zones using an in vitro cell closure model. Because dHACM was demonstrated to promote the proliferation of stem cells, the closure of the cell‐free zone observed is likely a result of a combination of both stem cell migration and proliferation. As a nonadherent cell type, HSCs were not examined for cell migration using the Oris™ assay; however, previously published studies have shown that dHACM recruits HSCs to the site of a wound in vivo in a mouse subcutaneous implantation model.3 Over 50 soluble growth factors and cytokine proteins have been identified in soluble extracts of dHACM,32, 33 suggesting that dHACM contains soluble components that elute from the tissue and stimulate the growth and chemotactic migration of different types of stem cells, thus amplifying the number of stem cells locally. However, due to the complexity of this unique material, a causative effect cannot be assigned to any single regulatory protein or other signaling molecule either currently identified or yet to be identified in dHACM tissues.
The focus of stem cell delivery has recently moved away from cellular differentiation and shifted to focus on paracrine signaling properties of these cells, including their influence on the inflammatory status of injured tissues.9 Thus, cytokine secretion from stem cells in response to treatment with dHACM was quantified to further characterize the array of biological responses elicited by dHACM treatment on host cells involved in healing and tissue repair. ADSCs, BM‐MSCs, and HSCs were shown to modulate secretion of a number of cytokines involved in immunoregulation and mitogenesis in response to dHACM extracts by tenfold or greater over basal controls, which was considered to be significant and most likely to have an impact on stem cell biology. Of these molecules, they can generally be classified into three unique functions: (1) chemokines and proteins related to leukocyte migration, (2) immunomodulatory cytokines, and (3) mitogenic growth factors and proteins related to tissue growth.
Upregulated chemokines, including I‐309, IL‐8, IL‐16, MCP‐1, MIP‐1α, and MIP‐1β, are known to direct chemotaxis of immune cells that are critical in healing, including monocytes and macrophages, neutrophils, T lymphocytes, dendritic cells, and eosinophils.37 Similarly ICAM‐1, which was upregulated in all three stem cell types, is an adhesion molecule that facilitates leukocyte endothelial transmigration,38 suggesting a potential role of ICAM‐1 in the extravasation of circulating stem cells from the blood vessels toward the dHACM treated site. In wound settings, these chemokines are commonly expressed early following establishment of hemostasis, often peaking at day 1 with expression sustained through the first week of healing, and have also been shown to promote angiogenesis.37
Immunomodulatory cytokines, including GDF‐15, IL‐1RA, and IL‐6, were also upregulated in MSCs, ADSCs, and HSCs. These cytokines are known to regulate various pro‐ and anti‐inflammatory cues that are required for healing, including regulation of apoptosis, T cell activation, B cell differentiation, macrophage differentiation, hematopoiesis, and additional downstream immunomodulatory cues.39, 40, 41
Finally, growth factors and growth factor regulatory molecules, including EGF, FGF‐4, GH, IGFBP‐1, IGFBP‐2, and SCF, were secreted in response to dHACM. These growth cytokines are known to stimulate cell proliferation, migration, differentiation, cell survival, and protein synthesis, and have been shown to enhance healing of wounds.42, 43, 44, 45 Additionally, TIMP‐1, which was also upregulated, is an inhibitor of MMPs and is actively involved in tissue remodeling processes.46
A sustained state of inflammation resulting from local hypoxia, bacterial colonization, repeated ischemia–reperfusion injury, and cellular as well as systemic changes of aging, is a hallmark of chronic wounds.47 Data suggests that a balance of stem cell secreted immunoregulatory proteins in a chronic wound may be crucial to transition the wound out of this sustained inflammatory phase and into a normal acute healing phase to achieve wound re‐epithelialization. In prospective, randomized, controlled clinical trials, dHACM tissue has demonstrated the ability to resolve chronic inflammation and accelerate healing in a variety of refractory wounds;29, 30, 31, 48, 49, 50 however, the mechanisms of action remain somewhat unclear. Data from preclinical wound models in mice has additionally shown that dHACM implanted subcutaneously leads to the recruitment of circulating host MSCs and HSCs, suggesting that adult stem cells may contribute to the mechanism by which dHACM promotes healing.3, 33 The results from the current study contribute to an understanding of how different types of stem cells respond to dHACM in vitro and how dHACM‐treated stem cells may contribute to the mode of action for resolving chronic inflammation and ultimately promoting healing.
The cascade of events elicited by dHACM treatment is a complex process resulting from the interplay between the wound environment and the properties of dHACM; therefore, it is possible that these results were unique to the in vitro culture conditions employed in this study. Although the in vitro experiments presented here do not fully reproduce the complex environment of a chronic wound, which may include high levels of proteolytic activity, microbial biofilm, poor vascularization, and ongoing chronic inflammation, these studies were intended to compare the responses of different stem cell types under controlled conditions, as simplified models that demonstrate the response of stem cells to dHACM tissues. Additional studies will be required to further characterize stem cell responses to dHACM and how these results translate in vivo.
Soluble extracts of dHACM resulted in increased proliferation and migration of ADSCs, BM‐MSCs, and HSCs, along with modulation of secreted regulatory proteins from those cells in vitro. The secretion of important growth factors and cytokines from stem cells may enhance wound healing in addition to the biological responses elicited by dHACM eluted factors to reset the immune and inflammatory status of a chronic wound to that of an acute healing environment. Thus, dHACM may impact wound healing by increasing host stem cell populations in treated tissues and, in turn modulating stem cell responses in the wound bed.
ACKNOWLEDGMENTS
MM, KC, JL, JJL, CSY, and TJK are employees of MiMedx. The authors would like to thank Lisa Godwin for daily maintenance of cell stocks and for her assistance plating and treating cells for these experiments.
How to cite this article: Massee M, Chinn K, Lei J, Lim JJ, Young CS, Koob TJ. 2016. Dehydrated human amnion/chorion membrane regulates stem cell activity in vitro . J Biomed Mater Res Part B 2016:104B:1495–1503.
REFERENCES
- 1. Sen CK, GM Gordillo, S Roy, R Kirsner, L Lambert, TK Hunt, F Gottrup, GC Gurtner, MT Longaker. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maan ZN, RC Rennert, TJ Koob, M Januszyk, WW Li, GC Gurtner. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res 2015;193:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maxson S, EA Lopez, D Yoo, A Danilkovitch-Miagkova, MA Leroux. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen L, EE Tredget, PY Wu, Y Wu. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dash NR, SN Dash, P Routray, S Mohapatra, PC Mohapatra. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–366. [DOI] [PubMed] [Google Scholar]
- 8. Kim WS, BS Park, JH Sung, JM Yang, SB Park, SJ Kwak, JS Park. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 10. Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gunsilius E, Gastl G, Petzer AL. Hematopoietic stem cells. Biomed Pharmacother 2001;55:186–194. [DOI] [PubMed] [Google Scholar]
- 12. Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993;81:2844–2853. [PubMed] [Google Scholar]
- 13. Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998;176(2A Suppl):26S–38S. [DOI] [PubMed] [Google Scholar]
- 14. DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock 1995;4:233–240. [PubMed] [Google Scholar]
- 15. Ueta M, MN Kweon, Y Sano, C Sotozono, J Yamada, N Koizumi, H Kiyono, S Kinoshita. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol 2002;129:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park CY, S Kohanim, L Zhu, PL Gehlbach, RS Chuck. Immunosuppressive property of dried human amniotic membrane. Ophthalmic Res 2009;41:112–113. [DOI] [PubMed] [Google Scholar]
- 17. Meller D, RT Pires, RJ Mack, F Figueiredo, A Heiligenhaus, WC Park, P Prabhasawat, T John, SD McLeod, KP Steuhl, SC Tseng. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology 2000;107:980–989; discussion 990. [DOI] [PubMed] [Google Scholar]
- 18. Diaz‐Prado S, ME Rendal-Vazquez, E Muinos-Lopez, T Hermida-Gomez, M Rodriguez-Cabarcos, I Fuentes-Boquete, FJ de Toro, FJ Blanco. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank 2010;11:183–195. [DOI] [PubMed] [Google Scholar]
- 19. Ramakrishnan KM, Jayaraman V. Management of partial‐thickness burn wounds by amniotic membrane: a cost‐effective treatment in developing countries. Burns 1997;23 Suppl 1:S33–S36. [DOI] [PubMed] [Google Scholar]
- 20. Niknejad H, H Peirovi, M Jorjani, A Ahmadiani, J Ghanavi, AM Seifalian. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 21. Alviano F, V Fossati, C Marchionni, M Arpinati, L Bonsi, M Franchina, G Lanzoni, S Cantoni, C Cavallini, F Bianchi, PL Tazzari, G Pasquinelli, L Foroni, C Ventura, A Grossi, GP Bagnara. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro . BMC Dev Biol 2007;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bacenkova D, J Rosocha, T Tothova, L Rosocha, M Sarissky. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011;13:1047–1056. [DOI] [PubMed] [Google Scholar]
- 23. Nazarov I, JW Lee, E Soupene, S Etemad, D Knapik, W Green, E Bashkirova, X Fang, MA Matthay, FA Kuypers, VB Serikov. Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Transl Med 2012;1:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Semenov OV, S Koestenbauer, M Riegel, N Zech, R Zimmermann, AH Zisch, A Malek. Multipotent mesenchymal stem cells from human placenta: critical parameters for isolation and maintenance of stemness after isolation. Am J Obstet Gynecol 2010;202:193 e1–193 e13. [DOI] [PubMed] [Google Scholar]
- 25. Insausti CL, M Blanquer, AM Garcia-Hernandez, G Castellanos, JM Moraleda. Amniotic membrane‐derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning 2014;7:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magatti M, S De Munari, E Vertua, C Nassauto, A Albertini, GS Wengler, O Parolini. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant 2009;18:899–914. [DOI] [PubMed] [Google Scholar]
- 27. Kang JW, HC Koo, SY Hwang, SK Kang, JC Ra, MH Lee, YH Park. Immunomodulatory effects of human amniotic membrane‐derived mesenchymal stem cells. J Vet Sci 2012;13:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang CJ, ML Yen, YC Chen, CC Chien, HI Huang, CH Bai, BL Yen. Placenta‐derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon‐gamma. Stem Cells 2006;24:2466–2477. [DOI] [PubMed] [Google Scholar]
- 29. Zelen CM, TE Serena, G Denoziere, DE Fetterolf. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long‐term follow‐up study. Wound Med 2014;4:1–4. [Google Scholar]
- 31. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 2014;11:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koob TJ, JJ Lim, M Massee, N Zabek, G Denoziere. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353–1362. [DOI] [PubMed] [Google Scholar]
- 33. Koob TJ, R Rennert, N Zabek, M Massee, JJ Lim, JS Temenoff, WW Li, G Gurtner. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013;10:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daniel J, inventor; MiMedx Group, Inc., Kennesaw, GA, assignee. Placental Tissue Grafts, US Patent 8,372,437, 2013. [Google Scholar]
- 35. Daniel J, Tofe R, Spencer R, Russo J, inventors; MiMedx Group, Inc., Kennesaw, GA, assignee. Placental Tissue Grafts, US Patent 8,357,403, 2013. [Google Scholar]
- 36. Daniel J, R Tofe, R Spencer, J Russo, Group inventors; MiMedx, Inc. Kennesaw, GA, assignee Placental Tissue Grafts, US Patent 8,409,626, 2013. [Google Scholar]
- 37. Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001;69:513–521. [PubMed] [Google Scholar]
- 38. Yang L, RM Froio, TE Sciuto, AM Dvorak, R Alon, FW Luscinskas. ICAM‐1 regulates neutrophil adhesion and transcellular migration of TNF‐alpha‐activated vascular endothelium under flow. Blood 2005;106:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kempf T, A Zarbock, C Widera, S Butz, A Stadtmann, J Rossaint, M Bolomini-Vittori, M Korf-Klingebiel, LC Napp, B Hansen, A Kanwischer, U Bavendiek, G Beutel, M Hapke, MG Sauer, C Laudanna, N Hogg, D Vestweber, KC Wollert. GDF‐15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 2011;17:581–588. [DOI] [PubMed] [Google Scholar]
- 40. Ishida Y, T Kondo, A Kimura, K Matsushima, N Mukaida. Absence of IL‐1 receptor antagonist impaired wound healing along with aberrant NF‐kappaB activation and a reciprocal suppression of TGF‐beta signal pathway. J Immunol 2006;176:5598–5606. [DOI] [PubMed] [Google Scholar]
- 41. Petersen AM, Pedersen BK. The role of IL‐6 in mediating the anti‐inflammatory effects of exercise. J Physiol Pharmacol 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- 42. Brown GL, LB Nanney, J Griffen, AB Cramer, JM Yancey, LJ Curtsinger, III, L Holtzin, GS Schultz, MJ Jurkiewicz, JB Lynch. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med 1989;321:76–79. [DOI] [PubMed] [Google Scholar]
- 43. Werner S, KG Peters, MT Longaker, F Fuller-Pace, MJ Banda, LT Williams. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA 1992;89:6896–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laron Z. Insulin‐like growth factor 1 (IGF‐1): a growth hormone. Mol Pathol 2001;54:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol 1995;58:14–22. [DOI] [PubMed] [Google Scholar]
- 46. Birkedal‐Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 1995;7:728–735. [DOI] [PubMed] [Google Scholar]
- 47. Schreml S, RM Szeimies, L Prantl, S Karrer, M Landthaler, P Babilas. Oxygen in acute and chronic wound healing. Br J Dermatol 2010;163:257–268. [DOI] [PubMed] [Google Scholar]
- 48. Forbes J, Fetterolf DE. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care 2012;21:290, 292, 294–296. [DOI] [PubMed] [Google Scholar]
- 49. Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J 2014;11:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care 2013;22:347–348, 350–351. [DOI] [PubMed] [Google Scholar]


