Summary
Background
Older subjects with type 2 diabetes mellitus (T2DM) have differential characteristics compared with middle‐aged or younger populations, and require tailored management of the disease.
Aims
To evaluate how clinical characteristics, degree of control of glycaemia and cardiovascular risk factors, presence of chronic complications and treatments differ between older T2DM patients and younger adults.
Methods
Cross‐sectional study using data from a population‐based electronic database. We retrieved data from 318,020 patients ≥ 30 years diagnosed with T2DM, attended during 2011 in primary care centres in Catalonia, Spain. We performed descriptive and comparative analyses stratified by gender and age subgroups: ≤ 65, 66–75, 76–85 and >85 years.
Results
Both men and women across older age subgroups (> 65 years) had longer diabetes duration than younger adults (8.0 vs. 5.6 in men and 8.4 vs. 6.9 years in women; p < 0.001), but better glycaemic control (mean glycated haemoglobin 7.1 vs. 7.7 in men and 7.1 vs. 7.4 in women; p < 0.001), and better combined control of different cardiovascular risk factors (p < 0.001). Moreover, older patients were more likely to achieve glycaemic targets irrespective of having cardiovascular disease. The use of oral antidiabetics decreased with increasing age, and insulin in monotherapy was more frequently prescribed among patients in the older age subgroups. Diabetes‐related complications were more frequent in men of all group ages. In the older age subgroups, patients of both sexes had a longer duration of T2DM but better glycaemic control. In this context, the prevalence of diabetic retinopathy decreased unexpectedly with increasing age.
Conclusion
Control of glycaemia and cardiovascular risk factors was better among older T2DM patients. There is a need for prospective studies to quantify the weight of risk factors in each complication to adapt the therapeutic and care approaches in elderly people.
What's known
The prevalence rates of T2DM increase with age, and older people are a growing population that account for a high proportion of cases among adults. Older patients are more likely to present cardiovascular complications and comorbid conditions, which entail specific goals to control the disease. However, elderly patients are systematically excluded from clinical trials, and there is also a lack of reliable data on the response to pharmacological treatments in this age group.
What's new
In a primary care real‐life setting, T2DM patients in the older age subgroup (> 65 years) had a better control of glycaemic targets and cardiovascular risk factors than younger patients in spite of having a higher prevalence of chronic complications. Moreover, this age subgroup was less intensively treated with glucose‐lowering and lipid‐lowering drugs than younger patients. T2DM in elderly people should be clinically managed taking into account the observed differential age‐related pattern of the disease.
Introduction
Type 2 diabetes mellitus (T2DM) has become one of the most serious and challenging public health issues of our time, and the human, social and economic burden associated with the disease has dramatically increased over the past few decades. According to the International Diabetes Federation 382 million people worldwide have diabetes, and 316 million are at high risk of developing T2DM 1. In Spain, a recent epidemiological survey estimated that the prevalence rate of T2DM is around 13.8%, and that about 6% of the Spanish population is unaware of their disease 2. Moreover, the study showed that diabetes is more frequent in men and prevalence rates increase with age 2.
The global prevalence of diabetes in people 60–79 years of age has been estimated to be 18.6% 1; the prevalence of diagnosed diabetes in the United States in subjects ≥ 75 years was 20% in 2012, which is more than eightfold the rate reported among adults aged 18–44 years (2.4%) 3. Similar prevalence rates have been found in Spain, with 40% of the population aged 75 years and over having known diabetes (41.3% of women and 37.4% of men) 2.
The strong link between age and diabetes is of concern if we take into account the progressive increase in life expectancy, which is likely to result in a substantial increase in the number of older people with diabetes, and a concomitant increase in the costs for the health system in the near future. There is compelling evidence that older onset‐diabetes has differential characteristics compared with onset in middle‐aged or earlier populations 4. On the one hand, the disease starts insidiously in people 65 years and over, and remains frequently undiagnosed until a routine analysis is performed or after the subject is admitted to a hospital for any other reason. On the other hand, older people are more likely to present cardiovascular complications, have higher rates of comorbid conditions, mortality, and prevalence of geriatric syndromes (e.g. cognitive dysfunction, functional impairment, frailty, falls and fractures, polypharmacy, depression, vision and hear impairment, persistent pain, urinary incontinence) than older people without diabetes 5. Finally, some studies report that older adults have a worse glycaemic control than other age groups with diabetes 6, and have the highest rates of hyperglycaemic crises and also of hypoglycaemia episodes requiring emergency department visits 5.
Although recommendations in clinical guidelines may vary per country, decision‐making should not be in general based on the age of the patient but on a combination of factors including general health status and functional and cognitive ability, among others 4, 5, 7, 8. Thus, in elderly individuals with preserved cognitive and functional abilities and a good life expectancy, the recommendation is a glycated haemoglobin goal similar to that recommended for younger adults. Conversely, the goal for glycaemic control in frail elderly subjects not meeting the above criteria or with greater hypoglycaemia vulnerability should be more relaxed, as the short life expectancy precludes the medium‐ and long‐term benefits resulting from very tight control goals 4, 9. Indeed, the benefits associated with glycaemic control require 5–10 years to reduce the incidence of microvascular complications 4, 10, and it is not yet certain whether it has an actual impact in the incidence of cardiovascular disease (CVD) in these patients.
The objective of the present population‐based cross‐sectional study was to retrospectively assess and compare the clinical characteristics, degree of glycaemic and cardiovascular risk factors control, treatments, and diabetes‐related complications between older T2DM patients and younger adults in a primary care population database in Catalonia, Spain. Secondarily, we aimed to compare these same variables stratifying by gender and different age subgroups.
Methods
Design and data sources
Descriptive, population‐based, cross‐sectional study at the primary care setting in Catalonia, Spain. Data were extracted from SIDIAP (Information System for the Development of Research in Primary Care) 11, which is a computerised database containing anonymised patient's records for the 5.8 million people attended by general practitioners in the Catalan Health Institute. SIDIAP includes data on demographic variables, diagnoses, clinical variables, prescriptions, specialist referrals, laboratory test results, and medications withdrawn from pharmacist offices, obtained from the CatSalut general database.
Data extraction
Data were obtained for patients ≥ 30 years diagnosed with T2DM by 31 December 2011 and attended a primary care centre during 2011. Patients with type 1 diabetes mellitus or gestational diabetes were excluded.
For the objective of the study, we extracted demographic data, including age (further categorised into age subgroups: ≤ 65, 66–75, 76–85, and > 85 years) and sex; clinical variables included diabetes duration; smoking status; body mass index (BMI); blood pressure (BP) (systolic and diastolic); standardised glycated haemoglobin (HbA1c) values; lipid levels including total cholesterol (TC), low‐density lipoproteins or LDL cholesterol (LDLc), high‐density lipoproteins or HDL cholesterol (HDLc), non‐HDL cholesterol, and triglycerides (TG), estimated glomerular filtration rate (eGFR) using the Modified Diet in Renal Disease (MDRD‐4) formula and urine albumin‐to‐creatinine ratio (ACR). Values of clinical variables corresponded to the most recent registered value in the last 15 months except for BMI, which was the most recent value in the last 24 months, and smoking status, which corresponded to the most recent information recorded in the medical history. As for comorbidities, the diagnose of hypertension and/or dyslipidaemia was considered if mentioned in an active record up to the cut‐off date, and we also extracted information on the presence of diabetes‐related chronic complications, namely ischaemic heart disease, heart failure, stroke, peripheral artery disease, diabetic retinopathy and chronic kidney disease (defined according to eGFR‐MDRD4 and ACR values).
Control of CV risk factors were defined as follows: no current smoking; BMI < 30 kg/m2; BP < 140/90 mmHg; HbA1c ≤ 7% (≤ 53.0 mmol/mol); TC ≤ 250 mg/dl; LDLc < 130 mg/dl for patients without CVD and < 100 mg/dl for those with CVD; HDLc > 50 mg/dl for women and > 40 mg/dl for men; and TG ≤ 150 mg/dl.
Statistical analysis
A descriptive analysis was performed stratified by gender and age subgroup. For qualitative variables, absolute and relative frequencies were calculated. For quantitative variables, mean values and standard deviation were obtained. Proportions and means were compared by Pearson's chi‐squared test and analysis of variance (ANOVA), respectively. All hypothesis contrasts were bi‐directional and the statistical significance level was set at 0.05. Moreover, the prevalence of diabetes‐related complications and the degree of glycaemic control was studied stratifying by T2DM duration (≤ 5, 5–10, 10–20 and > 20 years). All analyses were performed with Stata/SE version 13 for Windows (Stata Corp., College Station, TX, USA) and R software version 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of T2DM in ageing population
A total of 318,020 subjects with a diagnosis of T2DM were included in the study; 53.8% of them were males (N = 171,219; Table 1). Mean age of the overall population was 68.8 years (SD = 11.9); the mean age at diagnosis was 61.6 years (SD = 11.7), and the median disease duration was 6.7 years [interquartile range (IQR) = 6.2 years). According to prespecified age categories, 38.0% of subjects were ≤ 65 (62.9% males); 29.4% were 66–75 (54.3% males); 25.8% were 76–85 (45% males); and 6.8% were > 85 years (33.4% males).
Table 1.
Demographic and clinical characteristics of the study population by gender and age group
| Characteristic, mean (SD) | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ≤ 65 years | 66–75 years | 76–85 years | > 85 years | p‐value | Total | ≤ 65 years | 66–75 years | 76–85 years | > 85 years | p‐value | |
| N | 171,219 | 75,986 | 50,912 | 37,090 | 7231 | 146,801 | 44,641 | 42,817 | 45,143 | 14,200 | ||
| Age | 66.9 (11.7) | 56.2 (7.0) | 70.3 (2.9) | 79.8 (2.7) | 88.4 (2.5) | 71.1 (11.8) | 56.8 (7.0) | 70.6 (2.9) | 80.1 (2.8) | 89.0 (2.8) | ||
| Age at diagnosis | 60.0 (11.4) | 50.7 (7.3) | 62.8 (5.8) | 71.3 (6.5) | 79.5 (7.0) | < 0.001 | 63.5 (11.8) | 51.0 (7.4) | 62.8 (6.1) | 71.4 (6.8) | 79.9 (7.2) | < 0.001 |
| Diabetes duration | 6.9 (5.2) | 5.6 (4.2) | 7.5 (5.2) | 8.5 (5.9) | 9.0 (6.5) | < 0.001 | 7.7 (5.7) | 5.9 (4.5) | 7.8 (5.5) | 8.8 (6.2) | 9.1 (6.5) | < 0.001 |
| HbA1c (%) | 7.2 (1.4) | 7.4 (1.5) | 7.1 (1.3) | 7.1 (1.2) | 7.0 (1.2) | < 0.001 | 7.2 (1.3) | 7.4 (1.5) | 7.2 (1.3) | 7.1 (1.2) | 7.0 (1.2) | < 0.001 |
| Systolic BP (mmHg) | 134.6 (15.0) | 133.9 (14.7) | 135.3 (14.8) | 135.0 (15.3) | 134.3 (16.3) | < 0.001 | 134.9 (15.7) | 132.0 (15.0) | 135.7 (15.1) | 136.5 (15.9) | 135.7 (17.1) | < 0.001 |
| Diastolic BP (mmHg) | 75.4 (9.9) | 78.9 (9.3) | 74.4 (9.2) | 71.5 (9.5) | 69.6 (9.8) | < 0.001 | 74.9 (9.7) | 78.2 (9.1) | 75.2 (9.2) | 72.7 (9.6) | 71.1 (9.8) | < 0.001 |
| BMI (kg/m2) | 29.4 (4.4) | 30.1 (4.7) | 29.3 (4.1) | 28.4 (3.9) | 27.2 (3.7) | < 0.001 | 31.0 (5.7) | 32.3 (6.2) | 31.3 (5.5) | 30.0 (5.1) | 28.1 (4.7) | < 0.001 |
| TC (mg/dl) | 181.3 (38.5) | 188.9 (40.7) | 177.8 (35.7) | 173.2 (35.4) | 169.4 (35.3) | < 0.001 | 193.9 (38.1) | 200.4 (39.4) | 193.7 (36.8) | 189.6 (37.1) | 187.5 (38.1) | < 0.001 |
| HDLc (mg/dl) | 46.1 (12.1) | 44.7 (11.6) | 47.0 (12.2) | 47.4 (12.5) | 47.2 (12.8) | < 0.001 | 52.7 (13.3) | 51.3 (12.9) | 53.2 (13.0) | 53.5 (13.7) | 52.6 (14.1) | < 0.001 |
| LDLc (mg/dl) | 105.9 (32.1) | 111.0 (33.6) | 103.3 (30.5) | 100.7 (30.2) | 99.7 (29.7) | < 0.001 | 112.0 (32.7) | 118.3 (33.7) | 111.1 (31.9) | 107.9 (31.7) | 108.3 (32.0) | < 0.001 |
| Triglycerides (mg/dl) | 156.2 (115.8) | 182.0 (146.0) | 143.3 (85.8) | 129.3 (72.0) | 117.3 (59.0) | < 0.001 | 152.3 (86.4) | 163.6 (104.6) | 151.5 (79.4) | 145.6 (75.0) | 139.2 (71.9) | < 0.001 |
| eGFR‐MDRD (ml/min) | ||||||||||||
| < 15 | 335 (0.2) | 90 (0.2) | 106 (0.3) | 115 (0.4) | 24 (0.4) | 252 (0.2) | 44 (0.1) | 75 (0.2) | 101 (0.3) | 32 (0.3) | < 0.001 | |
| 15–29 | 1418 (1.0) | 194 (0.3) | 385 (0.9) | 604 (2.0) | 235 (4.2) | 2164 (1.8) | 114 (0.3) | 366 (1.0) | 1047 (2.8) | 637 (5.7) | < 0.001 | |
| 30–59 | 21,430 (15.8) | 3402 (5.8) | 6786 (16.3) | 8956 (29.8) | 2286 (40.6) | 29,442 (24.4) | 2765 (7.7) | 7394 (20.5) | 13,651 (36.3) | 5632 (50.2) | < 0.001 | |
| ≥60 | 112,236 (82.9) | 54,473 (93.7) | 34,289 (82.5) | 20,389 (67.8) | 3085 (54.8) | 88,765 (73.6) | 32,832 (91.8) | 28,209 (78.3) | 22,815 (60.7) | 4909 (43.8) | < 0.001 | |
| ACR (mg/g) | 45.3 (164.4) | 38.1 (153.6) | 43.9 (160.8) | 58.6 (183.6) | 72.0 (201.0) | < 0.001 | 35.7 (140.3) | 27.3 (118.0) | 30.3 (133.5) | 44.2 (157.5) | 61.6 (174.7) | < 0.001 |
The number of patients with available data varied depending on each studied variable. ACR, albumin‐to‐creatinine ratio; BMI, body mass index; BP, blood pressure; eGFR‐MDRD, estimated glomerular filtration rate (eGFR) using the Modified Diet in Renal Disease (MDRD‐4) formula; HbA1c, standardised glycated haemoglobin; HDLc, high‐density lipoproteins HDL cholesterol; LDLc, low‐density lipoproteins LDL cholesterol; TC, total cholesterol.
There was a progressive improvement in glycaemic control values (HbA1c) with age in both genders (p < 0.001) despite longer diabetes duration in older age groups. In the total sample, obesity was more common among women than men (mean BMI 29.4 kg/m2 vs. 31.0 kg/m2, respectively), and declined with age for both genders (p < 0.001). Of note, in both genders the mean values of diastolic BP, TC, LDLc and TG were significantly lower in the older age groups (p < 0.001). The average values of eGFR‐MDRD also decreased gradually with age in both genders; thus, the percentage of patients with renal failure (eGFR < 60 ml/min) and urine ACR increased gradually with age in both genders (p < 0.001).
Degree of control and treatment of T2DM with age
The degree of control of main cardiovascular risk factors for T2DM and pharmacological treatments is shown in Table 2. The percentage of subjects with fair glycaemic control (HbA1c ≤ 7%) was significantly higher among older age groups (p < 0.001); moreover, a lower proportion of patients in the older age groups were not well controlled (HbA1c > 10%) in spite of having more comorbid conditions. Overall, a 22% of patients in both genders were not taking any glucose‐lowering drugs. Its use was progressively reduced with increasing age, with a total of 71.5% of men and 68.4% of women older than 85 years taking any glucose‐lowering agent. However, there was also a parallel increase in the use of insulin with age, particularly in monotherapy. There were no substantial differences in the control of blood pressure (BP ≤ 140/90 mmHg) with age, although the control was slightly better among patients ≤ 65 years, particularly in women (67.3% vs. 71% of men), while 65.5% of men and 62.9% of women older than 65 years had their BP under control. As for the pharmacological treatment of hypertension, there was a greater proportion of older subjects being pharmacologically treated compared with younger adults, and more frequently treated with a combination of different drugs.
Table 2.
Degree of Control of Main Cardiovascular Risk Factors for T2DM and Pharmacological Treatment by Gender and Age Group
| Characteristic | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ≤ 65 years | 66–75 years | 76–85 years | > 85 years | p‐value | Total | ≤ 65 years | 66–75 years | 76–85 years | > 85 years | p‐value | |
| BMI, kg/m 2 , n (%) | ||||||||||||
| ≥ 30 | 50,847 (39.5) | 25,902 (46.3) | 15,438 (38.6) | 8547 (30.5) | 960 (20.9) | < 0.001 | 57,735 (52.6) | 21,152 (61.3) | 19,033 (55.4) | 15,125 (45.8) | 2425 (31.2) | < 0.001 |
| HbA1c, n (%) a | ||||||||||||
| ≤ 7 | 71,967 (54.2) | 29,409 (50.7) | 22,828 (56.2) | 16,513 (57.1) | 3217 (60.9) | < 0.001 | 64,362 (54.5) | 18,604 (52.3) | 19,030 (53.5) | 20,398 (55.9) | 6330 (60.3) | <0.001 |
| 7.1–7.5 | 19,357 (14.6) | 7856 (13.5) | 6292 (15.5) | 4456 (15.4) | 753 (14.3) | 17,405 (14.7) | 4734 (13.3) | 5500 (15.5) | 5704 (15.6) | 1467 (14.0) | ||
| 7.6–8.5 | 22,023 (16.6) | 9725 (16.8) | 6614 (16.3) | 4872 (16.8) | 812 (15.4) | 19,886 (16.8) | 5749 (16.2) | 6383 (17.9) | 6137 (16.8) | 1617 (15.4) | ||
| 8.6–10 | 12,999 (9.8) | 6789 (11.7) | 3546 (8.7) | 2295 (7.9) | 369 (7.0) | 11,314 (9.6) | 3951 (11.1) | 3384 (9.5) | 3175 (8.7) | 804 (7.7) | ||
| > 10 | 6488 (4.9) | 4252 (7.3) | 1316 (3.2) | 792 (2.7) | 128 (2.4) | 5169 (4.4) | 2528 (7.1) | 1285 (3.6) | 1070 (2.9) | 286 (2.7) | ||
| Glucose‐lowering drugs, n (%) | ||||||||||||
| No pharmacological treatment | 38,263 (22.3) | 18,590 (24.5) | 9679 (19.0) | 7935 (21.4) | 2059 (28.5) | < 0.001 | 33,436 (22.8) | 11,010 (24.7) | 8105 (18.9) | 9833 (21.8) | 4488 (31.6) | < 0.001 |
| Oral antidiabetics | 104,252 (60.9) | 45,588 (60.0) | 32,294 (63.4) | 22,356 (60.3) | 4014 (55.5) | 84,423 (57.5) | 26,023 (58.3) | 25,843 (60.4) | 25,505 (56.5) | 7052 (49.7) | ||
| Oral antidiabetics + Insulin | 17,804 (10.4) | 7705 (10.1) | 5900 (11.6) | 3739 (10.1) | 460 (6.4) | 18,638 (12.7) | 5505 (12.3) | 6358 (14.8) | 5693 (12.6) | 1082 (7.6) | ||
| Insulin | 10,900 (6.4) | 4013 (5.4) | 3039 (6.0) | 3060 (8.3) | 698 (9.7) | 10,304 (7.0) | 2103 (4.7) | 2511 (5.9) | 4112 (9.1) | 1578 (11.1) | ||
| Hypertension (≥ 140/90 mmHg), n (%) | 109,681 (64.1) | 40,698 (53.6) | 35,839 (70.4) | 27,745 (74.8) | 5399 (74.7) | < 0.001 | 109,533 (74.6) | 25,724 (57.6) | 33,522 (78.3) | 38,274 (84.8) | 12,013 (84.6) | < 0.001 |
| BP < 140/90 mmHg, n (%) | 93,888 (66.3) | 39,954 (67.3) | 28,816 (65.5) | 21,157 (65.7) | 3961 (65.9) | < 0.001 | 81,549 (65.3) | 26,103 (71.0) | 24,296 (64.0) | 24,186 (61.9) | 6964 (62.5) | < 0.001 |
| Antihypertensive drugs, n , % | ||||||||||||
| No pharmacological treatment | 54,517 (31.8) | 33,471 (44.0) | 12,223 (24.0) | 7364 (19.9) | 1459 (20.2) | < 0.001 | 36,888 (25.1) | 19,117 (42.8) | 8710 (20.3) | 6736 (14.9) | 2325 (16.4) | < 0.001 |
| Monotherapy | 39,134 (22.9) | 16,498 (21.7) | 12,067 (23.7) | 8728 (23.5) | 1841 (25.5) | 32,839 (22.4) | 10,047 (22.5) | 9922 (23.2) | 9647 (21.4) | 3223 (22.7) | ||
| Combined treatment | 77,568 (45.3) | 26,017 (34.2) | 26,622 (52.3) | 20,998 (56.6) | 3931 (54.4) | 77,074 (52.5) | 15,477 (34.7) | 24,185 (56.5) | 28,760 (63.7) | 8652 (60.9) | ||
| Dyslipidaemia, n (%) | 84,151 (49.1) | 37,409 (49.2) | 26,653 (52.4) | 17,473 (47.1) | 2616 (36.2) | < 0.001 | 79,290 (54.0) | 22,043 (49.4) | 25,339 (59.2) | 25,358 (56.2) | 6550 (46.1) | < 0.001 |
| LDLc control, n (%) | ||||||||||||
| < 130 mg/dl; no CVD | 157,488 (58.8) | 72,845 (52.2) | 46,373 (64.2) | 32,186 (65.1) | 6084 (62.9) | < 0.001 | 138,421 (57.2) | 43,706 (50.1) | 40,837 (60.2) | 41,247 (62.0) | 12,631 (56.3) | < 0.001 |
| < 100 mg/dl and CVD | 13,731 (47.2) | 3141 (45.6) | 4539 (48.3) | 4904 (48.1) | 1147 (43.0) | 8380 (39.2) | 935 (38.0) | 1980 (41.9) | 3896 (40.6) | 1569 (33.0) | ||
| Lipid‐lowering drugs, n (%) | 95,011 (55.5) | 38,745 (51.0) | 32,015 (62.9) | 21,332 (57.5) | 2919 (40.4) | < 0.001 | 80,916 (55.1) | 21,989 (49.3) | 27,200 (63.5) | 26,101 (57.8) | 5626 (39.6) | < 0.001 |
| Smoking status, n (%) | ||||||||||||
| Smoker | 32,898 (19.7) | 21,837 (29.7) | 7662 (15.4) | 3102 (8.5) | 297 (4.3) | <0.001 | 8156 (5.7) | 6516 (15.0) | 1162 (2.8) | 413 (0.9) | 65 (0.5) | <0.001 |
| Ex‐smoker | 61,845 (37.1) | 25,201 (34.2) | 20,641 (41.4) | 13,822 (38.1) | 2181 (31.4) | 9923 (6.9) | 5386 (12.4) | 2496 (5.9) | 1613 (3.7) | 428 (3.2) | ||
| Antiplatelet drugs, n (%) | 68,503 (40.0) | 22,419 (29.5) | 23,460 (46.1) | 18,727 (50.5) | 3897 (53.9) | < 0.001 | 48,580 (33.1) | 8987 (20.1) | 14,459 (33.8) | 18,567 (41.1) | 6567 (46.2) | < 0.001 |
| HbA1c + BP+ LDLc + smoking control, n (%) | 29,570 (27.6) | 10,095 (22.6) | 10,146 (29.7) | 7862 (32.5) | 1467 (35.2) | < 0.001 | 27,792 (28.5) | 7116 (24.9) | 8886 (29.0) | 9260 (30.4) | 2530 (32.0) | < 0.001 |
HbA1c 7% = 53 mmol/mol; HbA1c 7.5% = 58.5 mmol/mol; HbA1c 8.5% = 69.4 mmol/mol; HbA1c 10% = 85.8 mmol/mol. BMI, body mass index; BP, blood pressure; HbA1c, standardised glycated haemoglobin; LDLc, low‐density lipoproteins LDL cholesterol.
In both genders, the control of dyslipidaemia, both in patients without CVD and with CVD (LDLc levels < 130 mg/dl and < 100 mg/dl, respectively) was better among patients 66–85 years than in patients ≤ 65 years, while in the age group > 85 years the highest values across all age groups was observed. Additionally, lipid‐lowering drugs were used less frequently by both women and men in the older age groups (p < 0.001). As for the use of antiplatelet agents, their use was progressively higher with increasing age (p < 0.001), being used by 53.9% of men and 46.2% of women older than 85 years. Finally, the percentage of patients currently smokers decreased with age (p < 0.001), and in all age groups there was a much lower proportion of women smokers. As for the combined control of different cardiovascular risk factors (namely HbA1c ≤ 7%, BP ≤ 140/90 mmHg, LDLc < 130 mg/dl or 100 mg/dl, and no smoking), it was achieved in 22.6% of men and 24.9% of women ≤ 65 years, while these percentages were significantly higher among patients > 65 years, ranging between 29.7% and 35.2% in men, and 29% and 32% in women across older age groups (p < 0.001).
Presence of diabetes‐related complications with age
The prevalence of chronic micro and macrovascular complications associated with T2DM by gender and age subgroup is shown in Table 3. There was a sharp increase in the frequency of heart failure and all macrovascular complications (ischaemic heart disease, stroke and peripheral artery disease) with increasing age (p < 0.001 in all complications). The global prevalence of ischaemic heart disease was 8.6% in women vs. 15.9% in men; the prevalence of stroke was 6.4% in men vs. 5.3% in women; the prevalence of peripheral artery disease was 5.7% in men vs. 2.0% in women; and the prevalence of heart failure was 4.7% in men vs. 6.6% in women.
Table 3.
Prevalence of diabetes‐related complications by gender and age subgroup
| Complication, n (%) | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ≤ 65 years | 66–75 years | 76–85 years | > 85 years | p‐value | Total | ≤ 65 years | 66–75 years | 76–85 years | > 85 years | p‐value | |
| Heart failure | 8027 (4.7) | 1472 (1.9) | 2212 (4.3) | 3331 (9.0) | 1012 (14.0) | < 0.001 | 9619 (6.6) | 601 (1.3) | 1923 (4.5) | 4731 (10.5) | 2364 (16.6) | < 0.001 |
| Ischaemic heart disease | 27,195 (15.9) | 8116 (10.7) | 9068 (17.8) | 8345 (22.5) | 1666 (23.0) | < 0.001 | 12,640 (8.6) | 1638 (3.7) | 3467 (8.1) | 5488 (12.2) | 2047 (14.4) | < 0.001 |
| Stroke | 11,032 (6.4) | 2416 (3.2) | 3640 (7.1) | 3972 (10.7) | 1004 (13.9) | < 0.001 | 7822 (5.3) | 867 (1.9) | 1840 (4.3) | 3627 (8.0) | 1488 (10.5) | < 0.001 |
| Peripheral artery disease | 9799 (5.7) | 2705 (3.6) | 3331 (6.5) | 3174 (8.6) | 589 (8.1) | < 0.001 | 3003 (2.0) | 435 (1.0) | 732 (1.7) | 1364 (3.0) | 472 (3.3) | < 0.001 |
| Diabetic retinopathy | 11,963 (7.0) | 4857 (6.4) | 4012 (7.9) | 2681 (7.2) | 413 (5.7) | < 0.001 | 11,032 (7.5) | 2419 (5.4) | 3512 (8.2) | 4132 (9.2) | 969 (6.8) | < 0.001 |
| Chronic kidney disease | ||||||||||||
| Renal failure (eGFR‐MDRD < 60 ml/min) | 18,202 (24.3) | 6067 (18.4) | 5785 (24.3) | 5346 (34.1) | 1004 (41.2) | < 0.001 | 17,836 (27.4) | 3212 (16.0) | 5051 (24.1) | 7347 (37.9) | 2226 (47.7) | < 0.001 |
| Albuminuria (ACR > 30 mg/mmol) | 4497 (6.0) | 716 (2.2) | 1400 (5.9) | 1893 (12.1) | 488 (20.0) | 4309 (6.6) | 386 (1.9) | 950 (4.5) | 2073 (10.7) | 900 (19.3) | ||
| Any CVD | 13,731 (8.0) | 3141 (4.1) | 4539 (8.9) | 4904 (13.2) | 1147 (15.9) | < 0.001 | 8380 (5.7) | 935 (2.1) | 1980 (4.6) | 3896 (8.6) | 1569 (11.0) | < 0.001 |
ACR, albumin‐to‐creatinine ratio; CVD, cardiovascular disease; eGFR‐MDRD, estimated glomerular filtration rate (eGFR) using the Modified Diet in Renal Disease (MDRD‐4) formula.
As for microvascular complications, diabetic retinopathy was more frequent among patients > 65 years (p < 0.001), although taking into account the diabetes duration, there was a progressive decrease in frequency with increasing age, particularly in men (Figure 1). The assessment of kidney disease by eGFR‐MDRD showed a progressive increase in renal failure cases (eGFR‐MDRD < 60 ml/minute) with age, which was also the case for ACR values, and both complications together were observed up to a 20% of men and 19.3% of women older than 85 years compared with a 2.2% of men and 1.9% of women ≤ 65 years (p < 0.001). Furthermore, albuminuria (ACR > 30 mg/mmol) increased with age and was more frequent in men than in women in all age groups (p < 0.001).
Figure 1.
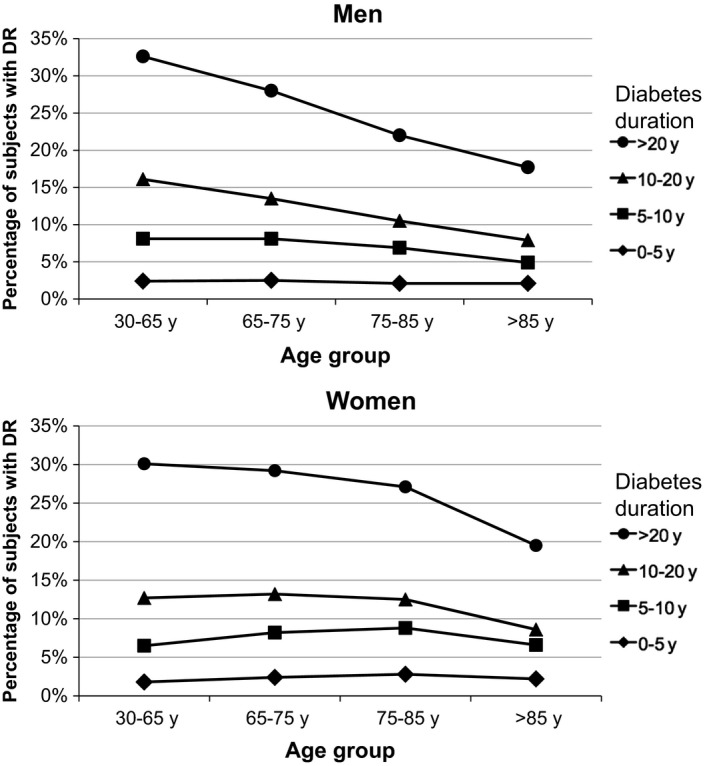
Impact of T2DM duration, age and gender on the prevalence of diabetic retinopathy (DR)
Impact of T2DM duration on glycaemic control and chronic complications with age
We further studied whether diabetes duration (≤ 5 years, 5–10 years, 10–20 years and > 20 years) was related to the degree of glycaemic control and presence of diabetes‐related complications.
The older the patients were the higher the percentage achieving a glycaemic goal of HbA1c values ≤ 7%, and this was true in all age subgroups regardless the duration of T2DM. Moreover, patients in older age subgroups were more likely to achieve target glycaemic values irrespective of having a CVD or heart failure. For instance, the proportion of patients with a disease duration ≤ 15 years and no CVD who achieved target HbA1c values ≤ 7% was 49% among those aged ≤ 65 years, and 54% among those aged 65–75 years; in patients with a disease duration > 15 years and CVD, 56% aged ≤ 65 years and 83% aged 65–75 years achieved target HbA1c values ≤ 7% and ≤ 8.5%, respectively.
As for individual diabetes‐related complications, there was a higher prevalence among subjects with a longer duration of T2DM and increasing age, particularly in those with a disease lasting for more than 20 years. This was true for heart failure (5.4% if T2DM lasting between 0 and 20 years vs. 10.1% after 20 years of diabetes across age groups, p < 0.001), coronary artery disease (12.3% vs. 21.3% after 20 years of diabetes, p < 0.001), stroke (5.8%, but 10.8% after 20 years of diabetes, p < 0.001) and peripheral artery disease (3.9% vs. 8.6% after 20 years of diabetes, p < 0.001). In the case of diabetic retinopathy, patients with a longer duration had also a higher prevalence of diabetic retinopathy (6.7% vs. 26% after 20 years of diabetes, p < 0.001). However, the prevalence decreased with increasing age (Figure 1).
Discussion
Older people are a growing population with T2DM that account for approximately 50% of all cases of diabetes in adults and have differential characteristics, requiring tailored management approaches 1, 5, 8. The present descriptive study assessed the clinical and characteristics, the degree of glycaemic control, the presence of chronic diabetes‐related complications and the use of pharmacological treatments in a population of 318,020 adults with T2DM treated in a real‐life clinical setting.
The results evidenced the existence of different profiles between age groups: except for the control of BP, diabetic patients older than 65 years had a better glycaemic control and a better control of dyslipidaemia, obesity and tobacco smoking than patients ≤ 65 years; they were less frequently treated with glucose‐lowering and lipid‐lowering drugs, but more frequently with antiplatelet agents. They also had a better control of glycaemic targets and cardiovascular risk factors in spite of a progressive increase in the prevalence of chronic complications with increasing age.
We observed a better glycaemic control among elderly patients that was independent of T2DM duration and the degree of obesity, as it has been previously reported in another cross‐sectional study 12. Moreover, glycaemic goals were more often achieved by patients in the older subgroups regardless the presence of a CVD, in accordance with the results from clinical trials and observational studies suggesting that a global control of cardiovascular risk factors in older patients provides a greater benefit regarding morbidity and mortality than an intensive glycaemic control 13, 14, 15, 16, 17. Antihypertensive treatment, for instance, has benefits even in very old patients 18, 19, 20, 21, and there are also compelling evidences of the benefit of statins and antiplatelet agents in older adults in secondary prevention of CVD, while its use in primary prevention is controversial, and individual characteristics and the risk of related adverse events should be taken into account 5, 7, 22, 23, 24, 25.
There is a lack of data regarding the benefits of the pharmacological treatment of T2DM among elderly people, mainly because they are systematically excluded from clinical trials, and evidences have been inferred from studies in middle‐aged adults 5. From our results, both men and women ≥ 65 years used less glucose‐lowering drugs, albeit with a progressive increase in insulin use with increasing age, which is consistent with the evidence that elderly patients eventually require insulin as a result of the natural progression of the disease but also because of the high prevalence of renal failure that contraindicates other antidiabetic drugs 26, 27. Moreover, as BP increases with age, older patients used more hypertensive agents than younger patients reaching a similar control of BP. In relation to lipid‐lowering drugs, they used less, which would be in agreement with the observed better control of dyslipidaemia, although the particular subgroup ≥ 85 years had the worse level of control across all age groups. This is probably because of the fact that they were using less lipid‐lowering drugs because general practitioners do not prescribe them based on the scarce evidences on the benefits of statins in very old people.
As expected, increasing age was associated with a parallel increase in the prevalence of micro and macrovascular complications except for retinopathy, which decreased after 85 years, and they were more frequent in men. This pattern was dependent on the diabetes duration and also on the degree of obesity in the case of heart failure, which suggests that control and prevention of CVDs must be an important goal based not only on the age of the patient but also on disease's duration.
The present study has several advantages and limitations. The main strength is that this is the largest cross‐sectional study conducted in Spain to study T2DM in older population through the use of a large primary care database with high quality records previously validated in other studies 28, 29, and that is closer to the real‐life clinical practice than randomised clinical trials, which usually exclude elderly patients. However, no causal associations between risk factors and presence of diabetes‐related complications can be drawn because of the cross‐sectional design, and we estimated the strength of these associations stratifying by other variables. Moreover, the retrospective design may have prevented analyses or biased results regarding some of the variables. Records for diabetic neuropathy were also scarce, probably because it is difficult to diagnose and there is a lack of uniform diagnostic criteria. In addition, and inherent to all cross‐sectional studies conducted in elderly populations, there is a survival bias because patients with diabetes‐related complications, poor control and/or severe forms of T2DM usually die at a younger age than patients with a late onset and/or well controlled disease. It is possible that those who survive have different metabolic characteristics and also a slower decline of beta cell function in the natural history of T2DM than those who do not survive. This could lead to significant bias. Avoidance of this bias could only be addressed through the design of prospective controlled long‐term follow‐up studies. Finally, mortality could have also impacted the prevalence of particular factors and complications, as populations at high risk have greater mortality rates and therefore survivors in older age groups have a lesser prevalence than actually expected.
Conclusion
Patients with T2DM older than 65 years have a better glycaemic control and a better global control of cardiovascular risk factors than younger adults. However, older age groups were also more likely to achieve glycaemic targets irrespective of having CVD and longer diabetes duration. Finally, the use of agents to control hyperglycaemia and dyslipidaemia was lower in older ages. This differential age‐ and gender‐related pattern stresses the need to individually adapt the therapeutic and care approaches of T2DM in elderly people to allow the best benefit and the lowest risk at all stages of the disease. Further research is warranted to investigate through prospective and interventional studies the observed differences in the clinical behaviour and treatment of T2DM in elderly people.
Author contributions
JB‐DLP, MM‐C and JF‐N wrote the manuscript and contributed to the discussion; XMT, AC and JMF‐R‐L contributed to the discussion; JF‐N, MM‐C and DM designed and conducted the study, reviewed/edited the manuscript and contributed to the discussion. MM‐C had full access to all data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis.
Acknowledgements
This study was funded by the Catalan Diabetes Association, the Catalan Health Department and Novartis Farmacéutica S.A. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data, or preparation, review or approval of the manuscript. CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM) and CIBER on Patophisiology, Obesity and Nutrition (CIBERON) are initiatives from Instituto de Salud Carlos III. We acknowledge Mònica Gratacòs and Maren White for providing support in the manuscript preparation and editing.
Disclosures
The authors declare that they have no conflict of interest associated with the contents of this manuscript.
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 6th edn Brussels, Belgium: International Diabetes Federation, 2013. [Google Scholar]
- 2. Soriguer F, Goday A, Bosch‐Comas A et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es study. Diabetologia 2012; 55: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . National Center for Health Statistics. Early Release of Selected Estimates Based on Data From the 2012 National Health Interview Survey: Diagnosed Diabetes. http://www.cdc.gov/nchs/nhis/released201306.htm (accessed 14 September 2015).
- 4. Sinclair AJ, Paolisso G, Castro M et al. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive Summary. Diabetes Metab 2011; 37(Suppl. 3): S27–38. [DOI] [PubMed] [Google Scholar]
- 5. Kirkman MS, Briscoe VJ, Clark N et al. Diabetes in older adults. Diabetes Care 2012; 35: 2650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care 2006; 29: 2415–9. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Standards of medical care in diabetes–2015: summary of revisions. Diabetes Care 2015; 38(Suppl. 1): s17–80.25537701 [Google Scholar]
- 8. International Diabetes Federation . Managing Older People With Type 2 Diabetes Global Guideline, 2013. http://www.idf.org/guidelines/managing-older-people-type-2-diabetes. (accessed 14 September 2015).
- 9. Huang ES, Zhang Q, Gandra N et al. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med 2008; 149: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clement M, Bhattacharyya O, Conway JR. Is tight glycemic control in type 2 diabetes really worthwhile? Yes Can Fam Physician 2009; 55: 580–4. [PMC free article] [PubMed] [Google Scholar]
- 11. Bolibar B, Fina Aviles F, Morros R et al. SIDIAP database: electronic clinical records in primary care as a source of information for epidemiologic research. Med Clin (Barc) 2012; 138: 617–21. [DOI] [PubMed] [Google Scholar]
- 12. Berkowitz SA, Meigs JB, Wexler DJ. Age at type 2 diabetes onset and glycaemic control: results from the National Health and Nutrition Examination Survey (NHANES) 2005‐2010. Diabetologia 2013; 56: 2593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerstein HC, Miller ME, Byington RP et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel A, MacMahon S, Chalmers J et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–72. [DOI] [PubMed] [Google Scholar]
- 15. Duckworth W, Abraira C, Moritz T et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–39. [DOI] [PubMed] [Google Scholar]
- 16. Greenfield S, Billimek J, Pellegrini F et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med 2009; 151: 854–60. [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Kamineni A, Carnethon M et al. Lifestyle risk factors and new‐onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009; 169: 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curb JD, Pressel SL, Cutler JA et al. Effect of diuretic‐based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276: 1886–92. [PubMed] [Google Scholar]
- 19. Cigolle CT, Blaum CS, Halter JB. Diabetes and cardiovascular disease prevention in older adults. Clin Geriatr Med 2009; 25: 607–41, vii–viii. [DOI] [PubMed] [Google Scholar]
- 20. Anderson RJ, Bahn GD, Moritz TE et al. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2011; 34: 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bulpitt C, Fletcher A, Beckett N et al. Hypertension in the Very Elderly Trial (HYVET): protocol for the main trial. Drugs Aging 2001; 18: 151–64. [DOI] [PubMed] [Google Scholar]
- 22. Collins R, Armitage J. High‐risk elderly patients PROSPER from cholesterol‐lowering therapy. Lancet 2002; 360: 1618–9. [DOI] [PubMed] [Google Scholar]
- 23. Shepherd J, Blauw GJ, Murphy MB et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360: 1623–30. [DOI] [PubMed] [Google Scholar]
- 24. Moreno G, Mangione CM. Management of cardiovascular disease risk factors in older adults with type 2 diabetes mellitus: 2002‐2012 literature review. J Am Geriatr Soc 2013; 61: 2027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. NICE (National Institute for Health and Clinical Excellence) . Clinical Guideline CG87. Type 2 Diabetes: The Management of Type 2 Diabetes. Last updated December 2014. http://guidance.nice.org.uk/CG87/NiceGuidance/pdf/English, 2014. (accessed 14 September 2015).
- 26. Spain M, Edlund BJ. Introducing insulin into diabetes management: transition strategies for older adults. J Gerontol Nurs 2011; 37: 10–5. [DOI] [PubMed] [Google Scholar]
- 27. Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab 2003; 284: E7–12. [DOI] [PubMed] [Google Scholar]
- 28. Vinagre I, Mata‐Cases M, Hermosilla E et al. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care 2012; 35: 774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mata‐Cases M, Mauricio D, Vinagre I et al. Treatment of hyperglycaemia in type 2 diabetic patients in a primary care population database in a Mediterranean area (Catalonia, Spain). J Diabetes Metab 2014; 5: 338. [Google Scholar]


