Abstract
Although the rates of vertical transmission of HIV in the developing world have improved to around 3% in countries like South Africa, resistance to antiretrovirals (ARV) used in Prevention of Mother‐to‐Child transmission (pMTCT) strategies may thwart such outcomes and affect the efficacy of future ARV regimens in mothers and children. This study conducted in Durban, South Africa, between 2010 and 2013 found a high rate of nevirapine (NVP) resistance among women receiving Zidovudine (AZT) from 14 weeks gestation, single dose nevirapine (sd NVP) at the onset of labor and a single dose of coformulated Tenofovir/Emtricitabine (TDF/FTC) postpartum. Using Sanger sequencing, high and intermediate levels of nevirapine (NVP) resistance were detected in 15/44 (34%) and in 1/44 (2%) of women tested, respectively. Most subjects selected the K103N mutation (22% (10/45) of all patients and 66% (10/15) of those with high‐level NVP resistance). Such rate of NVP resistance is comparable to studies where only sd NVP was used. In conclusion, a post‐partum single‐dose TDF/FTC tail does not prevent the selection of NNRTI resistance in women receiving pre‐partum ZDV and intrapartum sd NVP to prevent mother‐to‐child HIV‐1 transmission. J. Med. Virol. 87:1662–1667, 2015. © 2015 The Authors. Journal of Medical Virology published by Wiley Periodicals, Inc.
Keywords: HIV drug resistance, vertical transmission
INTRODUCTION
Prevention of Mother‐to‐Child HIV‐1 Transmission (pMTCT) programs in resource‐limited settings have enabled an overall reduction in the rates of vertical transmission to around 3% in countries like South Africa [Moodley et al., 2013]. However selection of antiretroviral (ARV) drug resistance during pMTCT due to issues like poor adherence, ARV access, complicated pMTCT strategies and interruptions in ARV supply remains a challenge [Paredes et al., 2013]. It is important that these challenges be overcome in order to preserve future treatment options for women exposed to ARVs used for pMTCT. Resistance associated with sd NVP is well documented [Eshleman et al., 2004; Eshleman et al., 2005; Flys et al., 2007] and occurs in 25% of women at 6–8 weeks postpartum [Eshleman et al., 2004]. A meta‐analysis of studies assessing resistance to sd NVP found the overall risk to be 37.5% [Arrive et al., 2007]. In addition, these drug resistant variants tend to persist and may result in treatment failure when the drug is re‐initiated [Flys et al., 2007; Lockman et al., 2007; Coovadia et al., 2009]. Studies that have assessed the optimal strategy for pMTCT in reducing the potential for NVP resistance have investigated, among others, Zidovudine(AZT) monotherapy according to the ACTG 076 protocol [ACTG076, 1997], AZT with Didanosine (DDI) [Lallemant et al., 2010] and short course AZT and Lamivudine (3TC) [Palmer et al., 2012].
A study by Chi et al investigated whether a single dose of Tenofovir (TDF) with Emtricitabine (FTC) given at delivery would reduce NVP resistance. NVP resistance was reduced by 53% at 6 weeks post‐delivery [Chi et al., 2007]. Subsequently the strategy of using a single dose of TDF/FTC has been included in pMTCT guidelines in certain countries. The South African pMTCT guidelines were revised in 2008, 2010 and 2013 (Table I). The 2010 guidelines provided ARV prophylaxis to women not eligible for triple therapy (ie CD4 count >350 cells/μl) .The pMTCT strategy included antenatal AZT from 14 weeks gestation, sd NVP at the onset of labor with 3 hourly AZT during labor and postpartum single dose of TDF/FTC. The aim of this study was to assess the rates of resistance in this group of patients since resistance to NVP, AZT or TDF/FTC will have clinical implications for women initiating Highly Active Antiretroviral Therapy (HAART) containing these ARVs. Although the 2013 guidelines now include the use of HAART for pMTCT the question of resistance in this group is still relevant for those women who have or will initiate HAART and for other countries in the developing world using similar strategies.
Table I.
South African pMTCT Guidelines Between 2008 and 2013
| National Department of Health (2008) | Clinical Guidelines (2010) | National Department of Health (2013) | |
|---|---|---|---|
| Gestation at initiation | 28 weeks | 14 weeks | Any |
| Regimen during pregnancy | AZT monotherapy | AZT monotherapy | Fixed Dose Combination(FDC) – FTC/TDF/EFV |
| Regimen during labor | sd NVP at onset of labor Continue with AZT 3hrly until delivery | sd NVP at onset of labor Continue with AZT 3hrly until delivery | Continue FTC/TDF/EFV |
| Post delivery | Stop all ARV's | Stat dose of Truvada (TDF and FTC) | Continue FTC/TDF/EFV |
| Postpartum period | No continuation during postpartum period | No continuation during postpartum period | Continue FDC for one week after cessation of breastfeeding |
| Infant regimen | sd NVP and AZT(for 7 or 28 days*) | NVP for 6 weeks or for duration of breastfeeding | Eligible to start HAART |
| Cd4 cutoff for initiation of HAART | ≤200 c/μl | ≤350 c/μl | ≤350 c/μl |
METHODS
This study was conducted at Lwazi clinic, Addington Hospital in Durban, South Africa. Full ethical permission was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu‐Natal (BF 069‐09). Permission from the Department of Health and management of Addington Hospital was obtained. Ninety seven patients were recruited. Patients with a CD4 count of >350cells/mm3 who did not qualify for HAART were included in the study. Full informed consent was obtained. A blood sample for viral load was collected at baseline and at 6 weeks post‐delivery. A sample for resistance testing was collected at 6 weeks post‐delivery. Information on adherence was captured at the 6 weeks post‐delivery visit where patients were asked specifically whether they received AZT and NVP during labor and TDF/FTC post‐delivery. Patients could have answered “yes”, “no” or “unsure”. Antenatal adherence counseling formed part of the routine follow up. Of the 97 patients recruited, 28 patients did not return for the 6 weeks visit, one sample was lost, 18 patients had a viral load of <1000 copies/ml and were excluded from resistance testing and six samples did not amplify. Forty four samples were included for resistance testing. Funding was provided by the Medical Research Council and the National Health Laboratory service Research Trust.
LABORATORY METHODS
Viral Load
Viral load testing was implemented at the Department of Virology, University of KwaZulu‐Natal, National Health Laboratory Services. Viral loads were performed initially using the automated Nuclisens EasyQ® (bioMerieux) HIV‐1 assay. RNA was extracted using the Nuclisens EasyMag® HIV‐1(bioMerieux). The Nuclisens Easy Q® platform was discontinued in January 2011 and was replaced with the Abbot m2000sp™ and Abbot M2000rt™ systems of extraction and real time amplification, respectively.
Primers
Primers were designed using a subtype C isolate, Genbank accession no AY772699 (http://www.ncbi.nlm.nih.gov/nuccore/AY772699). Nested primers were designed to cover reverse transcriptase (RT). Primer sequences were as follows: 1855‐F1 (1855–1882 in AY772699, 2444–2470 in HXB2), 5′‐AGAAATTTGTGGAAAAAAGGCTATAGG‐3′, 2754‐R2ON (2754–2724 in AY772699, 3343‐3314 in HXB2), 5′ TTTAATTTTCCCACTAACTTCTGTATATCA‐3′, 1863‐F1AIN (1863–1882 in AY772699, 2452–2476 in HXB2), 5′‐GTGGAAAAAAGGCTATAGGTACAGT‐3′, 2745‐R2 (2745–2720 in AY772699, 3334–3310 in HXB2), 5′‐CCCACTAACTTCTGTATATCATTGA‐3′.
RNA Preparation, Conventional RT‐PCR and PCR
Samples were collected in EDTA tubes and processed within 24–48 hr of collection. RNA was extracted from 1 ml of plasma using the Nuclisens EasyMag® HIV‐1(bioMerieux) assay and used to generate PCR products for Sanger sequencing. The extracted RNA (3.75 μl) was added to the RT‐PCR reaction mix (final volume of 12.5 μl) containing 2× buffer, MgSO4 (5 mM, final concentration of 0.6 mM), sterile water, RNAse out, forward(1855‐F1) and reverse(2754‐R2ON) primers (final concentration of 0.2 mM and SuperScript® III One‐Step RT PCR (0.5 μl) (Invitrogen, Carlsbad, CA). After a reverse transcription step at 55°C for 25 min, thermocycling was performed under the following conditions: 25 cycles of 94°C for 2 min thereafter for 30 sec, 57°C for 30 sec, 68°C for 30 sec and extension at 68°C for 1 min. One microlitre of the first round PCR product was added to the second round PCR reaction mix (final volume of 50 μl) containing 10× buffer, MgS04 (50Mm, final concentration 2 mM), dNTP (10 mM), Platinum® Taq High Fidelity enzyme (0.4 μl) (Invitrogen), sterile water and forward (1863‐F1AIN) and reverse (2745‐R2) primers (final concentration of 0.2mM) Cycling conditions were as for the first round excluding the RT step of 55°C for 25 min. 50 μl of PCR product was available for Sanger sequencing.
Sanger Sequencing
PCR products were purified using the Exo/SAP amplicon purification system [Werle et al., 1994]. Population sequencing was performed at Inqaba Biotechnical Industries (Pty) Ltd in Pretoria using the automated sequencer, ABI 3500XL genetic analyser. (Applied Biosystems, Foster City, CA). Genotypes were interpreted using the Geneious software (Biomatters Ltd. Auckland, New Zealand). Drug resistant mutations were interpreted using the Stanford database (http://hivdb.stanford.edu). To exclude sample contamination, phylogenetic analysis was performed by generating neighbor joining trees.
Statistical Analysis
Nonparametric methods, χ2 test or Fisher's exact test were used as appropriate for the type of data analyzed. SPSS (IBM, Armonk, NY) was use for all statistical analyses.
RESULTS
There was no statistically significant difference in the CD4 counts and viral load (baseline and 6 weeks) between the NVP resistant group and NVP susceptible groups (Table II).
Table II.
Patient Characteristics as Per Nevirapine Resistant Versus Susceptible Groups
| Parameter | Resistant (n = 16) | Susceptible (n = 28) | P‐value |
|---|---|---|---|
| Mean CD4 cells/mm3 | 480 | 510 | 0.871 |
| Viral load (log) | |||
| Baseline | 3.44 | 3.2 | 0.893 |
| 6 weeks | 3.87 | 3.93 | 0.598 |
| Average duration of exposure to AZT | 15 weeks | 17 weeks | 0.451 |
The average duration of exposure to AZT in the NVP resistant group was 15 weeks and in the NVP susceptible group was 17 weeks with no statistically significant difference. There was no association between the lack of intake of the stat dose of TDF/FTC and the development of NVP resistance (P = 0.621).
In the NVP resistant group only one patient reported not having received AZT in labor and one reported not having received TDF/FTC post‐delivery. In the group without NVP resistance, three patients were unsure whether they received AZT or TDF/FTC, one reported not having received any ARV's and one reported not having received TDF/FTC.
Specific information relating to antenatal AZT adherence was not captured for the purpose of the study since study visits were at baseline and at 6 weeks post‐delivery only. However, adherence to AZT was discussed at the routine antenatal visits.
Of 44 patients, resistance mutations were detected in 19 (43%) patients. All patients had a single mutation with the exception of four patients where dual mutations were found. (K103N and Y188C, K103N and G190AG, A98G and G190AG and Y188C and V90I). Sixteen (36%) patients had mutations associated with resistance to NNRTI's. Fifteen (34%) patients had mutations (viz K103N, Y181C, Y188C, Y188HY, and G190AG) associated with high level resistance to NNRTI, in particular NVP, whilst 1 (2%) patient had K101E associated with intermediate resistance to NNRTIs. The mutation most frequently detected was K103N, seen in 66% (10/15) of patients in whom a mutation conferring high‐level NNRTI resistance was detected. T69S, a mutation which confers resistance to all NRTI's was found in one patient. V90I and E138A were detected in each of two patients. These mutations are polymorphic accessory mutations and do not confer resistance to NNRTIs. No Thymidine analog mutations (TAMs) or M184V were detected.
The frequency of mutations is indicated in Figure 1.
Figure 1.
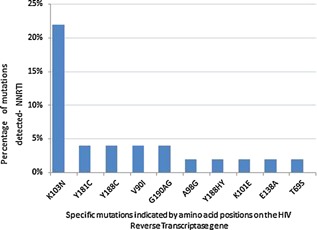
Percentage of mutations detected in 44 women exposed to pMTCT (antepartum AZT, intrapartum sdNVP and postpartum sd TDF/FTC).
DISCUSSION
In our study conducted in Durban, South Africa, among women receiving antenatal AZT for variable periods from 14 weeks gestation, sd NVP at the onset of labor and sd TDF/FTC postpartum, high level NVP resistance was detected in 34% of patients. Although the average risk of NVP resistance with the use of sd NVP is 35.7% [Arrive et al., 2007] our detection rate is still high considering that antepartum AZT and postpartum TDF/FTC were included in the pMTCT strategy and that the mean baseline CD4 counts were relatively high.
A study by Chi et al [2007], conducted in Zambia found that a postpartum single‐dose of TDF/FTC reduced NNRTI resistance by 53% at 6 weeks in women receiving pre‐partum short course AZT and intrapartum sd NVP. The pMTCT strategy used is very similar to that described in our study. However, despite the use of TDF/FTC and pre‐partum AZT, we still detected high rates of NNRTI resistance. In comparison to the study by Chi et al, AZT was administered earlier in pregnancy in our study (as early as 14 weeks gestation compared to 32 weeks) and the mean log viral load at 6 weeks was lower, (3.87 compared to 4.40), factors which should have reduced NNRTI resistance but was not found in our study. There are a number of reasons that may explain the high rate of NVP resistance detected including poor adherence to this complex pMTCT strategy, previous exposure to sd NVP and higher rates of transmitted drug resistance (TDR) in KwaZulu‐Natal. In Zambia in 2007, there probably was not as high levels of TDR (NNRTI) as we are seeing in KZN and the effect of NVP in successive pregnancies may not have been that apparent.
Although adherence to the regimen was documented at the 6 week visit by self‐reporting this method of assessment of adherence is not very accurate [Brouwer, 2011] and given the complex pMTCT strategy, poor adherence may have contributed to the higher rates of resistance detected. A study in KZN, among patients failing first line therapy found ARV resistance in 83% of patients where the majority (82.7%) of patients reported > 95% adherence [Marconi, 2008].
Previous exposure to NVP in successive pregnancies may result in higher rates of NVP resistance [Martinson, 2009].Given the length of time that sd NVP has been used for pMTCT in SA it is likely that a proportion of women may have had prior exposure to sd NVP.
The 2012 WHO report on TDR indicates that the rate of transmitted resistance in KwaZulu‐Natal increased from <5% (low) in 2005 to 5–15% (moderate) in 2010. The rate in 2010 is much higher than that of Gauteng, South Africa. Factors such as poor adherence, interruptions in ARV supply, population level based rates of viral load suppression and lost to follow up are among some of the issues that could be contributing to a higher rate of transmitted resistance in KwaZulu‐Natal although these factors are not unique to KZN alone [WHO, 2012]. A 2013 study in Durban among women being screened for HIV prevention trials found baseline resistance of 7.4% confirming higher levels of TDR in KZN [Parikh, 2013].
Besides higher rates of TDR, perhaps more importantly and pertinent to the pMTCT program is that the most common TDR mutations detected are those conferring NNRTI resistance, in particular, K103N. The WHO report (2012) indicates that the rate of NNRTI resistance in the African region has increased from 1% in 2003 to 6.4% in 2010 with K103N/S being the most commonly detected mutation [WHO, 2012]. A pooled analysis of sequences submitted to genbank from TDR surveys conducted in SA over 10 years also found K103N followed by Y181C to be the most common TDR mutation in SA [Manasa, 2012]. The development of NVP resistance after exposure to sd NVP may feed the increasing pool of TDR which could, in turn, compromise first line NNRTI‐containing ART.
There have also been higher rates of NVP resistance reported in subtype C [Flys et al., 2006]. Eshleman et al compared the rates of NVP resistance in subtypes A, D, and C. The number of women with NVP resistance was much higher in subtype C (69.2%) compared to subtype A (19.4%) and D (36.1%). [Eshleman et al., 2005] Furthermore, using LigAmp to detect minority K103N variants, higher levels of K103N were detected in subtype C compared to A and D [Flys et al., 2006]. The role of subtype C may be a contributor but does not by itself explain the high rates of resistance detected in this study since other studies assessing ARV drug resistance after similar pMTCT strategies in South Africa did not find as high rates of NVP resistance [van Zyl, 2008].
The use of sd NVP is problematic for both the mother and her baby. The development of NNRTI resistance after exposure to sd NVP can lead to poor clinical response to NNRTI containing regimens in the mother [Lockman et al., 2007; Stringer et al., 2010], transmitted NNRTI resistance to the infected infant [Micek et al., 2014] and poor clinical response to NNRTI‐containing ART in the infant [Musiime et al., 2009; Lehman et al., 2012]. High rates of NVP resistance in infants exposed to sd NVP in South Africa have been reported [Martinson, 2007; Hunt et al., 2011].
The study did not detect TAMs consistent with other studies investigating AZT resistance used in the pMTCT context where either no or low level TAMS were detected [Coffie, 2008; van Zyl, 2008]. In addition, no K65R mutation associated with resistance to TDF was observed.
The limitations of this study include the lack of baseline resistance information, the variable timing of the 6 week visit since patients may have returned earlier or later to the clinic depending on logistical factors and the lack of adherence information regarding antepartum AZT. In addition, the true prevalence of resistance mutations may be under‐estimated since Sanger sequencing will only detect mutations that are present in at least 20% of the HIV quasisispecies. Thus more sensitive, next generation sequencing tools are required to assess HIV minority variants.
The pMTCT strategy described in this study is similar to that of the World Health Organisation (WHO) Option A [WHO, 2012], with the exception of a stat dose TDF/FTC instead of 7 days of AZT/3TC to cover the NVP tail. Given the high rate of NVP resistance despite antepartum AZT and postpartum TDF/FTC, Option B [WHO, 2012] and B+[WHO, 2012] need to be considered to optimize treatment options for patients initiating ART after exposure to pMTCT. HAART is superior to AZT (with or without sd NVP) for pMTCT resulting in improved pMTCT and HIV‐free survival [Dryden‐Peterson et al., 2011]. Although the South African pMTCT guidelines of 2013 moved to Option B (with Option B+ to be implemented on 1st January 2015), similar strategies are still being used in other developing countries. In addition, the clinical impact of efavirenz containing first line treatment in women exposed to such pMTCT strategies remains to be seen.
In conclusion, in this resistance study conducted among pMTCT recipients receiving pre‐partum AZT, intrapartum sd NVP and post‐partum single‐dose TDF/FTC, high rates of NVP resistance was detected, in particular K103N. Whether this is attributable to poor adherence, higher levels of transmitted resistance, previous exposure to sd NVP or the association of K103N with subtype C, sd NVP, although simple and effective for pMTCT, still remains problematic.
The copyright line for this article was changed on 17 November 2015 after original online publication.
REFERENCES
- ACTG076 and reduction of perinatal transmission. Update Natl Minor AIDS Counc, 1997. p. 4–7, 9. [PubMed]
- Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, Leroy V, Perre PV, Rouzioux C, Dabis F. 2007. Prevalence of resistance to nevirapine in mothers and children after single‐dose exposure to prevent vertical transmission of HIV‐1: A meta‐analysis. Int J Epidemiol 36:1009–1021. [DOI] [PubMed] [Google Scholar]
- Brouwer ES, Napravnik S, Smiley SG, Corbett AH, Eron JJ, Jr . 2011. Self‐report of current and prior antiretroviral drug use in comparison to the medical record among HIV‐infected patients receiving primary HIV care. Pharmacoepidemiol Drug Saf 20:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Sinkala M, Mbewe F, Cantrell RA, Kruse G, Chintu N, Aldrovandi GM, Stringer EM, Kankasa C, Safrit JT, Stringer JS. 2007. Single‐dose tenofovir and emtricitabine for reduction of viral resistance to non‐nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: An open‐label randomised trial. Lancet 370:1698–1705. [DOI] [PubMed] [Google Scholar]
- Clinical Guidelines : PMTCT (Prevention of Mother‐to‐child tranmission). South Africa: National Department of Health 2010.
- Coffie PA, Ekouevi DK, Chaix ML, Tonwe‐Gold B, Clarisse AB, Becquet R, Viho I, N'Dri‐Yoman T, Leroy V, Abrams EJ, Rouzioux C, Dabis F. 2008. Maternal 12‐month response to antiretroviral therapy following prevention of mother‐to‐child transmission of HIV type 1, Ivory Coast, 2003–2006. Clin Infect Dis 46:611–621. [DOI] [PubMed] [Google Scholar]
- Coovadia A, Hunt G, Abrams EJ, Sherman G, Meyers T, Barry G, Malan E, Marais B, Stehlau R, Ledwaba J, Hammer SM, Morris L, Kuhn L. 2009. Persistent minority K103N mutations among women exposed to single‐dose nevirapine and virologic response to nonnucleoside reverse‐transcriptase inhibitor‐based therapy. Clin Infect Dis 48:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden‐Peterson S, Jayeoba O, Hughes MD, Jibril H, Keapoletswe K, Tlale J, Modise TA, Asmelash A, Moyo S, van Widenfelt E, Makhema J, Essex M, Shapiro RL, Lockman S. 2011. Highly active antiretroviral therapy versus zidovudine for prevention of mother‐to‐child transmission in a programmatic setting, Botswana. J Acquir Immune Defic Syndr 58:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman SH, Guay LA, Mwatha A, Brown ER, Cunningham SP, Musoke P, Mmiro F, Jackson JB. 2004. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV‐1 6‐8 weeks after single‐dose nevirapine (HIVNET 012). J Acquir Immune Defic Syndr 35:126–130. [DOI] [PubMed] [Google Scholar]
- Eshleman SH, Guay LA, Mwatha A, Cunningham SP, Brown ER, Musoke P, Mmiro F, Jackson JB. 2004. Comparison of nevirapine (NVP) resistance in Ugandan women 7 days vs. 6‐8 weeks after single‐dose nvp prophylaxis: HIVNET 012. AIDS Res Hum Retroviruses 20:595–599. [DOI] [PubMed] [Google Scholar]
- Eshleman SH, Hoover DR, Chen S, Hudelson SE, Guay LA, Mwatha A, Fiscus SA, Mmiro F, Musoke P, Jackson JB, Kumwenda N, Taha T. 2005. Nevirapine (NVP) resistance in women with HIV‐1 subtype C, compared with subtypes A and D, after the administration of single‐dose NVP. J Infect Dis 192:30–36. [DOI] [PubMed] [Google Scholar]
- Flys TS, Mwatha A, Guay LA, Nakabiito C, Donnell D, Musoke P, Mmiro F, Jackson JB, Eshleman SH. 2007. Detection of K103N in Ugandan women after repeated exposure to single dose nevirapine. AIDS 21:2077–2082. [DOI] [PubMed] [Google Scholar]
- Flys TS, Donnell D, Mwatha A, Nakabiito C, Musoke P, Mmiro F, Jackson JB, Guay LA, Eshleman SH. 2007. Persistence of K103N‐containing HIV‐1 variants after single‐dose nevirapine for prevention of HIV‐1 mother‐to‐child transmission. J Infect Dis 195:711–715. [DOI] [PubMed] [Google Scholar]
- Flys TS, Chen S, Jones DC, Hoover DR, Church JD, Fiscus SA, Mwatha A, Guay LA, Mmiro F, Musoke P, Kumwenda N, Taha TE, Jackson JB, Eshleman SH. 2006. Quantitative analysis of HIV‐1 variants with the K103N resistance mutation after single‐dose nevirapine in women with HIV‐1 subtypes A, C, and D. J Acquir Immune Defic Syndr 42:610–613. [DOI] [PubMed] [Google Scholar]
- Hunt GM, Coovadia A, Abrams EJ, Sherman G, Meyers T, Morris L, Kuhn L. 2011. HIV‐1 drug resistance at antiretroviral treatment initiation in children previously exposed to single‐dose nevirapine. AIDS 25:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemant M, Ngo‐Giang‐Huong N, Jourdain G, Traisaithit P, Cressey TR, Collins IJ, Jarupanich T, Sukhumanant T, Achalapong J, Sabsanong P, Chotivanich N, Winiyakul N, Ariyadej S, Kanjanasing A, Ratanakosol J, Hemvuttiphan J, Kengsakul K, Wannapira W, Sittipiyasakul V, Pornkitprasarn W, Liampongsabuddhi P, McIntosh K, Van Dyke RB, Frenkel LM, Koetsawang S, Le Coeur S, Kanchana S. 2010. Efficacy and safety of 1‐month postpartum zidovudine‐didanosine to prevent HIV‐resistance mutations after intrapartum single‐dose nevirapine. Clin Infect Dis 50:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman DA, Wamalwa DC, McCoy CO, Matsen FA, Langat A, Chohan BH, Benki‐Nugent S, Custers‐Allen R, Bushman FD, John‐Stewart GC, Overbaugh J. 2012. Low‐frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine‐based treatment. J Acquir Immune Defic Syndr 60:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, Chand F, Makhema J, Moffat C, Asmelash A, Ndase P, Arimi P, van Widenfelt E, Mazhani L, Novitsky V, Lagakos S, Essex M. 2007. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med 356:135–147. [DOI] [PubMed] [Google Scholar]
- Manasa J, Katzenstein D, Cassol S, Newell ML, de Oliveira T, Southern Africa T, Resistance N. 2012. Primary drug resistance in South Africa: Data from 10 years of surveys. AIDS Res Hum Retroviruses 28:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi VC, Sunpath H, Lu Z, Gordon M, Koranteng‐Apeagyei K, Hampton J, Carpenter S, Giddy J, Ross D, Holst H, Losina E, Walker BD, Kuritzkes DR. 2008. Prevalence of HIV‐1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis 46:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson NA, Morris L, Gray G, Moodley D, Pillay V, Cohen S, Dhlamini P, Puren A, Bhayroo S, Steyn J, McIntyre JA. 2007. Selection and persistence of viral resistance in HIV‐infected children after exposure to single‐dose nevirapine. J Acquir Immune Defic Syndr 44:148–153. [DOI] [PubMed] [Google Scholar]
- Martinson NA, Morris L, Johnson J, Gray GE, Pillay V, Ledwaba J, Dhlamini P, Cohen S, Puren A, Steyn J, Heneine W, McIntyre JA. 2009. Women exposed to single‐dose nevirapine in successive pregnancies: Effectiveness and nonnucleoside reverse transcriptase inhibitor resistance. AIDS 23:809–816. [DOI] [PubMed] [Google Scholar]
- Micek MA, Dross S, Blanco AJ, Beck IA, Matunha L, Seidel K, Montoya P, Matediana E, Gantt S, Gloyd S, Frenkel L. 2014. Transmission of Nevirapine‐Resistant HIV Type 1 via Breast Milk to Infants After Single‐Dose Nevirapine in Beira, Mozambique. J Infect Dis 210:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley P, Parboosing R, Moodley D. 2013. Reduction in Perinatal HIV Infections in KwaZulu‐Natal, South Africa, in the Era of More Effective Prevention of Mother to Child Transmission Interventions (2004–2012). J Acquir Immune Defic Syndr 63:410–415. [DOI] [PubMed] [Google Scholar]
- Musiime V, Ssali F, Kayiwa J, Namala W, Kizito H, Kityo C, Mugyenyi P. 2009. Response to nonnucleoside reverse transcriptase inhibitor‐based therapy in HIV‐infected children with perinatal exposure to single‐dose nevirapine. AIDS Res Hum Retroviruses 25:989–996. [DOI] [PubMed] [Google Scholar]
- Policy and Guidelines for the Implementation of the PMTCT Programme. South Africa. National Department of Health. 2008.
- Updates on Revised Antiretroviral Treatment Guidelines 2013. South Africa : National Department of Health. 2013. (27 March).
- Palmer S, Boltz VF, Chow JY, Martinson NA, McIntyre JA, Gray GE, Hopley MJ, Mayers D, Robinson P, Hall DB, Maldarelli F, Coffin JM, Mellors JW. 2012. Short‐course Combivir after single‐dose nevirapine reduces but does not eliminate the emergence of nevirapine resistance in women. Antivir Ther 17:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. 2013. Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis 207:S93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh UM, Kiepiela P, Ganesh S, Gomez K, Horn S, Eskay K, Kelly C, Mensch B, Gorbach P, Soto‐Torres L, Ramjee G, Mellors JW, Team MTNP, Taskforce IP. 2013. Prevalence of HIV‐1 drug resistance among women screening for HIV prevention trials in KwaZulu‐Natal, South Africa (MTN‐009). PloS ONE 8:e59787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JS, McConnell MS, Kiarie J, Bolu O, Anekthananon T, Jariyasethpong T, Potter D, Mutsotso W, Borkowf CB, Mbori‐Ngacha D, Muiruri P, Ong'ech JO, Zulu I, Njobvu L, Jetsawang B, Pathak S, Bulterys M, Shaffer N, Weidle PJ. 2010. Effectiveness of non‐nucleoside reverse‐transcriptase inhibitor‐based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi‐country, prospective cohort study. PLoS Med 7:e1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, HIV Drug Resistance Report 2012.
- WHO. Use of Antiretroviral Drugs for treating Pregnancy women and preventing HIV infection in Infants. 2012. [Google Scholar]
- Werle E, Schneider C, Renner M, Volker M, Fiehn W. 1994. Convenient single‐step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res 22:4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl GU, Claassen M, Engelbrecht S, Laten JD, Cotton MF, Theron GB, Preiser W. 2008. Zidovudine with nevirapine for the prevention of HIV mother‐to‐child transmission reduces nevirapine resistance in mothers from the Western Cape, South Africa. J Med Virol 80:942–946. [DOI] [PubMed] [Google Scholar]


