Abstract
Objective
The effect of providing antenatal dietary and lifestyle advice on secondary measures of maternal anthropometry was evaluated and their correlation with both gestational weight gain and infant birth weight was assessed.
Methods
In a multicenter, randomized controlled trial, pregnant women with BMI of ≥25 kg/m2 received either Lifestyle Advice or Standard Care. Maternal anthropometric outcomes included arm circumference, biceps, triceps, and subscapular skinfold thickness measurements (SFTM), percentage body fat (BF), gestational weight gain, and infant birth weight. The intention to treat principles were utilized by the analyses.
Results
The measurements were obtained from 807 (74.7%) women in the Lifestyle Advice Group and 775 (72.3%) women in the Standard Care Group. There were no statistically significant differences identified between the treatment groups with regards to arm circumference, biceps, triceps, and subscapular SFTM, or percentage BF at 36‐week gestation. Maternal anthropometric measurements were not significantly correlated with either gestational weight gain or infant birth weight.
Conclusions
Among pregnant women with a BMI of ≥25 kg/m2, maternal SFTM were not modified by an antenatal dietary and lifestyle intervention. Furthermore, maternal SFTM correlate poorly with both gestational weight gain and infant birth weight.
Introduction
Overweight and obesity represent a considerable disease burden during pregnancy, with half of all women having a body mass index (BMI) of ≥25 kg/m2 on entering pregnancy 1, 2. The literature highlights the associations between maternal obesity and an increased risk of adverse outcomes for both women and their infants, all of which increase as maternal BMI increases 3, 4. Additionally, there is evidence of a persisting longer‐term health legacy, maternal obesity being associated with an increased risk of high infant birth weight 4 and, in turn, subsequent obesity 5, 6.
The US Institute of Medicine has summarized the observational literature relating to maternal gestational weight gain, reporting average total weight gains between 10 and 15 kg 7. However, there was considerable interindividual variation with many women, particularly those with a BMI of ≥25 kg/m2, exceeding this average 7. Nevertheless, current recommendations suggest a weight gain of 7.0‐11.5 kg for women with a BMI of 25‐29.9 kg/m2 and 5.0‐9.0 kg for women with a BMI of 30 kg/m2 or more 7.
In nonpregnant individuals, there is a clear relationship between measured weight gain and adiposity gain. In contrast, gestational weight gain while used as a surrogate marker of adiposity 8 in fact reflects a combination of maternal fat deposition, pregnancy‐related plasma volume expansion, peripheral edema, placental mass, fetal mass, and amniotic fluid volume 9. Furthermore, from a practical perspective, determining the relative contribution of each of these individual components is extremely difficult 9.
Although a number of techniques have been proposed to differentiate between the various components and more accurately determine maternal adiposity, all have limitations, particularly during pregnancy. Although BMI correlates well with the markers of adult health and disease risk, it does not differentiate between adipose and lean tissue mass or between maternal and fetal contributions to weight gain. Bioimpedance analysis, dual‐energy X‐ray absorptiometry, computed tomography, magnetic resonance imaging, and three or four compartment model assessments all more accurately determine adipose and lean tissues. Limitations include the lack of differentiation between maternal and fetal tissues, exposure to ionizing radiation 9, 10, and lack of portability and expense 9, 11, 12, all of which make these assessments impractical to carry out on a large scale.
In contrast, skinfold thickness measurements (SFTM) have been proposed for use in pregnancy as they are reproducible with specific training and adherence to defined protocols, and through the measurement of between three and seven body sites, they allow estimation of body fat (BF) percentage 9, 13. In particular, use of the biceps, triceps, and subscapular sites allows the evaluation of pregnancy‐related changes in adipose tissue that are more independent of fetal growth 9, 14. Although some have suggested that SFTM lack reproducibility 15, we have documented acceptable interobserver variability from a population of pregnant women with a BMI of ≥25 kg/m2 16.
Observational studies involving lean pregnant women suggest deposition of adipose tissue in the second trimester of pregnancy with subsequent mobilization in late gestation 17, 18, 19, 20. It is unclear if this is also the case among women with a BMI of ≥25 kg/m2, who have increased reliance on lipid metabolism during pregnancy 21. Similarly, the relationship between changes in adipose tissue deposition and SFTM, particularly among women with a BMI of ≥25 kg/m2 19, 22, and any correlation with either gestational weight gain or infant birth weight 23, 24 remain to be determined.
We have reported the primary findings of the LIMIT randomized trial evaluating the provision of antenatal dietary and lifestyle advice to women with a BMI of ≥25 kg/m2, which indicate a significant 18% relative risk reduction in infant birth weight of >4 kg 25, mediated by improvements in maternal diet and physical activity 26. The aims of this study were twofold. First, we report the effect of providing antenatal dietary and lifestyle advice on the measures of maternal SFTM; and second, we assess the correlation between maternal SFTM and both gestational weight gain and infant birth weight.
Methods
Study design
We conducted a multicenter, randomized trial involving three maternity hospitals across Adelaide, South Australia. The methods 27 and primary findings 25, 26, 28 from the LIMIT randomized trial have been reported, and the trial registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12607000161426).
Inclusion and exclusion criteria
Women with BMI of ≥25 kg/m2 and singleton pregnancy at 10+0‐20+0 weeks gestation were eligible.
Trial entry
All women presenting for antenatal care had their height and weight measured, and BMI calculated at the first antenatal appointment. Eligible women were presented with written information, and all women provided written informed consent.
Randomization, masking, and group allocation
Randomization occurred by telephoning the central randomization service, which used a computer‐generated schedule, with balanced variable blocks. Stratification occurred for parity (0 vs. 1/more), BMI at antenatal booking (25‐29.9 vs. ≥30 kg/m2), and collaborating center. Women were randomized to either “Lifestyle Advice” or “Standard Care.”
Treatment schedules
Lifestyle Advice Group
Women randomized to receive Lifestyle Advice participated in a comprehensive dietary and lifestyle intervention over the course of pregnancy that included a combination of dietary, physical activity, and behavioral strategies, delivered by a research dietician and trained research assistants 25, 26. Within 2 weeks of randomization, women attended a planning session with a research dietician, during which a detailed dietary and physical activity history was obtained 25, 26. Women were provided with individualized dietary advice consistent with current Australian standards, to maintain a balance of carbohydrates, fat, and protein, to reduce intake of foods high in refined carbohydrates and saturated fats, while increasing intake of fiber, and promoting consumption of two servings of fruit, five servings of vegetables, and three servings of dairy each day. Physical activity advice focussed on the benefits of exercise in pregnancy, and tips to increase incidental activity and walking. Women were encouraged to set achievable goals for change, and to self‐monitor their progress. This information was reinforced during subsequent inputs provided by the research dietician (at 28‐week gestation) and trained research assistants (via telephone call at 22‐, 24‐, and 32‐week gestation and a face‐to‐face visit at 36‐week gestation).
Standard Care Group
Women receiving Standard Care continued their pregnancy care according to local hospital guidelines, which did not include routine provision of advice related to diet, exercise, or gestational weight gain.
Study end points: maternal anthropometry
At trial entry and 36‐week gestation, maternal anthropometric measures were obtained 16, using a standardized protocol on the right‐hand side of the woman's body 13.
Arm circumference
With the woman standing with her arms relaxed at her side, the midpoint between the most superior and the lateral point of the acromion border and the most proximal and lateral border of the head of the radius was identified and the circumference measured using the crosshand technique, with the reading taken at eye level with constant tension applied to the tape 13, 16. Two separate marks were made on the woman's arm on the most anterior point of the biceps and the most posterior point of the triceps, to assist in locating these skinfold landmarks 13, 16.
Skinfold thickness measurements
SFTM were obtained using Harpenden Callipers, with a dial graduation of 0.2 mm and measuring range 0‐80 mm, which was viewed at 90° to avoid errors of parallax. Two measurements were taken, with a third measurement obtained if the difference was >7.5% 13, 16. The measurements were recorded to the nearest 0.1 mm and reported as the average of two measurements or the median of 3 13, 16.
Biceps
With the woman standing relaxed, with arms at her side, the biceps skinfold was visualized, and the site located on the anterior surface of the arm, in line with the mid‐arm point (as marked when the arm circumference was measured) and parallel to the long axis 13, 16.
Triceps
With the woman standing relaxed and her arm slightly pronated, the triceps skinfold was visualized from the posterior surface of the arm, in line with the mid‐arm point in the horizontal plane (as marked when the arm circumference was measured), and parallel to the long axis 13, 16.
Subscapular
The inferior angle of the scapula was palpated, and a site marked 2 cm and 45° inferiorly 13, 16. If required, the woman was asked to reach behind her back with her right arm to better expose the scapula 13, 16.
BF percentage
Using the following equation for use with pregnant women with a BMI of ≥25 kg/m2, percentage BF was calculated, where SFTM were reported in millimeters, and arm circumference and height were reported in centimeters 16.
BF% = 12.7 + 0.457 (Triceps SFTM) + 0.352 (Subscapular SFTM) + 0.103 (Biceps SFTM) − 0.057 (Height) + 0.265 (Arm circumference).
Gestational weight gain
Gestational weight gain was calculated as the difference in measured weight by subtracting the weight obtained at trial entry from the weight obtained at 36 weeks of gestation. Weight was obtained with the woman in light clothing and without shoes, using digital scales, and recorded to the nearest 0.1 kg. Height was obtained without shoes, using a wall‐mounted stadiometer, and recorded to the nearest 0.5 cm.
Analysis and reporting of results
The analyses were performed on an intention to treat basis, with women analyzed according to their randomized treatment group. Women were included in the analysis if they consented to anthropometric measurements being taken, and did not withdraw consent to use their data or have a miscarriage, or termination of pregnancy. The outcomes considered were the anthropometric measurements taken at 36‐week gestation, and both the total and the average weekly change in anthropometric measurements between trial entry and 36 weeks. The analyses were performed using linear regression models to determine the effect of treatment group on each outcome, adjusting for center, parity, BMI category, age, socioeconomic status, the anthropometric measurement at trial entry, and either the actual gestational age at measurement (for anthropometric measurements at 36 weeks), or the time between measurements (for changes in anthropometric measurements). Exploratory analyses were conducted to assess whether the effect of treatment varied by BMI category (overweight vs. obese), by including an interaction between treatment group and BMI category in the regression model. Statistical significance was assessed at the two‐sided P < 0.05 level and no adjustment was made for multiple comparisons. Pearson's correlation coefficients were calculated to measure the strength of association between anthropometric measurements, total gestational weight gain, and infant birth weight. All analyses were performed using SAS v9.3 (Cary, NC, USA).
Sample size
The sample size of 2,180 women was predetermined based on the primary outcome, large for gestational age infant 25.
Ethics
Ethics approval was provided by the Women's and Children's Local Health Network Human Research and Ethics Committee at the Women's and Children's Hospital, the Central Northern Adelaide Health Service Ethics of Human Research Committee (Lyell McEwin Hospital) and the Flinders Clinical Research Ethics Committee (Flinders Medical Centre).
Results
Between June 2008 and December 2011, 2,212 women were randomized, with 1,108 allocated to receive Lifestyle Advice, and 1,104 Standard Care. There were 2,154 women (1,080 Lifestyle Advice; 1,072 Standard Care) available for inclusion in the analyses, after excluding women who withdrew consent to use their data (ten women) or had a miscarriage, or termination of pregnancy (50 women) 25. The measurements were obtained from 872 (80.7%) women at trial entry, and 807 (74.7%) at 36 weeks of gestation in the Lifestyle Advice Group, and 835 (77.9%) women at trial entry, and 775 (72.3%) at 36 weeks of gestation in the Standard Care Group, who were included in the analyses (Figure 1). The median time between trial entry and 36‐week gestation measurements was 18.4 weeks (interquartile range, 16.0‐21.6 weeks). Baseline demographic and anthropometric measures of the women who had their measurements obtained were similar between treatment groups (Table 1) and their clinical characteristics similar to the full randomized groups (data not shown) 25.
Figure 1.
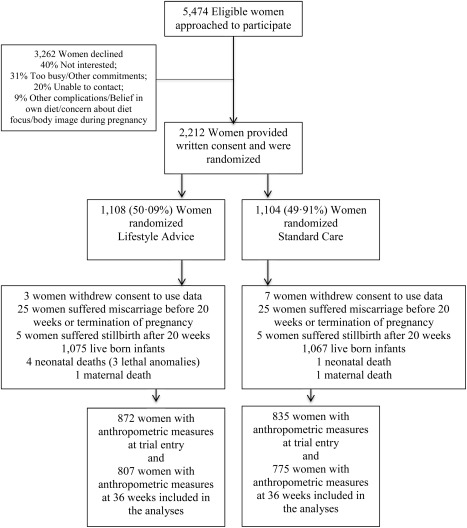
Flow of participants.
Table 1.
Baseline demographic and anthropometric measurements at trial entry
| Characteristic | Lifestyle Advice, N = 872a | Standard Care, N = 835a | Total, N = 1,707a |
|---|---|---|---|
| Maternal age (years)b | 29.6 (5.3) | 29.6 (5.5) | 29.6 (5.4) |
| Gestational age at entry (weeks)c | 14.1 (12.0‐16.9) | 14.3 (12.0‐17.0) | 14.1 (12.0‐17.0) |
| BMI (kg/m2)c | 31.0 (28.0‐35.9) | 31.1 (27.6‐35.8) | 31.0 (27.8‐35.8) |
| BMI category d | |||
| 25.0‐29.9 | 365 (41.9) | 360 (43.1) | 725 (42.5) |
| 30.0‐34.9 | 249 (28.6) | 238 (28.5) | 487 (28.5) |
| 35.0‐39.9 | 167 (19.2) | 135 (16.2) | 302 (17.7) |
| ≥40.0 | 91 (10.4) | 102 (12.2) | 193 (11.3) |
| Public patientd | 855 (98.1) | 814 (97.5) | 1669 (97.8) |
| Weight (kg)b | 88.4 (17.0) | 88.3 (17.6) | 88.3 (17.3) |
| Height (cm)b | 164.9 (6.6) | 164.9 (6.5) | 164.9 (6.5) |
| Raced | |||
| Caucasian | 789 (90.5) | 764 (91.5) | 1553 (91.0) |
| Asian | 19 (2.2) | 21 (2.5) | 40 (2.3) |
| Indian | 30 (3.4) | 27 (3.2) | 57 (3.3) |
| Other | 34 (3.9) | 23 (2.8) | 57 (3.3) |
| Smokerd | 110 (12.6) | 93 (11.1) | 203 (11.9) |
| Nulliparousd | 354 (40.6) | 358 (42.9) | 712 (41.7) |
| Index of socio economic disadvantage e | |||
| Unknown | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Quintile 1 (most disadvantaged) | 262 (30.0) | 240 (28.7) | 502 (29.4) |
| Quintile 2 | 207 (23.7) | 212 (25.4) | 419 (24.5) |
| Quintile 3 | 133 (15.3) | 125 (15.0) | 258 (15.1) |
| Quintile 4 | 126 (14.4) | 133 (15.9) | 259 (15.2) |
| Quintile 5 (least disadvantaged) | 143 (16.4) | 124 (14.9) | 267 (15.6) |
| Arm girth at trial entry (cm)b | 35.5 (4.4) | 35.4 (4.4) | 35.5 (4.4) |
| Biceps SFTM at trial entry (mm)b | 18.2 (7.5) | 17.4 (6.9) | 17.8 (7.2) |
| Triceps SFTM at trial entry (mm)b | 27.8 (7.3) | 28.4 (7.4) | 28.1 (7.4) |
| Subscapular SFTM at trial entry (mm)b | 28.8 (9.1) | 29.4 (9.7) | 29.1 (9.4) |
| Percent BF at trial entryb | 37.4 (7.2) | 37.7 (7.5) | 37.6 (7.3) |
| GA at trial entry measurement (weeks)c | 17.0 (14.3‐19.9) | 18.9 (14.9‐20.4) | 17.6 (14.6‐20.3) |
Includes all women randomized who did not withdraw consent to use their data and who had anthropometric measurements taken at baseline.
Mean and standard deviation.
Median and interquartile range.
Number and percentage.
Socio economic index as measured by SEIFA.
There were no statistically significant differences between those women receiving Lifestyle Advice and those receiving Standard Care, with regards to arm circumference, and biceps, triceps, and subscapular SFTM obtained at 36‐week gestation (Table 2). There was no difference observed between groups in the change in these measurements from baseline to 36 weeks, when expressed either as total change, or as an average weekly change to account for variation in gestational age at measurement. The calculated percentage BF at 36‐week gestation did not significantly differ between the two treatment groups (37.66 ± 7.12% Lifestyle Advice vs. 37.25 ± 7.02% Standard Care; adjusted mean difference 0.48; 95% CI −0.01 to 1.00; P = 0.07), with no difference observed in the change in percentage BF expressed either as total change across gestation, or as average weekly change. There was no evidence to suggest that the intervention effect was modified by maternal BMI category (data not shown).
Table 2.
Maternal anthropometric outcomes by treatment groupa
| Outcome | Lifestyle Advice Group | Standard Care Group | Unadjusted treatment effect (95% CI) | Unadjusted P‐value | Adjusted treatment effect (95% CI) | Adjusted P‐value |
|---|---|---|---|---|---|---|
| Arm girth at 36 weeks (cm) | 35.61 (4.13) | 35.47 (4.24) | 0.14 (−0.27, 0.56) | 0.4909 | −0.03 (−0.19, 0.12) | 0.6709 |
| Total change in arm girth (cm) | 0.06 (1.66) | 0.14 (1.61) | −0.08 (−0.25, 0.09) | 0.3474 | −0.03 (−0.19, 0.13) | 0.7188 |
| Average weekly change in arm girth (cm) | 0.00 (0.09) | 0.01 (0.09) | −0.00 (−0.01, 0.00) | 0.3199 | −0.00 (−0.01, 0.01) | 0.6952 |
| Biceps skinfold at 36 weeks (mm) | 16.32 (6.26) | 15.95 (5.97) | 0.38 (−0.23, 0.98) | 0.2230 | 0.06 (−0.46, 0.58) | 0.8124 |
| Total change in biceps skinfold (mm) | −1.88 (6.54) | −1.38 (6.55) | −0.50 (−1.17, 0.17) | 0.1409 | 0.02 (−0.50, 0.55) | 0.9289 |
| Average weekly change in biceps skinfold (mm) | −0.10 (0.35) | −0.08 (0.36) | −0.03 (−0.06, 0.01) | 0.1356 | −0.00 (−0.03, 0.03) | 0.8673 |
| Triceps skinfold at 36 weeks (mm) | 27.91 (7.60) | 27.44 (7.29) | 0.48 (−0.26, 1.21) | 0.2032 | 0.58 (−0.07, 1.23) | 0.0790 |
| Total change in triceps skinfold (mm) | −0.00 (7.71) | −0.83 (7.42) | 0.83 (0.06, 1.61) | 0.0346 | 0.53 (−0.13, 1.18) | 0.1149 |
| Average weekly change in triceps skinfold (mm) | −0.00 (0.42) | −0.05 (0.42) | 0.05 (0.01, 0.09) | 0.0203 | 0.03 (−0.00, 0.07) | 0.0806 |
| Subscapular skinfold at 36 weeks (mm) | 29.91 (8.82) | 29.66 (8.98) | 0.25 (−0.63, 1.14) | 0.5773 | 0.55 (−0.12, 1.23) | 0.1052 |
| Total change in subscapular skinfold (mm) | 0.90 (7.74) | 0.05 (7.24) | 0.85 (0.07, 1.62) | 0.0325 | 0.50 (−0.17, 1.18) | 0.1429 |
| Average weekly change in subscapular skinfold (mm) | 0.05 (0.43) | −0.00 (0.42) | 0.05 (0.01, 0.09) | 0.0290 | 0.03 (−0.01, 0.07) | 0.1244 |
| Percent BF at 36 weeks | 37.66 (7.12) | 37.25 (7.02) | 0.40 (−0.30, 1.11) | 0.2619 | 0.48 (−0.04, 1.00) | 0.0711 |
| Total change in percent BF | 0.12 (5.84) | −0.45 (5.65) | 0.56 (−0.03, 1.16) | 0.0650 | 0.43 (−0.09, 0.95) | 0.1082 |
| Average weekly change in percent BF | 0.00 (0.32) | −0.03 (0.32) | 0.03 (0.00, 0.07) | 0.0448 | 0.03 (−0.00, 0.06) | 0.0821 |
Values are mean (SD) and treatment effects are differences in means. Adjusted for center, parity, BMI category, age, socioeconomic status quintile, smoking status, baseline measurement, and either GA at measurement (for 36‐week measurement outcomes) or time between measurements (for total and average weekly change). Includes all women randomized who had anthropometric data collected, did not have a miscarriage or termination, and did not withdraw consent to use their data.
All SFTM and arm circumference measurements were not significantly correlated with either gestational weight gain or infant birth weight, correlation coefficients ranging between −0.239 and 0.111 (Table 3).
Table 3.
Correlations between maternal morphology measurements at trial entry and 36‐week gestation, gestational weight gain, and infant birth weight
| Pearson's correlation coefficients | ||
|---|---|---|
| Total gestational weight gain (kg) | Infant birth weight (g) | |
| Total gestational weight gain (kg) | 0.223 | |
| Arm girth at trial entry (cm) | −0.239 | 0.046 |
| Arm girth at 36 weeks (cm) | 0.002 | 0.087 |
| Biceps SFTM at trial entry (mm) | −0.185 | 0.009 |
| Biceps SFTM at 36 weeks (mm) | 0.070 | 0.055 |
| Triceps SFTM at trial entry (mm) | −0.159 | 0.036 |
| Triceps SFTM at 36 weeks (mm) | 0.111 | 0.107 |
| Subscapular SFTM at trial entry (mm) | −0.221 | −0.010 |
| Subscapular SFTM at 36 weeks (mm) | 0.028 | 0.057 |
| Percentage BF at trial entry | −0.230 | 0.007 |
| Percentage BF at 36 weeks | 0.066 | 0.081 |
Discussion
Our randomized trial is the largest reported to date evaluating the effects of an antenatal lifestyle intervention for women with a BMI of ≥25 kg/m2 during pregnancy on maternal anthropometric measures, and it utilized robust methodology. Our findings indicate that providing an intervention during pregnancy was not effective in reducing gestational weight gain 25 or maternal measures of peripheral adipose tissue deposition. Nor was there any effect on maternal percentage BF, with no evidence to suggest this differed among women with a BMI of 25–29.9 kg/m2 and ≥30 kg/m2. Furthermore, SFTM and anthropometric measures were not significantly correlated with either gestational weight gain or infant birth weight.
Our study has a number of strengths, including the large sample size of pregnant women with a BMI of ≥25 kg/m2, which has been a limitation of the literature to date. Although there are limitations to the use of skinfold measurements as an accurate predictor of adiposity, we adhered to a validated protocol, with acceptable reported interobserver variability 16, allowing comparisons between treatment groups to be made. Use of alternative methods of the assessment of maternal adiposity may have yielded more accurate estimates of the relative proportions of adipose and lean tissue mass although these were not considered feasible for use on such a large scale.
It is widely assumed that women deposit adipose tissue during pregnancy, with mobilization of peripheral stores as a fuel source for fetal growth in late gestation and during lactation 29. A number of observational studies suggest that there is maximal subcutaneous fat accumulation as measured by skinfold thickness, during the second trimester of pregnancy, followed by a decline in the third trimester as an indicator of tissue mobilization 17, 18, 19, 20. However, these studies have largely been confined to relatively lean pregnant women, with the mean reported early pregnancy BMI within the normal range 17, 18, 19, 20.
In contrast, description of the subcutaneous accumulation of fat stores across gestation among women with a BMI of ≥25 kg/m2 is less conclusive. Although some suggest that there is a decrease in the measures of subcutaneous adipose tissue over pregnancy 19, others have reported relative stability in SFTM, particularly involving the triceps and subscapular sites 22. However, changes in SFTM across pregnancy appear to be site specific, with increases in central adipose tissue deposition reported by some, as measured at the suprailiac, subscapular 30, and thigh skinfold sites 22. Importantly, there appears to be considerable interindividual variation, with pregnant women with high BMI reported to have a greater variation in the change in fat mass over time when compared with women of normal BMI 31, 32. Although we assessed upper body measures only, our findings are consistent with those reported by Sidebottom et al. 22, indicating relatively little change in SFTM and subcutaneous adipose tissue accumulation in the second and third trimesters of pregnancy. Additional assessment of maternal SFTM at 28 weeks of gestation may have allowed greater assessment of mobilization of peripheral adipose tissue stores during gestation although it was not considered feasible in the context of this study, where participant burden was considerable.
Although measured weight gain in nonpregnant individuals is more closely reflective of adiposity gain, measuring gestational weight gain in pregnancy is a relatively poor surrogate for maternal adiposity 8, highlighting the considerable variability which exists across individual women in the composition of that weight gain 11, 12. Among relatively lean pregnant women, subcutaneous measures of adipose tissue deposition have been demonstrated to correlate poorly with gestational weight gain 14, 22 although this observation is not universal, with others suggesting a positive association 32, 33. Furthermore, measured gains in maternal adipose tissue appear to be poorly predictive of infant birth weight 32, 33, 34, conferring little advantage over traditional assessment of prepregnancy or early pregnancy BMI 14, 20, perhaps with the exception of women with low prepregnancy weight, where there appears to be a negative correlation with low infant birth weight 35, 36.
To date, associations between measured maternal adiposity, gestational weight gain, and infant birth weight among women with a BMI of ≥25 kg/m2 have been poorly investigated. Although observational data indicate women with obesity have greater gain in central adiposity (compared with women of normal BMI), there is poor correlation with infant birth weight 23, 24. These findings are consistent with ours. From a metabolic perspective, changes which occur during pregnancy are highly complex as it is their relationship to maternal BMI, adiposity gain, gestational weight gain, and infant birth weight. It is therefore not surprising that although these measures may be associated with each other, changes in one may not necessarily correspond to observable or measurable changes in another.
As we have reported previously, providing antenatal lifestyle advice among women with a BMI of ≥25 kg/m2 was associated with an 18% relative risk reduction in infant birth weight of >4 kg 25, an effect mediated by changes in maternal diet quality and physical activity 26 despite the fact that both maternal gestational weight gain 25 and total energy intake during pregnancy 26 did not differ significantly between the randomized groups. Our intervention consisted of three face‐to‐face sessions and three telephone contacts over the course of pregnancy and was of similar intensity to those utilized in the previously published studies 37, 38, 39. Although increasing the intensity of the intervention may have been associated with greater effects on maternal gestational weight gain and, in turn, maternal SFTM, it was not considered feasible to implement within current maternity care models. Furthermore, our findings suggest that focusing attention solely on gestational weight gain, rather than achievable, albeit modest changes in dietary intake and physical activity, may reduce important opportunities to significantly impact clinically relevant infant outcomes 25, 28.
Conclusion
As indicated previously, the use of gestational weight gain is a surrogate measure of maternal adiposity 8, 9. Although both gestational weight gain and maternal adiposity as assessed by peripheral SFTM are relatively simple measures to obtain, they were not impacted by our antenatal lifestyle intervention. Furthermore, among women with a BMI of ≥25 kg/m2, maternal SFTM and measures of anthropometry correlated poorly with both gestational weight gain and infant birth weight. Further mechanistic information is required to elucidate the specific changes in maternal metabolic pathways over the course of pregnancy and their relationship, if any, to peripheral measures of adiposity.
Acknowledgments
The following persons and institutions (except where indicated, in Adelaide, South Australia) participated in the LIMIT Trial:
Steering Group—J.M. Dodd (Chair), D. Turnbull, A. McPhee, R.M. Grivell, C. Crowther, M. Gillman (Obesity Prevention Program, and Harvard University, Boston, Massachusetts, USA), G. Wittert, J.A. Owens, and J.S. Robinson.
Co‐ordinating Team—J.M. Dodd, A. Deussen, R.M. Grivell, L. Yelland, L. Moran, C. Cramp, A. Newman, L. Kannieappan, S. Hendrijanto, M. Kelsey, J. Beaumont, C. Danz, J. Koch, A. Webber, C. Holst, K. Robinson, S. Zhang, V. Ball, K. Ball, H. Deussen, N. Salehi, R. Bartley, R. Stafford‐Green, S. Ophel, M. Cooney, M. Szmeja, A. Short, A. Melrose, S. Han, I. Mohamad, and L. Chapple.
Statistical Analyses—L. Yelland
Serious Adverse Events Committee—R.M. Grivell, J. Svigos, V. Bhatia, N. Manton.
Writing Group—J.M. Dodd, D. Turnbull, A. McPhee, A. Deussen, R.M. Grivell, L. Yelland, C. Crowther, G. Wittert, J.A. Owens, and J.S. Robinson.
Collaborating Hospitals (total number of women recruited from each site in parentheses). Asterisk indicates named associate investigator for the NHMRC grant.
Flinders Medical Centre (South Australia) (669): J. McGavigan*, R. Bryce, S. Coppi, C. Fanning, G. Hannah, M. Ignacio, H. Pollard, F. Schmidt, Y. Shinners.
Lyell McEwin Hospital (South Australia) (505): G. Dekker*, S. Kennedy‐Andrews, R. Beaven, J. Niven, S. Burgen, J. Dalton, N. Dewhurst, L. Forst, V. Mugg, C. Will, H. Stone
Women's and Children's Hospital (South Australia) (1,038): J.M. Dodd, J.S. Robinson, A. Deussen, C. Crowther*, C. Wilkinson*, H. Purcell, J. Wood, D. Press, K. Ralph, S. Donleavy, S. Seager, F. Gately, A. Jolly, L. Lahnstein, S. Harding, K. Daw, M. Hedges, R. Fraser‐Trumble.
We are indebted to the 2,212 women who participated in this randomized trial.
Funding agencies: This study was sponsored by a four‐year project grant from the National Health and Medical Research Council (NHMRC), Australia (ID 519240). J.M.D. is supported through a NHMRC Practitioner Fellowship (ID 627005); R.M.G. is supported through a NHMRC Early Career Fellowship (ID 1073514); L.J.M. is supported through a South Australian Cardiovascular Research Development Program (SACVRDP) Fellowship (AC11S374), a program collaboratively funded by the National Heart Foundation of Australia, the South Australian Department of Health, and the South Australian Health and Medical Research Institute; L.N.Y. is supported through a NHMRC Early Career Fellowship (ID 1052388). Infrastructure support was provided by The University of Adelaide, the Women's and Children's Hospital, Flinders Medical Centre, and Lyell McEwin Hospital, Adelaide.
Disclosure: The authors declared no conflicts of interest.
Author contributions: J.M.D., L.M.K., R.M.G., A.R.D., L.J.M., L.N.Y., and J.A.O. are all members of the LIMIT randomized trial group. The primary investigator of the LIMIT randomized trial (J.M.D.) prepared the initial draft of the manuscript, had full access to all of the study data, and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.M.D. and L.N.Y. were responsible for conducting the statistical analyses. All members of the LIMIT randomized trial group listed above were involved in the study concept and design of the trial, supervision of conduct of the trial, the acquisition of data, the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and providing approval of the final submitted version.
Clinical trial registration: Australian and New Zealand Clinical Trials Registry (ACTRN12607000161426).
References
- 1. Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004‐2005. Maternal Child Health J 2008;13(5):614–620. [DOI] [PubMed] [Google Scholar]
- 2. National Health and Medical Research Council. NHMRC Strategic Plan 2013‐2015. Report no. NH160. Canberra, Australia: Commonwealth of Australia; 2012. [Google Scholar]
- 3. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust 2006;184:56–59. [DOI] [PubMed] [Google Scholar]
- 4. Dodd JM, Grivell RM, Nguyen A‐M, Chan A, Robinson JS. Maternal and perinatal health outcomes by body mass index category. Aust N Z J Obstet Gynaecol 2011;51:136–140. [DOI] [PubMed] [Google Scholar]
- 5. Mollberg M, Hagberg H, Bager B, Lilja H, Ladfors L. High birthweight and shoulder dystocia: the strongest risk factors for obstetrical brachial plexus palsy in a Swedish population‐based study. Acta Obstet Gynecol Scand 2005;84:654–659. [DOI] [PubMed] [Google Scholar]
- 6. Winter JD, Langenberg P, Krugman SD. Newborn adiposity by body mass index predicts childhood overweight. Clin Pediatr 2010;49:866–870. [DOI] [PubMed] [Google Scholar]
- 7. Institute of Medicine and National Research Council . Weight Gain During Pregnancy: Reexamining the Guidelines. Washington D.C: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 8. van der Wijden CL, Delemarre‐van der Waal HA, van Mechelen W, van Poppel MN. The concurrent validity between leptin, BMI and skin folds during pregnancy and the year after. Nutr Diab 2013;3:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Widen EM, Gallagher D. Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 2014;68:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fattah C, Farah N, Barry S, O'Connor N, Stuart B, Turner MJ. The measurement of maternal adiposity. J Obstet Gynaecol 2009;29:686–689. [DOI] [PubMed] [Google Scholar]
- 11. Hopkinson JM, Butte NF, Ellis KJ, Wong WW, Puyau MR, Smith EO. Body fat estimation in late pregnancy and early postpartum: comparison of two‐, three‐, and four‐component models. Am J Clin Nutr 1997;65:432–438. [DOI] [PubMed] [Google Scholar]
- 12. Kopp‐Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four‐component model. J Appl Physiol 1999;87:196–202. [DOI] [PubMed] [Google Scholar]
- 13. Marfell‐Jones M, Olds T, Stewart A, Carter L. International Standards for Anthropometric Assessment. The International Society for the Advancement of Kinanthropometry; 2006. [Google Scholar]
- 14. Anderson GD, Blidner IN, McClemont S, Sinclair JC. Determinants of size at birth in a Canadian population. Am J Obstet Gynecol 1984;150:236–244. [DOI] [PubMed] [Google Scholar]
- 15. Hu FB. Obesity Epidemiology. Oxford: Oxford University Press; 2008. p 53–83. [Google Scholar]
- 16. Kannieappan LM, Deussen AR, Grivell RM, Yelland LN, Dodd JM. Developing a tool for obtaining maternal skinfold thickness measurements and assessing inter‐observer variability among pregnant women who are overweight and obese. BMC Pregnancy Childbirth 2013;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hediger ML, Scholl TO, Schall JI, Healey MF, Fischer RL. Changes in maternal upper arm fat stores are predictors of variation in infant birth weight. J Nutr 1994;124:24–30. [DOI] [PubMed] [Google Scholar]
- 18. Sadurskis A, Kabir N, Wager J, Forsum E. Energy metabolism, body composition, and milk production in healthy Swedish women during lactation. Am J Clin Nutr 1988;48:44–49. [DOI] [PubMed] [Google Scholar]
- 19. Straughen JK, Trudeau S, Misra VK. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutr Diab 2013;3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villar J, Cogswell M, Kestler E, Castillo P, Menendez R, Repke JT. Effect of fat and fat‐free mass deposition during pregnancy on birth weight. Am J Obstet Gynecol 1992;167:1344–1352. [DOI] [PubMed] [Google Scholar]
- 21. Okereke NC, Huston‐Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab 2004;287:E472–E479. [DOI] [PubMed] [Google Scholar]
- 22. Sidebottom AC, Brown JE, Jacobs DR, Jr. Pregnancy‐related changes in body fat. Eur J Obstet Gynecol Reprod Biol 2001;94:216–223. [DOI] [PubMed] [Google Scholar]
- 23. Farah N, Stuart B, Donnelly V, Kennelly MM, Turner MJ. The influence of maternal body composition on birth weight. Eur J Obstet Gynecol Reprod Biol 2011;157:14–17. [DOI] [PubMed] [Google Scholar]
- 24. Kent E, O'Dwyer V, Fattah C, Farah N, O'Connor C, Turner MJ. Correlation between birth weight and maternal body composition. Obstet Gynecol 2013;121:46–50. [DOI] [PubMed] [Google Scholar]
- 25. Dodd JM, Turnbull DA, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: the LIMIT randomised trial. Br Med J 2014;348:g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dodd JM, Cramp CS, Sui Z, et al. The effects of antenatal lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med 2014;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dodd JM, Turnbull DA, McPhee AJ, Wittert G, Robinson JS. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: the LIMIT randomised controlled trial. BMC Pregnancy Childbirth 2011;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dodd JM, McPhee AJ, Turnbull DA, et al. The effects of antenatal lifestyle advice for women who are overweight or obese on neonatal health: the LIMIT randomised trial. BMC Med 2014;12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Raaij JM, Schonk CM, Vermaat‐Miedema SH, Peek ME, Hautvast JG. Body fat mass and basal metabolic rate in Dutch women before, during, and after pregnancy: a reappraisal of energy cost of pregnancy. Am J Clin Nutr 1989;49:765–772. [DOI] [PubMed] [Google Scholar]
- 30. Ehrenberg HM, Huston‐Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol 2003;189:944–948. [DOI] [PubMed] [Google Scholar]
- 31. Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr 2000;84:95–101. [DOI] [PubMed] [Google Scholar]
- 32. Maple‐Brown LJ, Roman NM, Thomas A, Presley LH, Catalano PM. Perinatal factors relating to changes in maternal body fat in late gestation. J Perinatol 2013;33:934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langhoff‐Roos J, Lindmark G, Gebre‐Medhin M. Maternal fat stores and fat accretion during pregnancy in relation to infant birthweight. Br J Obstet Gynaecol 1987;94:1170–1177. [DOI] [PubMed] [Google Scholar]
- 34. Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr 1988;47:942–947. [DOI] [PubMed] [Google Scholar]
- 35. Kelly A, Kevany J, de Onis M, Shah PM. A WHO collaborative study of maternal anthropometry and pregnancy outcomes. Int J Gynaecol Obstet 1996;53:219–233. [DOI] [PubMed] [Google Scholar]
- 36. Sanin Aguirre L, Reza‐Lopez S, Levario‐Carrillo M. Relation to maternal body composition and birth weight. Biol Neonat 2004;86:55–62. [DOI] [PubMed] [Google Scholar]
- 37. Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. Br J Obstet Gynaecol 2010;117:1316–1326. [DOI] [PubMed] [Google Scholar]
- 38. Oteng‐Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta‐analysis. BMC Med 2012;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta‐analysis of randomised evidence. Br Med J 2012;344:e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]


