Abstract
Background
Acute heart failure (AHF) is a leading cause of death in critically ill patients and is often accompanied by significant renal dysfunction. Few data exist on the predictive value of measures of renal dysfunction in large cohorts of patients hospitalized for AHF.
Methods
Six hundred and eighteen patients hospitalized for AHF (300 male, aged 73.3 ± 10.3 years, 73% New York Heart Association Class 4, mean hospital length of stay 12.9 ± 7.7 days, 97% non‐ischaemic AHF) were included in a retrospective single‐centre data analysis. Echocardiographic data, serum creatinine/urea levels, estimated glomerular filtration rate (eGFR), and clinical/laboratory markers were recorded. Mean follow‐up time was 2.9 ± 2.1 years. All‐cause mortality was recorded, and univariate/multivariate analyses were performed.
Results
Normal renal function defined as eGFR > 90 mL/min/1.73 m2 was noted in only 3% of AHF patients at baseline. A significant correlation of left ventricular ejection fraction with serum creatinine levels and eGFR (all P < 0.002) was noted. All‐cause mortality rates were 12% (90 days) and 40% (at 2 years), respectively. In a multivariate model, increased age, higher New York Heart Association class at admission, higher total cholesterol levels, and lower eGFR independently predicted death. Patients with baseline eGFR < 30 mL/min/1.73 m2 had an exceptionally high risk of death (odds ratio 2.80, 95% confidence interval 1.52–5.15, P = 0.001).
Conclusions
In a large cohort of patients with mostly non‐ischaemic AHF, enhanced serum creatinine levels and reduced eGFR independently predict death. It appears that patients with eGFR < 30 mL/min/1.73 m2 have poorest survival rates. Our data add to mounting data indicating that impaired renal function is an important risk factor for non‐survival in patients hospitalized for AHF.
Keywords: ICU, Cardiac failure, Cardiac shock, Cardiorenal syndrome
Introduction
Acute heart failure (AHF) is a leading cause of death and implies an important burden on healthcare systems worldwide. AHF is of epidemiologic importance as it accounts for over 1 million hospitalizations and about 300 000 heart‐failure‐related deaths annually in the USA alone.1, 2 Among the key findings in critically ill patients with AHF on today's intensive care units is the fact that it may often be accompanied by comorbidities1, 3, 4, 5 such as impaired renal function.4, 6, 7, 8, 9, 10, 11, 12, 13, 14
Although the pathophysiology of AHF‐induced acute kidney injury remains incompletely understood, a number of pathomechanisms including both haemodynamic and neurohormonal mechanisms were proposed.1, 4, 7, 8, 9, 10, 11, 12, 15, 16 Importantly, chronic kidney disease (CKD) may often be present as underlying comorbidity in patients with stable chronic heart failure (CHF).4, 7, 10, 11 Data from large CHF cohorts demonstrate that up to 60% of CHF patients develop some degree of CKD.17 Importantly, in patients with stable CHF, presence of CKD significantly affects survival rates, and previous investigations demonstrate a strong correlation between glomerular filtration rate (GFR) and risk of death.4, 10, 11, 14, 18 In CHF, data indicate that the risk of death may even be stronger correlated to a decline in GFR than to declining left ventricular ejection fraction (LVEF).19
In addition, acute renal failure (ARF) may develop in AHF that may require renal replacement therapy (RRT).12, 20, 21 ARF may induce progressive uraemia that worsens the prognosis of affected patients.22 Importantly, development of ARF accounts for excess mortality not only in heart failure patients but also in a general population of critically ill patients.7, 12, 18, 21, 23, 24 However, in cases of dialysis‐dependent ARF, optimal timing of RRT25 and optimal mode (i.e. continuous vs. intermittent RRT) remain currently unclear.26, 27, 28 This may especially be the case in haemodynamically instable patients with AHF.
Little is known about the predictive value of measures of routine renal function in the subgroup of patients hospitalized for non‐ischaemic AHF. In a large cohort of patients hospitalized for non‐ischaemic AHF, we therefore aimed to investigate the prognostic impact of impaired renal function assessed by serum creatinine/urea levels and estimated GFR (eGFR) on all‐cause mortality.
Methods
Study patients and study design
Between 2001 and 2003, n = 638 Caucasian patients were hospitalized for AHF. Of those, 618 patients [mean age 73.3 ± 10.3 years, 300 male, 449 in New York Heart Association (NYHA) Class 4] had available data for evaluation of renal function (i.e. baseline creatinine). Data from admission charts in a single centre, that is, the General Hospital Murska Sobota (Slovenia), were analysed in a retrospective fashion. The retrospective data analysis was approved by the National Ethics Committee and was performed in accordance with the Declaration of Helsinki. Patients with clinical and radiological evidence of decompensated AHF were included on the basis of clinical symptoms as established in respective international guidelines.29, 30 Patients were hospitalized in medical intensive care/intermediate care units and were treated according to current international recommendations. Patients on ambulatory chronic hemodialysis were excluded from the analysis.
Assessment of renal function, outcome parameters, and laboratory indices
At admission, serum creatinine levels, serum urea levels, eGFR, LVEF, high‐sensitivity C‐reactive protein, white blood cell count, and other prognostically important laboratory indices were assessed. Serum creatinine and serum urea levels were assessed by photometry from heparin plasma samples. For serum creatinine levels, the Jaffé method of detection was used (Roche Diagnostics, Mannheim, Germany). For assessment of outcome, the 28 days mortality, length of hospital stay, total follow‐up time, NYHA status at discharge, and other clinical indices were recorded. eGFR was assessed using the modified diet in renal disease formula31: GFR (mL/min/1.73 m2) = 186 × (Scr/88.4)−1.154 × (age)−0.203 × (0.742 if female). Although the degree of CKD remains unclear in the present cohort (please also refer to limitations section of discussion), study patients were grouped according to disease severity Stages 1–5 equivalent to severity stages proposed for CKD. The following stages applied: Stage 1 (increased risk; GFR > 90 mL/min/1.73 m2), Stage 2 (mild severity; GFR 60–89 mL/min/1.73 m2), Stage 3 (moderate severity; GFR 30–59 mL/min/1.73 m2), Stage 4 (severe disease; GFR 15–29 mL/min/1.73 m2), and Stage 5 (very severe disease; GFR < 15 mL/min/1.73 m2).
Statistical analysis
Statistical analyses were performed using MedCalc 9.0.1.1 software (MedCalc Software, Mariakerke, Belgium) and StatView 5 (SAS Institute, Cary, North Carolina, USA). All data are expressed as means ± SD and were tested for normal distribution using the Kolmogorov–Smirnov test, if not indicated otherwise. The relationship of baseline variables with survival was assessed by Cox proportional‐hazard analysis (univariate and multivariate analyses). Odds ratios (ORs) and 95% confidence interval (CI) for risk factors are given. Kaplan–Meier cumulative survival curves are displayed for illustrative purposes. Comparison between curves was performed by Mantel–Haensel log‐rank test. Significance was assigned when P < 0.05.
Results
Study population
A total of 618 Caucasian patients [mean age 73.3 ± 10.3 years, 300 male (48.5%), mean length of in‐hospital stay 12.9 ± 7.7 days, 100% NYHA Classes 3 and 4, CHADS score 2.4 ± 1.0] hospitalized on a medical intensive care unit for AHF were included in this analysis. Data on patients' demographics in respective equivalent stages of renal dysfunction and laboratory data are presented in Table 1. Of the overall sample, n = 152 (25%) patients presented with clinical signs of severe fluid overload (pleural effusion, pulmonary oedema, and peripheral oedema). Eighteen patients of the overall sample (3%) were considered to have AHF because of reasons related to acute ischaemia, that is, acute myocardial infarction (AMI). n = 160 patients (26%) of the overall samples initially presented in a hypertensive (initial systolic blood pressure ≥ 140 mmHg) state (mean systolic blood pressure in hypertensive subjects 151.4 ± 13.4 mmHg). At initial presentation, atrial fibrillation was present in n = 320 patients (52%), and n = 281 (i.e. 88%) of these patients were found to have chronic atrial fibrillation. At hospital admission, 22% of all patients had previous evidence for ischaemic heart disease or history of AMI (11%). Major concomitant diseases at hospital admission were as follows: arterial hypertension (44%), diabetes mellitus (33%), anaemia 29% (according to World Health Organization definitions), chronic obstructive pulmonary disease (17%), hyper‐lipoproteinaemia (12%), thyroid disease (7%), and gout (5%). In the study population, patients with advanced age and male gender were more likely to be found in higher renal disease severity categories (Table 1). Co‐medication at admission consisted in the following drugs at baseline: angiotensin‐converting‐enzyme inhibitors 78% plus angiotensin II receptor antagonists 8% (mean enalapril equivalent dose 11.0 ± 10.1 mg), 48% anti‐arrhythmic drugs/beta‐blockers, statins (20%), diuretics (83%), spironolactone (44%), and digoxin (37%). Mean amount of total medications 6.2 ± 1.9 per patient (with 4.4 ± 1.6 cardiovascular medications).
Table 1.
Patients' demographics and prognosis relevant indices
| All patients n = 618 (100%) | >90 mL/min/1.73 m2 (equivalent stage 1) n = 20 (3.2%) | 60–89 mL/min/1.73 m2 (equivalent stage 2) n = 157 (25.4%) | 30–59 mL/min/1.73 m2 (equivalent stage 3) n = 370 (59.9%) | 15–29 mL/min/1.73 m2 (equivalent stage 4) n = 62 (10.0%) | <15 mL/min/1.73 m2 (equivalent stage 5) n = 9 (1.5%) | |
|---|---|---|---|---|---|---|
| Age | 73.3 ± 10.3 | 69.7 ± 11.9 | 72.3 ± 11.0 | 73.2 ± 10.1 | 78.7 ± 7.3 | 70.1 ± 9.0 |
| Gender | 300 male (48.5%) | 6 male (30%) | 48 male (31%) | 201 male (54%) | 39 male (63%) | 6 male (67%) |
| LVEF (%) | 43.4 ± 12.1 | 52.0 ± 14.9 | 44.2 ± 11.3 | 43.3 ± 12.4 | 39.5 ± 9.8 | 20.0 ± 2.5 |
| NYHA at admission | 3.7 ± 0.5 | 3.6 ± 0.50 | 3.7 ± 0.48 | 3.7 ± 0.45 | 3.9 ± 0.36 | 4.0 ± 0.0 |
| Atrial fibrillation at admission | n = 320 (51.8%) | n = 9 (45%) | n = 71 (45%) | n = 199 (51%) | n = 33 (53%) | n = 1 (11%) |
| CHADS score | 2.4 ± 0.96 | 2.6 ± 0.95 | 2.5 ± 1.1 | 2.36 ± 0.93 | 2.46 ± 0.92 | 2.1 ± 0.6 |
| Length of in‐hospital stay (days) | 12.9 ± 7.7 | 19.0 ± 16.7 | 12.8 ± 5.5 | 12.9 ± 7.9 | 12.4 ± 6.9 | 13.1 ± 7.3 |
| Serum creatinine (µmol/L) | 113.5 ± 65.1 | 53.5 ± 7.2 | 75.55 ± 7.4 | 110.9 ± 18.4 | 189.0 ± 32.3 | 504.6 ± 217.5 |
| Serum urea (mg/dL) | 9.2 ± 4.8 | 4.5 ± 1.9 | 6.6 ± 3.2 | 9.4 ± 3.8 | 15.5 ± 5.8 | 17.2 ± 6.6 |
| eGFR (mL/min/1.73 m2) | 51.6 ± 19.6 | 107.6 ± 20.4 | 70.75 ± 7.9 | 45.9 ± 8.3 | 24.4 ± 4.1 | 9.45 ± 4.2 |
| Serum potassium (mmol/L) | 4.4 ± 0.5 | 4.1 ± 0.4 | 4.3 ± 0.5 | 4.5 ± 0.5 | 4.6 ± 0.5 | 4.9 ± 0.6 |
| C‐reactive protein (mg/L) | 31.6 ± 43.3 | 46.0 ± 37.45 | 33.34 ± 38.75 | 29.54 ± 46.91 | 35.46 ± 38.96 | 23.78 ± 14.42 |
| White blood cell count (x109/L) | 8.9 ± 5.3 | 8.2 ± 3.6 | 8.8 ± 6.1 | 8.8 ± 3.5 | 10.2 ± 10.3 | 8.5 ± 3.75 |
| Platelet count (x109/L) | 236.9 ± 93.8 | 246.3 ± 94.5 | 248.6 ± 94.2 | 230.8 ± 88.6 | 238.1 ± 91.7 | 262.1 ± 228.9 |
| Total cholesterol (mg/dL) | 4.8 ± 1.6 | 4.5 ± 1.21 | 5.07 ± 1.76 | 4.78 ± 1.44 | 4.64 ± 2.01 | 4.01 ± 1.13 |
| Total bilirubin (µmol/L) | 20.1 ± 19.4 | 32.4 ± 49.5 | 17.9 ± 16.4 | 20.56 ± 18.94 | 19.42 ± 11.73 | 15.4 ± 11.75 |
| Uric acid (mg/dL) | 420.2 ± 139.9 | 293.9 ± 99.2 | 337.3 ± 110.0 | 440.5 ± 127.1 | 548.5 ± 129.9 | 411.5 ± 233.1 |
| Creatinine phosphokinase (U/L) | 1.4 ± 2.4 | 1.28 ± 0.78 | 1.39 ± 3.12 | 1.32 ± 2.08 | 1.25 ± 0.83 | 4.22 ± 6.67 |
| Aspartate aminotransferase (U/L) | 0.53 ± 2.0 | 0.51 ± 0.42 | 0.78 ± 3.92 | 0.42 ± 0.42 | 0.63 ± 1.35 | 0.38 ± 0.41 |
eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Assessment of cardiac function and cardiac injury
Assessment of cardiac function by means of echocardiography was performed in 63% of all cases at admission. A significant decline of LVEF was found with advanced stages of renal dysfunction (Stages 1 vs. 4, P = 0.01, Table 1). A direct correlation of LVEF with serum creatinine (r = −0.2, P = 0.001) and eGFR (r = 0.2, P = 0.002) but not with urea levels (r = −0.07, P = 0.27) was identified. Mean left ventricular end‐diastolic diameter (LVEDD) was 25.9 ± 26.8 mm (median 6.6, 25th–75th percentile: 5.2–55.0), and mean left ventricular end‐systolic diameter (LVESD) was 20.6 ± 22.1 mm (median 5.4, 25th–75th percentile: 3.9–44.0) at admission. LVEDD and LVESD were both found to correlate significantly with LVEF (both P < 0.0003) but not with serum creatinine, serum urea, or eGFR (all P > 0.4). Overall, mean systolic and diastolic blood pressures were 129.3 ± 18.8 and 77.7 ± 10.0 mmHg, respectively. The mean heart rate was 113.3 ± 29.9 beats per minute at admission. For laboratory assessment of cardiac injury, serum creatinine phosphokinase (CPK) levels were assessed in 80.1% of all admissions (Table 1). CPK was not found to correlate with LVEF, eGFR, serum creatinine, or serum urea levels (all P > 0.25).
An explorative subgroup analysis was performed in AHF patients grouped for diastolic (defined as LVEF > 40%) vs. systolic AHF. A significant difference in eGFR was noted between these two subgroups. In detail, eGFR was 53.4 ± 18.9 (diastolic AHF) vs. 47.6 ± 16.0 (systolic AHF) mL/min/1.73 m2 (P = 0.0069). Correspondingly, serum creatinine levels were higher in patients with systolic AHF [i.e. 117.7 ± 46.9 vs. 104.3 ± 33.5 (diastolic AHF) mL/min/1.73 m2, P = 0.01]. Nevertheless, mean age in patients with systolic AHF was higher (72.6 ± 9.8 vs. 69.7 ± 11.4 years, P = 0.02), and NYHA class at admission was higher also (3.8 ± 0.4 vs. 3.7 ± 0.5, P = 0.004). No between group differences were noted in regard to serum levels of uric acid, total cholesterol, alanine aminotransferase, and C‐reactive protein (all n.s.).
Assessment of renal function, renal injury, and associated comorbidities
Of 618 hospitalized patients for AHF, n = 20 patients (i.e. 3.2%) had an eGFR of above 90 mL/min/1.73 m2, which was considered the lower limit of normal (Table 1). The major proportion (59.5%) of patients presented with Stage 3 equivalent renal dysfunction (eGFR 30–59 mL/min/1.73 m2). This demonstrates severe renal impairment in most study patients (Table 1). A highly significant correlation of eGFR with serum creatinine (r = −0.677), serum urea (r = −0.581), and serum potassium (r = −0.2284) was found (all P < 0.0001). Throughout CKD, equivalence stages of renal dysfunction, white blood cell counts, platelet counts, total cholesterol, CPK, aspartate aminotransferase, and alanine aminotransferase (data not shown) were rather unchanged (Table 1).
Impact of measures of renal and cardiac function on outcome prognostication
Details on univariate and multivariate analyses are given in Table 2. In the respective single predictor model for survival, pronounced effects on survival were observed for the following variables: age, NYHA class at admission, eGFR, serum levels of urea, serum levels of uric acid, and total cholesterol (Table 2). Interestingly, higher cholesterol levels were associated with improved survival rates, a fact previously known as the cholesterol paradox.32 Moreover, LVEF, serum creatinine levels, potassium, white blood cell count, haemoglobin, and diastolic blood pressure were noted to significantly impact on survival. In the multivariate model for survival, the following variables were included: age, NYHA class at admission, eGFR, haemoglobin, and total cholesterol (please refer to Table 2). For illustrative purposes, Kaplan–Meier survival estimate curves were constructed for 2 years (Figure 1 A) and 1 year follow‐up (Figure 1 B and C). All‐cause mortality 30 days after hospital admission was 6% and was 12% (Day 90), 18% (Day 180), 27% (after 1 year), 40% (after 2 years), and 49% (after 3 years), respectively. The mean survival time of patients surviving the initial episode of AHF was 1115.5 ± 753.7 days (median 1115.5 days, 25th–75th percentile 322.0–1689.0). For further assessment of impact of renal function on outcome measures, ORs were calculated after grouping of patients to the following eGFR categories: <30 mL/min/1.73 m2 and <60 mL/min/1.73 m2. In patients with an initial eGFR < 30 mL/min/1.73 m2, the OR for death was determined as OR 2.80, 95% CI 1.52–5.15, P = 0.001. In the subgroup of patients with baseline eGFR < 60 mL/min/1.73 m2, the odds for death were 1.94 (95% CI 1.36–2.76, P = 0.0003).
Table 2.
Univariate and multivariate survival models in patients hospitalized for acute heart failure
| Single predictor model for non‐survival | Multivariable model for non‐survival | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P‐value | χ 2 | Hazard ratio (95% CI) | P‐value | χ 2 |
| Age (1 year increase) | 1.034 (1.022–1.045) | <0.0001 | 38.2 | 1.028 (1.015–1.041) | <0.0001 | 19.0 |
| Gender (male) | 1.09 (0.891–1.332) | 0.40 | 0.7 | — | — | — |
| Aetiology of heart failure (non‐ischaemic) | 1.204 (0.936–1.548) | 0.15 | 2.1 | — | — | — |
| LVEF (>40%/≤40%)*, increase by 1% | 0.986 (0.973–0.999) | 0.03 | 4.6 | * | * | * |
| NYHA class at admission (per 1 class up) | 1.981 (1.547–2.536) | <0.0001 | 33.0 | 1.68 (1.261–2.239) | 0.0004 | 12.6 |
| Creatinine (10 µmol/L increase) | 1.014 (1.004–1.024) | 0.006 | 6.0 | — | — | — |
| eGFR (per 1 mL/min/1.73 m2 increase) | 0.987 (0.981–0.992) | <0.0001 | 21.2 | 0.988 (0.982–0.995) | 0.0006 | 11.9 |
| Urea (10 mg/dL increase) | 1.062 (1.043–1.082) | <0.0001 | 34.3 | — | — | — |
| Uric acid (10 µmol increase) | 1.017 (1.009–1.025) | <0.0001 | 17.1 | — | — | — |
| Potassium (1 mmol/L increase) | 1.379 (1.131–1.682) | 0.0016 | 9.9 | — | — | — |
| White blood cell count (1/nL increase) | 1.027 (1.009–1.046) | 0.0033 | 6.2 | — | — | — |
| Haemoglobin (1 g/dL increase) | 0.992 (0.987–0.997) | 0.0012 | 10.0 | 0.996 (0.991–1.002) | 0.20 | 1.6 |
| Diastolic BP (10 mmHg increase) | 0.979 (0.967–0.991) | 0.0004 | 12.4 | — | — | — |
| Total cholesterol (10 mg/dL increase) | 0.835 (0.763–0.912) | <0.0001 | 17.6 | 0.885 (0.808–0.97) | 0.009 | 6.8 |
BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Not included to multivariable model.
Not included to multivariable model due to missing data.
Figure 1.
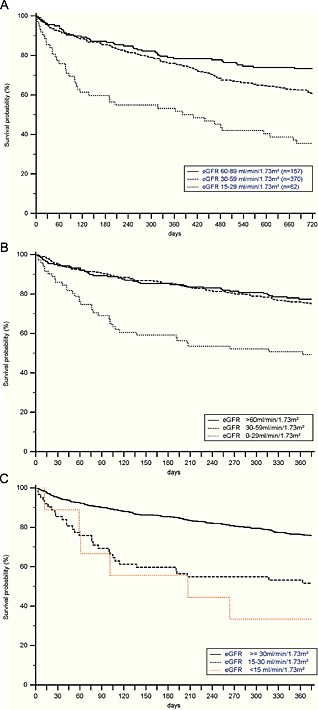
The 2 years (A) and 1 year (B) and (C) Kaplan–Meier survival estimates in patients hospitalized for acute heart failure according to estimated glomerular filtration rate categories are given (overall sample n = 618). (A) The 2 years survival estimates for acute heart failure patients with mild (full line), moderate (dashed line), and severe (dotted line) renal dysfunction. (B) The 1 year survival estimates for patients with normal to mildly reduced (full line), moderately reduced (dashed line), and severely to very severely (dotted line) reduced renal function. (C) The 1 year survival estimates for patients with normal to moderately (full line), severely (dashed line), and very severely (dotted line) reduced renal function.
Discussion
In the current analysis, we investigate the impact of renal dysfunction as assessed by a baseline single‐serum creatinine measurement on the outcomes of critically ill patients hospitalized for decompensated AHF. In a large cohort of AHF patients, we observed that mortality is significantly increased with advancing stages of renal dysfunction when patients were grouped according to GFR stages equivalent to the stages suggested for patients with CKD. Our findings add to few previous reports demonstrating a relationship between outcomes and renal dysfunction in patients hospitalized for AHF. As the vast majority of our study population suffered from non‐ischaemic AHF, our results suggest that these effects apply also in this subpopulation of AHF.
Interestingly, 97% of AHF patients presented with impaired renal function as defined by eGFR of >90 mL/min/1.73 m2 at baseline. In detail, the study cohort investigated here consists of AHF patients with mostly moderate kidney disease at admission (60%, Table 1). When compared to previously published data, the overall severity of renal impairment seems in line with most published CHF cohorts, and distribution of age and gender as well as all‐cause mortality rates may be considered comparable.4, 7, 10, 21 Thus, we believe our study cohort reflects a rather typical cohort of patients hospitalized for AHF. Nevertheless, as mentioned before, our cohort may be distinguished from previous investigations as in our investigation, mostly patients with non‐ischaemic AHF were included.
Epidemiological data including data from larger cohorts of patients with CHF demonstrate that CKD may be present in up to 50% of affected patients.7, 17, 22, 33, 34, 35 In addition, it seems pivotal to note that estimation of acute‐on‐chronic renal dysfunction in this specific cohort may be regarded especially difficult. Nevertheless, although we aimed to investigate the effect of initial eGFR on long‐term outcomes, we cannot fully elucidate the degree of CKD in the cohort under investigation. On the other hand, we believe that is important to investigate the impact of renal dysfunction on the cohort under investigation. In our univariate and multivariate outcome models, we identified eGFR as one of the most important risk factors for death in this cohort of acute care patients. Additional independent predictors of death in our multivariate outcome model were increased age and NYHA class at admission as well as decreased haemoglobin levels, and total cholesterol levels. The latter association was previously referred to as ‘cholesterol paradox’.32
The authors are well aware of respective limitations that are primarily driven by study design. First, as discussed before, we cannot comment on the degree of underlying CKD in our cohort because of reasons of missing pre‐clinical serum creatinine data. In addition, assessment of course of both serum creatinine levels and urinary output was beyond the scope of our analysis. We are therefore unable to investigate our cohort in regard to the recently established AKIN/RIFLE criteria and thus focus on initial eGFR as read out. Second, our analysis is of retrospective single‐center nature, and respective limitations apply. Nevertheless, we focused on long‐term follow‐up. With a mean follow‐up period of 2.9 ± 2.1 years, we believe that a rather long observational period may be regarded a strength of our analysis. Third, in the clinical setting of acute decompensated heart failure, serum creatinine levels and eGFR may only partially reflect renal dysfunction. This seems the fact as respective indices should not be considered in a steady state. However, this well‐known effect is a major challenge in regard to all respective investigations both of retrospective and prospective nature. Fourth, we used eGFR rather than measured GFR for assessment of renal dysfunction. Nevertheless, due to the specific kinetics of the underlying condition, GFR estimating equations may be considered even more accurate than 24 h urine creatinine clearance studies. Nevertheless, eGFR equations theoretically assume stable kidney function, and respective data must therefore be interpreted with caution. In addition, a potential best eGFR equation is still under debate. Importantly, we would like to highlight the fact that here, GFR categories are used in equivalence to well‐known severity stages as proposed for CKD and should not be misinterpreted as CKD stages. Fifth, we focused on all‐cause mortality as read‐out because cardiovascular events, re‐hospitalization, or renal‐related outcome measures were not recorded. Sixth, a minority of patients had some previous evidence for ischaemic heart disease or history of AMI. Thus, a minor effect of ischaemia‐induced AHF may influence our data. Seventh, due to the specifics of the underlying condition (i.e. CHF), serum creatinine levels and thus eGFR may be influenced by additional factors such as malnutrition or muscle wasting,36 limb amputation, liver cirrhosis, or others. Although we are convinced that our study population consists in a typical cohort of AHF patients, we cannot exclude an effect of some degree on respective data.
Conclusions
In the present retrospective investigation in a large cohort of patients with mostly non‐ischaemic AHF, we observed that eGFR independently predicts survival. It appears that patients with eGFR < 30 mL/min/1.73 m2 have poorest survival rates. Our results add to mounting data indicating that the degree of renal dysfunction is of pivotal importance in a population of patients with (mostly non‐ischaemic) AHF.
Conflict of interest
All authors declare that they have no conflict of interest.
Schefold, J. C. , Lainscak, M. , Hodoscek, L. M. , Blöchlinger, S. , Doehner, W. , and von Haehling, S. (2015) Single baseline serum creatinine measurements predict mortality in critically ill patients hospitalized for acute heart failure. ESC Heart Failure, 2: 122–128. doi: 10.1002/ehf2.12058.
References
- 1. Redfield MM. Heart failure—an epidemic of uncertain proportions. N Engl J Med 2002; 347: 1442–1444. [DOI] [PubMed] [Google Scholar]
- 2. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117: e25–e146. [DOI] [PubMed] [Google Scholar]
- 3. Lainscak M, Hodoscek LM, Dungen HD, Rauchhaus M, Doehner W, Anker SD, von Haehling S. The burden of chronic obstructive pulmonary disease in patients hospitalized with heart failure. Wien Klin Wochenschr 2009; 121: 309–313. [DOI] [PubMed] [Google Scholar]
- 4. Holzmann T, Eltenton G, Anderson S, Bonny SJ. Problem‐based review: the patient with acute heart failure. Acute medicine 2013; 12: 44–50. [PubMed] [Google Scholar]
- 5. von Haehling S, Schefold JC, Hodoscek LM, Doehner W, Mannaa M, Anker SD, Lainscak M. Anaemia is an independent predictor of death in patients hospitalized for acute heart failure. Clin Res Cardiol Off J German Cardiac Soc 2010; 99: 107–113. [DOI] [PubMed] [Google Scholar]
- 6. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Kissela B, Kittner S, Lloyd‐Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed A, Campbell RC: Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin 2008; 4: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang KV, Williams AW, Greene EL, Redfield MM. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med 2008; 36: S75–S88. [DOI] [PubMed] [Google Scholar]
- 9. Marik PE, Flemmer M. Narrative review: the management of acute decompensated heart failure. J Intensive Care Med 2012; 27: 343–353. [DOI] [PubMed] [Google Scholar]
- 10. Butler J, Chirovsky D, Phatak H, McNeill A, Cody R. Renal function, health outcomes, and resource utilization in acute heart failure: a systematic review. Circ Heart Fail 2010; 3: 726–745. [DOI] [PubMed] [Google Scholar]
- 11. Summers RL, Amsterdam E. Pathophysiology of acute decompensated heart failure. Heart Fail Clin 2009; 5: 9–17, v. [DOI] [PubMed] [Google Scholar]
- 12. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 13. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007; 13: 422–430. [DOI] [PubMed] [Google Scholar]
- 14. Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta‐analysis. J Am Coll Cardiol 2006; 47: 1987–1996. [DOI] [PubMed] [Google Scholar]
- 15. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999; 341: 577–585. [DOI] [PubMed] [Google Scholar]
- 16. Schmieder RE, Mitrovic V, Hengstenberg C. Renal impairment and worsening of renal function in acute heart failure: can new therapies help? The potential role of serelaxin. Clin Res Cardiol Off J German Cardiac Soc 2015; 104: 621–631. [DOI] [PubMed] [Google Scholar]
- 17. Adams KF, Jr. , Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 18. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 19. Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000; 102: 203–210. [DOI] [PubMed] [Google Scholar]
- 20. Kellum JA. Acute kidney injury. Crit Care Med 2008; 36: S141–S145. [DOI] [PubMed] [Google Scholar]
- 21. Kellum JA, Hoste EA. Acute kidney injury: epidemiology and assessment. Scand J Clin Lab Invest Suppl 2008; 241: 6–11. [DOI] [PubMed] [Google Scholar]
- 22. Cowie MR, Komajda M, Murray‐Thomas T, Underwood J, Ticho B. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J 2006; 27: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 23. Druml W: Acute renal failure is not a ‘cute’ renal failure! Intensive Care Med 2004; 30: 1886–1890. [DOI] [PubMed] [Google Scholar]
- 24. Druml W. Long term prognosis of patients with acute renal failure: is intensive care worth it? Intensive Care Med 2005, 31: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 25. Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: a meta‐analysis. Am J Kidney Dis Off J Nat Kidney Found 2008; 52: 272–284. [DOI] [PubMed] [Google Scholar]
- 26. Lins RL, Elseviers MM, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J: Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dialysis Transplantation Off Publ Eur Dialysis & Transplant Assoc Eur Renal Assoc 2009; 24: 512–518. [DOI] [PubMed] [Google Scholar]
- 27. Schefold JC, Haehling S, Pschowski R, Bender T, Berkmann C, Briegel S, Hasper D, Jorres A. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): a prospective randomized controlled trial. Crit Care 2014; 18: R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple‐organ dysfunction syndrome: a multicentre randomised trial. Lancet 2006; 368: 379–385. [DOI] [PubMed] [Google Scholar]
- 29. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008 10: 933–989. [DOI] [PubMed] [Google Scholar]
- 30. Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005; 46: e1–e82. [DOI] [PubMed] [Google Scholar]
- 31. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 32. von Haehling S, Schefold JC, Springer J, Anker SD. The cholesterol paradox revisited: heart failure, systemic inflammation, and beyond. Heart Fail Clin 2008; 4: 141–151. [DOI] [PubMed] [Google Scholar]
- 33. Amin A: Hospitalized patients with acute decompensated heart failure: recognition, risk stratification, and treatment review. J Hosp Med 2008; 3: S16–S24. [DOI] [PubMed] [Google Scholar]
- 34. Flaherty JD, Bax JJ, De Luca L, Rossi JS, Davidson CJ, Filippatos G, Liu PP, Konstam MA, Greenberg B, Mehra MR, Breithardt G, Pang PS, Young JB, Fonarow GC, Bonow RO, Gheorghiade M. Acute heart failure syndromes in patients with coronary artery disease early assessment and treatment. J Am Coll Cardiol 2009; 53: 254–263. [DOI] [PubMed] [Google Scholar]
- 35. Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail 2005; 7: 119–125. [DOI] [PubMed] [Google Scholar]
- 36. Schefold JC, Bierbrauer J, Weber‐Carstens S. Intensive care unit‐acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachex Sarcopenia Muscle 2010; 1: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]


