Abstract
Aim
Results from clinical trials support the use of oral antipsychotics for treatment of early or first‐episode psychosis in patients with schizophrenia. This paper will review literature on the advantages of early initiation of treatment for schizophrenia and the clinical benefits of early use of long‐acting injectable antipsychotics (LAIs).
Method
A comprehensive literature review was conducted to identify published literature on the use of LAIs early in the treatment of schizophrenia.
Results
Although there is a higher response rate to initial antipsychotic treatment for a first‐episode of schizophrenia than with subsequent antipsychotic treatment, we have not effectively addressed this issue. Poor adherence to treatment is a primary cause of relapse and rehospitalization in subsequent years and was associated with higher relapse rates resulting in devastating effects and substantial economic burden. The costs of nonadherence were estimated to be $1.48 billion. Thus, a major challenge with the treatment of schizophrenia is changing poor adherence to persistence with antipsychotic therapy. LAIs are known to be at least as effective as oral antipsychotics for treating schizophrenia, and yet are underutilized. Further, LAIs address many of the problems associated with adherence to oral therapy. Recent evidence suggests that LAIs are effective for treating first‐episode psychosis and for early initiation of treatment for schizophrenia.
Conclusion
Although consistent antipsychotic treatment represents a critical part of treatment, a person‐centred approach to treating schizophrenia is essential for all aspects of care, including establishing and maintaining a therapeutic alliance, strengthening shared decision‐making and adherence, and achieving long‐lasting recovery.
Keywords: antipsychotic, clinical, early use, long‐acting injectable, schizophrenia
Introduction
The prevalence of schizophrenia is 4 cases/1000 of the adult population over the age of 18, representing over 50 million people worldwide who suffer from schizophrenia.1, 2 The challenges of living with schizophrenia are magnified by the significant stigma associated with the illness. Impairment of functional and cognitive capacity in schizophrenia impacts a substantial burden on the patient, their family and society, as well as the direct and indirect costs of the disease.2 People with schizophrenia face a number of challenges in managing their lives and illness, including lack of insight into their illness and cognitive deficits that interfere with treatment adherence – both psychosocial and pharmacologic. These challenges increase the risk of relapse, with each relapse resulting in significant personal and economic costs.
Antipsychotic (AP) medications, which are an important part of treatment, have been shown to improve clinical outcomes in people with schizophrenia and reduce economic burden secondary to reduced relapse and hospitalization rates and fewer emergency room (ER) visits.3, 4, 5 Results from recent meta‐analyses support the use of APs for treatment of early or first‐episode psychosis in people with schizophrenia.6, 7 In addition, the benefits of an early intervention programme for psychosis support higher recovery rates at substantially lower personal and economic costs.8, 9 Although response to treatment often is favourable for someone with a first‐episode of schizophrenia, a major challenge with schizophrenia treatment is transforming poor adherence to consistent treatment, including oral AP therapy.4 Nonadherence is associated with devastating personal costs, increased relapse rates,10, 11 greater use of health‐care resources12, 13 and higher costs of care.14, 15
Long‐acting injectable (LAI) APs are known to be at least as effective as oral APs for treating schizophrenia.16 A recent systematic review and meta‐analysis found that second‐generation LAIs were superior to first‐generation LAIs for relapse prevention.17 In addition, recent evidence suggests that LAIs are effective for treating first‐episode psychosis and for early initiation of treatment for schizophrenia.18, 19 Further, LAIs provide the added benefit versus oral APs of addressing problems with daily adherence.20, 21 Although LAIs traditionally have been reserved for patients at later periods of their disease, increasingly, LAI use is being advocated for early episodes of schizophrenia including after first hospitalization to achieve optimal outcomes such as reduced rates of recurrence, rehospitalization, and comorbidities and complications of untreated/undertreated illness.22, 23
An important question is whether LAIs have beneficial effects on the clinical course, medical resource use and costs of therapy when used early for treatment or for treatment of first‐episode of schizophrenia. Newer literature has centred on a single‐minded focus of collaborating with the person with schizophrenia, suggesting that the individual and practitioner develop a strategy for shared decision‐making related to treatment, including but not limited to which medications best suit the individual's needs24 and when those medications should be utilized. In order to provide the best possible care, all available treatment options should be discussed and considered.
This paper aims to review literature on the benefits of early treatment and the clinical benefits of early use of LAIs for the treatment of schizophrenia.
Person‐centred approach
A person‐centred approach to the treatment of schizophrenia is central to achieving both short‐ and long‐term beneficial outcomes because it encompasses individual experiences and self‐understanding of psychosis.25 A key concept in managing schizophrenia is the importance of the recovery model of care. Thus, initiating a treatment plan for schizophrenia must happen within a strong therapeutic alliance. Maintaining the therapeutic relationship over the long term is critical for assisting in achieving long‐lasting recovery, which is the most important goal of therapy.26 Results from a recent, randomized study demonstrated significant improvement among persons with schizophrenia in social functioning after 5 years with a formal programme providing a person‐centred approach to management.27
The Substance Abuse and Mental Health Services Administration (SAMHSA), in collaboration with individuals and health‐care professionals, developed a working definition of recovery from schizophrenia as a means to advance recovery opportunities and to clarify the concept of recovery for persons with schizophrenia and their health‐care providers.26 Their working definition of recovery is ‘a process of change through which individuals improve their health and wellness, live a self‐directed life, and strive to reach their full potential’.26 This definition of recovery includes considerations relevant to health, home, purpose and community. A person‐centred approach is central to this process.
In the overall management of schizophrenia, the approach to delivering the message about the need for treatment is more important than the message itself.28, 29 Additionally, health‐care providers must also take into account the importance of relationships between the person with schizophrenia and their families/significant others when selecting the most appropriate treatment.30 With a strong therapeutic alliance, the person with schizophrenia, their families and their provider collaborate to identify issues about care that need to be addressed and potential solutions.28 The goal is to get better and live better, that is, decrease symptoms, increase function, engage in meaningful activity and improve well‐being.
A study of the impact of attitudes towards AP medication use found that a poor relationship between hospitalized patients and prescribers, coercion to take AP medications during admission, and low insight about their condition were predictive of negative attitudes towards treatment.31 Poor insight into their disease process occurs in 30–50% of people with schizophrenia and is related to suboptimal outcomes.32, 33, 34 In fact, studies of adherence to AP medication found that poor insight among people with schizophrenia is the best predictor of nonadherence.32 A quality relationship between the person with schizophrenia and their health‐care provider (Fig. 1) has been shown to be associated with positive attitudes and better outcomes, including improved adherence to treatments (pharmacologic and other), decreased rehospitalization and improved symptoms.35, 36, 37, 38, 39
Figure 1.
Interaction between therapeutic alliance and treatment to provide a positive outcome.
The use of medication is one tool that addresses specific symptoms. People with schizophrenia will be encouraged to take medication if they feel that it is helpful and the benefits outweigh the risks. LAIs offer an important alternative to oral APs, because they can strengthen treatment adherence, by decreasing the burden of daily pill taking, and alerting providers to nonadherence. Compared with oral APs, use of an LAI was associated with improved quality of life (QoL) and a reduced burden on caregivers among outpatients with schizophrenia.40 Caregivers can have the opportunity to decrease worry and policing of medication adherence and relapse, and enhance communication. A study of 30 patients with schizophrenia who were randomized to treatment with oral or LAI APs evaluated the effects on working alliance.41 Despite clinical improvement, at a 2‐year follow up, working alliance had deteriorated in the LAI group, which emphasized the importance of maintaining and enhancing the therapeutic alliance after initiating drug therapy.
Unfortunately, patients are often not involved in the decision to use LAIs. Interviews conducted with people with schizophrenia and prescribers in a community mental health centre found that LAIs often were not offered to patients, and when offered, patients and caregivers were not involved in the decision.42 Thus, a person‐centred approach, which focuses on shared decision‐making and recovery from schizophrenia, considers choices about treatment, the role of oral versus LAI APs, and the impact on medication adherence is optimal.43
Attitudes and use of early LAI treatment in schizophrenia
An extensive body of literature supports the clinical benefits of oral APs for early intervention or treatment of first‐episode psychosis.5, 44, 45, 46, 47 A growing body of literature demonstrates that LAIs also are effective for early intervention or when used for first‐episode treatment of schizophrenia rather than delaying treatment.48, 49, 50, 51, 52 A higher response rate is associated with initial AP treatment in first episode with significantly less response to subsequent AP treatment.22, 53
Although LAIs may have advantages compared with oral APs, especially with respect to more convenient monitoring of adherence, LAI use often is limited by negative attitudes of health‐care professionals, specifically regarding earlier use in the course of therapy.54, 55, 56 A systematic review of patient and clinician attitudes towards first‐episode use of LAIs identified six relevant studies.55 Four of the six studies reported negative attitudes of clinicians towards LAIs for first‐episode psychosis, but none of the six studies described patient attitudes. Reluctance of clinicians to use LAIs for first‐episode psychosis was based on a presumption that patients would not accept treatment with LAIs. This is especially confounding given the American Psychiatric Association guidelines for LAI use that are based on patient preference. Although LAIs are used with greater frequency outside of the United States, a survey of attitudes towards LAIs from health‐care practitioners across Europe found that only 40% would use LAIs for first‐episode psychosis whereas 90% would use LAIs for chronic schizophrenia.54
Clinical experience with LAIs for first‐episode treatment
Neuroprotective potential
A scientific rationale may exist for using LAIs early in the treatment of schizophrenia. Treatment with APs may cause an initial increase in frontal lobe intracortical myelin volume early in the course of the disease, followed by a decrease as the disease progresses to a chronic phase.57 It has been proposed that use of LAIs versus oral APs may slow the decline in intracortical myelin during chronic schizophrenia. Five studies, including three randomized studies, have evaluated the effect of LAIs for treating first‐episode schizophrenia; the other two studies only evaluated attitudes and adherence to LAIs (Table 1). Although data are limited with LAIs for first‐episode psychosis, results from these trials suggest a beneficial brain effect.
Table 1.
Studies reporting the use of LAIs for first‐episode schizophrenia
| Reference | Patient population | Treatment | Assessments | Outcomes |
|---|---|---|---|---|
| Bartzokis et al., 201157 | 24 schizophrenia patients with first psychotic episode | LAI versus oral and matched controls | White matter volume by MRI | White matter remained stable with LAI but decreased significantly with oral |
| Kane et al., 198258 | 28 patients recovering from a first episode | Randomized to LAI, oral or placebo for 1 year | Relapse rate | 41% on placebo relapsed compared with none on active treatment |
| Kim et al., 200818 | 50 patients with first‐episode schizophrenia | Open‐label treatment with LAI or oral therapy for 2 years | PANSS, CGI, GAF, relapse, remission, adherence | Significant improvement in all parameters with LAI |
| Weiden et al., 200959 | 37 patients with ≤16 weeks lifetime exposure to antipsychotics | Randomized to LAI or oral treatment for 12 weeks | Adherence and attitudes | No difference in adherence behaviour; however, acceptance of LAI associated with better adherence |
| Weiden et al., 201260 | Continuation of previous study for 104 weeks | Randomized to LAI or oral treatment for 104 weeks | Adherence and attitudes | No difference in adherence between oral and LAI |
In one clinical study of 23 patients with schizophrenia treated with either a LAI or oral AP, intracortical myelination volume increased significantly (P = 0.005) with the LAI compared with a control group, but no significant difference was noted between oral APs and the control group.57 These results support the hypothesis that LAIs promote intracortical myelination and may exert a neuroprotective effect when used in first‐episode schizophrenia patients. The authors suggest that better adherence to medication or variations in the pharmacokinetic (PK) profile of LAIs versus oral APs may account for the differences.
Relapse reduction
In a randomized trial, 28 patients recovering from an acute first‐episode of schizophrenia were treated with an oral first‐generation antipsychotic (FGA), a LAI or placebo for 1 year.58 Seven of 17 (41%) patients in the placebo group relapsed, whereas none of the patients treated with oral or LAI APs relapsed.
A prospective, naturalistic study was conducted to evaluate the effectiveness of a LAI for relapse prevention in first‐episode schizophrenia.18 Fifty patients with schizophrenia were treated with the LAI or an oral AP for up to 2 years. Although symptom reductions were similar in LAI and oral treatment groups, a significantly (P < 0.05) lower relapse rate and higher medication adherence were observed with the LAI versus oral therapy. Good adherence was noted in 68% with the LAIs and 32% with oral therapy. Relapse rates at 1 year were 18% and 50% and at 2 years were 23% and 75% with LAIs versus oral therapy, respectively (Fig. 2).
Figure 2.
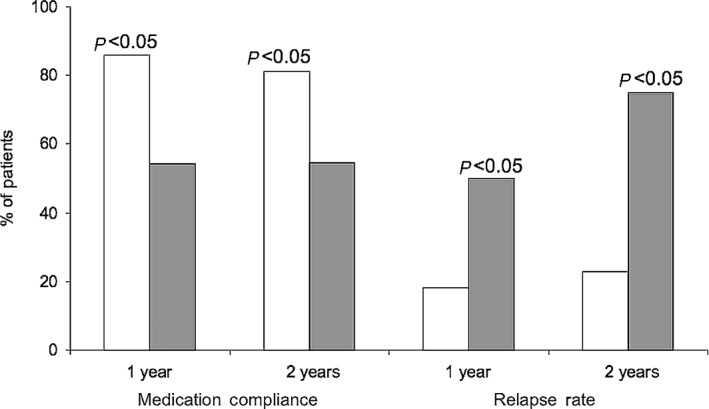
Impact of LAI or oral antipsychotic use on medication compliance and relapse rate at 1 and 2 years.  , LAI;
, LAI;  , oral.18
, oral.18
Adherence
A prospective, randomized trial in 37 patients with schizophrenia evaluated the effects of a LAI on adherence in first‐episode schizophrenia.59 Patients had no more than 16 weeks of previous exposure to AP therapy and were randomized to LAI or oral APs. Patients switching to the LAI were significantly (P < 0.05) more likely to remain adherent versus those staying on oral therapy. In a follow‐up report, the effect of maintenance treatment with a LAI on medication adherence was evaluated in 37 patients who were observed for up to 104 weeks.60 Although no differences in adherence between LAI and oral treatment were observed, nonadherence was more easily detected in the LAI group.
Early initiation or recent‐onset use of LAIs in schizophrenia
Sixteen published reports describe early initiation of treatment or recent‐onset treatment of schizophrenia with LAIs (Table 2). All reports are open‐label, observational or case–control studies, and none used a prospective, randomized trial design. Those studies reporting findings for recent‐onset schizophrenia used definitions of recent onset ranging from 2 to 5 years. Some reports are of the same cohort of patients and report different treatment phases or outcomes.63, 64, 65 A systematic review of studies reporting the effects of LAIs for the early treatment of first episodes of schizophrenia included some of these studies.51 Of 10 studies (cohort, randomized or open) that met selection criteria, LAIs were found to be effective for early schizophrenia. The authors concluded that LAIs for treating early schizophrenia improved symptom control and reduced the risk of relapse, especially when medication adherence was a concern or when the patient made the decision to use LAIs. Person‐centred choice and shared decision‐making can strengthen patients' empowerment and responsibility in the management of their illness and lives.
Table 2.
Summary of studies reporting use of LAIs for recent‐onset or early‐initiation therapy in schizophrenia
| Reference | Patient population | Treatment | Assessments | Outcomes |
|---|---|---|---|---|
| Barrio et al., 201361 | 26 patients with recent onset treated with LAI versus 26 control recent onset treated with oral | Case–control study of LAI versus oral for 2 years | PANSS total and subscales, PAS, hospitalization, remission | LAI showed significant improvement in PANSS and subscales, PAS; higher remission, lower hospital |
| Dubois et al., 201462 | 155 patients with schizophrenia (≤3 years) versus 253 (>3 years) | Observational study of LAI for 12 or 24 months from the TIMORES and eSTAR studies | CGI‐S, GAF, hospitalization, remission | Significant benefits for early for CGI‐S and GAF, remission; number of hospital days |
| Emsley et al., 200863 | 50 newly diagnosed patients | Open‐label treatment with LAI for 2 years | Remission, relapse, PANSS, CGI‐S, function, SF‐12 | Remission in 64%, and 97% maintained remission; remission associated with improvements in function |
| Emsley et al., 200864 | 50 patients with ≤12 months since diagnosis and ≤12 weeks drug; 54% were drug naïve | Open‐label study of LAI × 24 months | Clinical outcomes, EPS, prolactin | 72% completed 24 months; 78% had a response; 64% achieved a remission |
| Emsley et al, 200865 | 50 patients with ≤12 weeks drug treatment; 46% were antipsychotic naïve | Open label with LAI × 24 months; compared with oral antipsychotic | PANSS, EPS, BMI | LAI group had fewer discontinuations, lower PANSS scores, higher remission rate, lower relapse rate; EPS lower with LAI but BMI higher |
| Lasser et al., 200748 | 66 young adults with ≥4 weeks of antipsychotic treatment; mean duration of 131 days | Open‐label trial of LAI for 50 weeks | PANSS, SF‐36 | Significant improvement from baseline for PANSS and SF‐36 |
| Macfadden et al., 201049 | 57 recent diagnosed (≤3 years) versus 266 late diagnosed (>3 years) | Open‐label treatment with LAI for 1 year | Relapse, PANSS, CGI‐S | Recent diagnosed had greater improvement in PANSS and CGI‐S |
| Malla et al., 201366 | 85 patients in the early phase of schizophrenia spectrum disorder | Randomized to oral or LAI treatment for 2 years | PANSS, CGI‐S | Improvement in both groups, but no differences between groups |
| Napry‐eyenko et al., 201067 | 294 patients with ≤2 years duration of schizophrenia | Open‐label study of LAI for 6 months | PANSS, CGI‐S, GAF, SF‐36 | Significant improvement in all parameters from baseline |
| Rabinowitz et al., 201168 | 294 patients with ≤2 years duration of schizophrenia | Open‐label study of LAI for 6 months | Premorbid Adjustment Scale (PAS), PANSS, CGI‐S, GAF, SF‐36 | Premorbid functioning associated with better response |
| Olivares et al., 200969 | Patients with recent (≤2 years) or long term (>2 years) | Observational study of LAI for 24 months | CGI‐S, GAF, hospitalization | Greater improvement with recent for CGI‐S, hospital rate and days; GAF not different |
| Parellada et al., 200550 | 382 patients with ≤3 years diagnosis; mean 1.5 years | Open‐label trial of LAI for 6 months | PANSS total and subscales, CGI‐S, GAF, QoL, patient satisfaction | Significant improvement from baseline in PANSS total and subscale scores, CGI‐S, QoL, patient satisfaction |
| Sliwa et al., 201270 | Compared recent diagnosis (n = 216, ≤5 years) with chronic illness (n = 429, >5 years) | Open‐label LAI and followed for 1 year | Tolerability | Improved tolerability with LAI compared with baseline levels |
| Tiihonen et al., 201171 | 2588 hospitalized for the first time for schizophrenia | Registry‐based linkage study of depot versus oral treatment for mean follow up of 2 years | Hospitalization and discontinuation | Rehospitalization was one‐third with depot versus oral medication |
| Viala et al., 201252 | 25 schizophrenia patients hospitalized for the first time | Open‐label study of switch from oral to LAI | CGI, GAF at 6, 12 and 18 months; hospitalization | Significant improvement from baseline in CGI‐S and GAF; 16% relapsed; fewer and shorter hospitalizations |
Clinical symptoms and function
Among 85 patients in the early phase of a schizophrenia spectrum disorder who were randomized to treatment with oral or LAI APs for 2 years, symptoms on Positive and Negative Syndrome Scale (PANSS) or Clinical Global Impressions (CGI) scales improved in both groups, but no difference was observed between oral and LAI use.66 Although no differences were observed for effectiveness or tolerability, the authors concluded that use of an LAI was more likely to be associated with improved adherence.
Young adults who were expected to be in the early stages of schizophrenia or schizoaffective disorder were evaluated in an open‐label 50‐week study of a LAI.48 Of 66 patients who entered the trial and received at least one dose of drug, 64% completed 50 weeks of treatment. Mean PANSS scores significantly improved (P < 0.05) from baseline, and 64% of the patients demonstrated clinical improvement (≥20% reduction in PANSS total score) at end‐point. Patient assessments of QoL also improved during the study. Treatment with a LAI was associated with clinical benefits in stable young adults with early‐onset schizophrenia or schizoaffective illness.
Patients in early phases of schizophrenia and schizoaffective disorders (i.e. duration ≤3 years) were evaluated for their response to a LAI in a 6‐month, open‐label study.50 Of 382 patients who were enrolled, 73% completed 6 months of treatment. In addition to significant (P ≤ 0.0001) improvements in total PANSS and subscale scores, significant (P < 0.05) improvements were also observed in Global Assessment of Functioning (GAF), QoL, patient satisfaction and movement disorder symptoms. Thus, initiation of a LAI in patients within 3 years of diagnosis significantly improved symptoms and might represent an option for patients in the early phases of psychosis.
A series of reports have described the effects of treatment of early‐onset schizophrenia.63, 64, 65 In an open‐label study comparing patients treated with flexible doses of a LAI (n = 50) versus flexible doses of oral risperidone or haloperidol (n = 47), responders treated with a LAI had significantly (P < 0.05) fewer all‐cause discontinuations (26.0% vs. 70.2%), greater reductions on the PANSS total score (−39.7 vs. −25.7), a higher remission rate (64.0% vs. 40.4%) and a lower relapse rate (9.3% vs. 42.1%) (Fig. 3).64 A follow‐up analysis observed remission in 32 (64%) patients that was maintained for 2 years in 97% of those patients. Early symptom improvement on the PANSS was a significant predictor of remission (Fig. 4).
Figure 3.
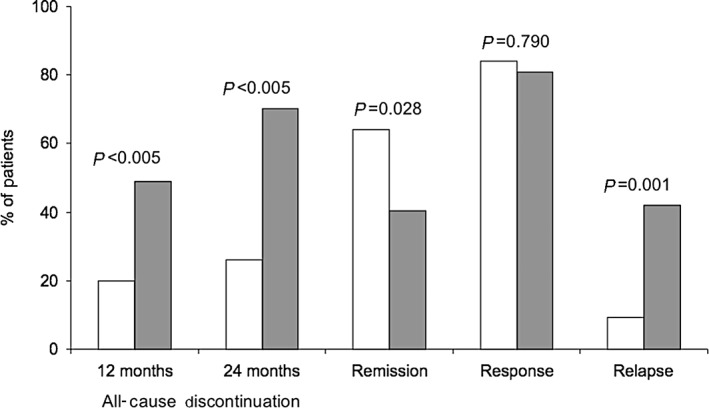
Outcomes after treatment with LAI or oral antipsychotics.  , LAI (n = 50);
, LAI (n = 50);  , oral (n = 47).64
, oral (n = 47).64
Figure 4.
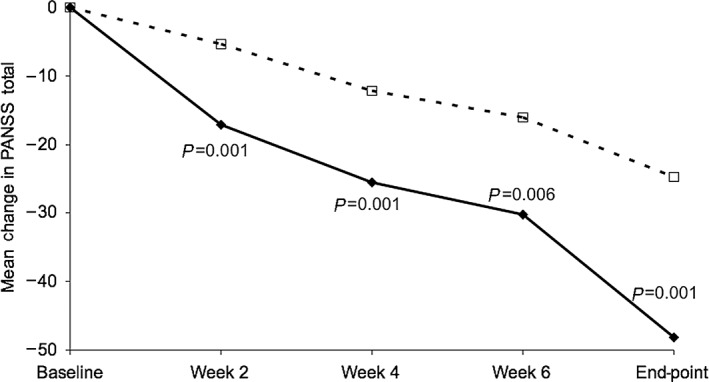
Improvement in symptoms (PANSS total) among those with remission and those with no remission after treatment with a LAI.  , remission (n = 32);
, remission (n = 32);  , no remission (n = 18).63
, no remission (n = 18).63
An open‐label, 6‐month study examined the effects of a LAI administered to 294 inpatients and outpatients early in the course of schizophrenia.67 Overall, 86% of enrolled patients completed the study. Among completers, significant (P < 0.05) improvements from baseline were found for the PANSS, CGI‐S, GAF and Short Form 36 (SF‐36). The authors concluded that use of LAIs early in the course of treatment may reduce discontinuation rates, which could result in increased effectiveness during this crucial period.
The effect of LAI versus oral AP use on remission rates, number of hospital readmissions, and personal and social functioning was evaluated in patients with recent‐onset schizophrenia (<2 years).61 In this case–control study, patients with recent‐onset schizophrenia who initiated LAI treatment (n = 26) were compared with a control group matched for age and sex, and treated with oral APs (n = 26). PANSS, Personal and Social (PAS) Functioning Scale, the number of hospital readmissions, and remission rate were assessed. After 2 years of treatment, the LAI group showed a significantly (P < 0.05) greater reduction in the PANSS total score (47.7 vs. 66.2) and in the negative (mean 14.3 vs. 19.4) and general psychopathology (mean 23.4 vs. 32.7) subscales compared with the oral AP group. PAS Functioning Scale scores also were significantly (P < 0.001) higher in the LAI group (72.4 vs. 59.7). Fewer hospital readmissions and a higher remission rate were observed in the LAI group. Reduction of negative symptoms and enhanced personal and social functioning are critical to illness management and recovery. These results indicate that treatment with a LAI in recent‐onset schizophrenia may have beneficial effects on clinical symptoms and social functioning compared with oral therapy.
Post‐hoc analyses of two observational studies (TIMORES and eSTAR) were conducted to evaluate outcomes among recently diagnosed and long‐term diagnosed patients who were treated with a LAI.62 Symptoms, function and hospitalization rates were examined at 12 and 24 months. Among 155 patients with schizophrenia ≤3 years versus 253 with >3 years duration, significant (P < 0.05) improvement in symptoms (CGI‐Severity), GAF and length of hospitalization were observed with the use of a LAI for recent versus late diagnosis.
A 6‐month, open‐label, multicentre study was conducted in patients with recent‐onset schizophrenia and good pretreatment functioning to determine the effects of a LAI.68 Assessments included the Premorbid Adjustment Scale (PAS) Structured Interview, PANSS, CGI‐S scale, GAF scale and SF‐36. Pretreatment functioning was categorized as stable‐good (n = 142), stable‐poor (n = 116) and deteriorating (n = 36). All groups showed significant (P < 0.05) improvement from baseline for efficacy measures, but improvement was significantly higher in the stable‐good group. The PAS also exhibited a significant linear association with symptom criteria (excellent, good, fair, poor) at baseline (P = 0.003); sustained remission rates at 3 months were excellent 47.7%, good 49.3%, fair 29.6% and poor 22.2% (P = 0.006). In patients with recent‐onset schizophrenia, pretreatment functioning was associated with a better treatment response. All patients showed significant improvement with a LAI, but the greatest improvement was observed in a subgroup with stable‐good functioning at baseline.
Twenty‐five schizophrenic patients hospitalized for the first time were treated with a LAI and subsequently followed for up to 18 months.52 Patients were assessed with CGI and GAF scales. Clinical improvement was associated with fewer relapses and a lower rate of rehospitalization. The authors recommended that implementation of a LAI as early as possible after a first episode of psychosis can reduce relapses and the number and duration of hospitalizations and improve cognitive symptoms and QoL.
A cohort study of 2588 consecutive patients in Finland with a first hospitalization for schizophrenia compared outcomes with oral versus LAI APs.71 Use of LAIs was associated with a significantly lower risk of rehospitalization (P = 0.007) and all‐cause discontinuation (P < 0.0001) compared with oral medication.
Economic benefits of early LAI use in schizophrenia
The impact of schizophrenia treatment on medical resource use including relapse rate, hospitalization, ER visits, physician visits, and direct and indirect costs of treatment is an important consideration for health‐care providers.72 The cost of schizophrenia in the United States in 2002 was estimated to be $62.7 billion and continues to increase, and after adjusting for inflation, the cost is currently estimated to be $100 billion annually.73 A potential avenue for managing costs is early treatment with LAIs. A recent systematic review of cost‐effectiveness studies in schizophrenia concluded that LAIs should be cost‐effective as a first‐line therapy for managing schizophrenia compared with FGA injectable or oral APs.74 Others have found that use of LAIs versus oral APs reduces schizophrenia‐related costs, including hospitalization and ER visits, often because of better medication adherence.69, 75, 76, 77, 78, 79 Consideration of economic burden must be comprehensive rather than our current cost system where costs are allocated in silos.
LAI APs – clinical relevance of formulation differences
LAI formulations are available for both typical and atypical APs (Table 3). Despite approval for the same condition, schizophrenia, differences exist in their profiles that are important considerations in the clinical setting.80, 81, 82, 83 In addition to intrinsic differences in their pharmacologic profile, which contribute to adverse effect (AE) profiles (e.g. metabolic, hyperprolactinaemia, extrapyramidal effects and neurological), each LAI has a distinct PK profile, including duration of action, frequency of dosing and ability to use a loading dose versus oral equivalent overlap, that guides its use in clinical practice.81
Table 3.
Comparison of properties of marketed and late‐stage long‐acting injectable antipsychotics80, 81, 82, 83
| Product | Vehicle | Injection site | Injection technique | Time to peak levels (days) | Duration of overlap with oral | Dose (mg) | Loading dose | Time between injections (weeks) |
|---|---|---|---|---|---|---|---|---|
| Fluphenazine decanoate | Sesame oil | Deltoid or gluteal | Z‐track | 0.3–1.5 | 2–3 months | 12.5–100 | Yes | 2 |
| Haloperidol decanoate | Sesame oil | Deltoid or gluteal | Z‐track | 3–9 | 2–3 months | 25–400 | Yes | 4 |
| Risperidone microspheres | Water | Deltoid or gluteal | Standard | 28 | 6–8 weeks | 12.5–50 | No | 2 |
| Olanzapine pamoate | Water | Gluteal only | Standard | 7 | 3 months | 150–300 or 300–405 | Yes, initiation required | 2–4 |
| Paliperidone palmitate | Water | Deltoid or gluteal | Standard | 13 | 36 days | 39–234 | Yes, initiation required | 4 |
| Aripiprazole monohydrate | Water | Gluteal only | Standard | 5–7 | 3–4 months | 300–400 | No | 4 |
| Aripiprazole lauroxyla | Water | Deltoid or gluteal | Standard | Estimated 7 days | Estimated 30 days | 300–600 | No | 4 |
| Paliperidone palmitate – 3 monthsa | Water | Deltoid or gluteal | Standard | NA | NA | 175–525 | No | 12 |
Not yet approved in the United States.
NA, information not available.
The older typical APs are less expensive than newer atypical drugs, but they are associated with more frequent AEs, especially extrapyramidal symptoms, cognitive dulling and neurological complaints.80, 83 Fluphenazine is characterized by large interpatient variability in its PK profile, which makes it difficult to determine the proper dose when converting from oral AP.80, 84 In addition, both fluphenazine and haloperidol must be administered using a Z‐track method to reduce drug leakage and tissue irritation, which requires training for proper administration.
Because of their long half‐life, LAIs that cannot be administered with a loading dose necessitate a period of overlap treatment with an oral AP while awaiting the LAI to reach steady‐state levels.80 A loading dose may be used with both fluphenazine and haloperidol and with olanzapine and paliperidone. Because of its microsphere formulation, a loading dose cannot be used with risperidone, which necessitates concomitant oral dosing for 3–4 weeks. Aripiprazole monohydrate must be reconstituted and requires a 2‐week overlap with oral medication.
There are also other factors that differ among the LAI formulations. For example, aripiprazole monohydrate must be reconstituted at the time of use.80 Because of the risk of serious AEs, in particular, postinjection delirium/sedation syndrome, patients must be enrolled in a registry prior to using olanzapine.84 Risperidone LAI requires refrigeration prior to use. Thus, each LAI AP formulation offers unique features and benefits, but also potential risks, to be considered within the context of each patient when selecting a specific medication.
Limitations of existing LAIs
Currently available LAIs require injection every 2–4 weeks, may cause pain and discomfort at the injection site in many patients, and continue to have tolerability and safety issues primarily related to cardiovascular, metabolic and endocrine disorders. These limitations may contribute to poor attitudes by patients and prescribers towards LAIs and act as barriers to wider and earlier use.54, 85 In addition, barriers to administering LAIs in the community psychiatric setting, such as lack of training for administering injectable drugs, are impediments to broader use of LAIs.86
A need exists for randomized controlled trials (RCTs) of an adequate duration with LAIs in first‐episode schizophrenia including trials comparing LAIs with oral medication. Adequately controlled trials should report on clinical outcomes as well as patient satisfaction and preferences. A need also exists for high‐quality cost‐effectiveness studies in first‐episode schizophrenia comparing LAIs with oral APs.
A need also exists to understand whether early intervention influences long‐term outcomes in schizophrenia. For instance, does early treatment with LAIs change the course of clinical and neurological deterioration in schizophrenia that occurs within the first 3–5 years after onset of the disease? Can LAIs improve clinical outcomes by reducing early relapse and loss of function in first‐episode patients?
Finally, additional LAIs that provide more reliable and convenient methods of drug delivery are needed. The future of the pharmacotherapy for schizophrenia should provide longer extended‐release injectable formulations, transdermal patches, subcutaneous implants of APs and AP pumps to more effectively address the high risk of relapse due to nonadherence early in the course of illness.
Summary and Conclusion
Early in the course of schizophrenia, patients often are nonadherent to oral AP therapy.70 LAIs provide a treatment option for these patients that has comparable effectiveness and tolerability while offering the potential for improving daily adherence. LAIs also provide clinicians and caregivers with the opportunity to easily detect nonadherence, which may allow for intervention. An additional advantage of LAIs is that the long pharmacological half‐life provides a margin of error for missed doses before plasma levels drop below critical thresholds, where the risks for relapse, hospitalization and suicide may be increased.
Evidence from RCTs for the effectiveness of LAIs as early intervention is limited. However, a number of meta‐analyses have concluded that LAIs are at least comparable to oral APs for relapse prevention and reduced hospitalization.17, 87 Further, results from naturalistic studies, which are more representative of a real‐world setting, consistently indicated superior efficacy for LAIs versus oral APs.87 Despite limited evidence for early use of LAIs, their demonstrated effectiveness for schizophrenia together with convincing evidence for improved adherence argues for their earlier use in the management of schizophrenia.87, 88, 89, 90, 91.
Evidence from clinical studies demonstrates potential clinical and economic benefits from early initiation or first‐episode use of LAIs in schizophrenia with respect to lower relapse rates, fewer hospitalizations, reduced illness‐related complications and comorbidities, and decreased medical resource use compared with oral APs. LAIs have the added benefit of improved daily adherence versus oral APs in many circumstances. With LAIs, the combination of clinical benefits and improved adherence contributes to greater cost advantages.
Although the majority of those with schizophrenia require treatment with AP medication as a component of their overall management, some recent reports suggest that some individuals experience acceptable outcomes without medication.92, 93 Predictors of good outcome without medication were availability of better psychosocial support, fewer previous psychiatric treatments, better neurocognitive skills and absence of psychiatric hospitalization in the previous 5 years. Clinicians should have an awareness of individuals who fit this profile and individualize treatment accordingly.
Unmet needs remain for improved LAI formulations that will provide optimal efficacy to support early use, less frequent injection for better patient comfort and convenience, fewer office visits for injection, an improved safety and tolerability profile, and further enhancements in medication. A person‐centred approach to treating schizophrenia can facilitate establishing and maintaining a therapeutic alliance. Through this relationship, clinicians can assist patients in understanding the clinical and personal benefits of being treated. The clinician has an important role in offering the option of an LAI early in the course of therapy and encouraging its use. An important component is educating the patient on the value of an LAI in treating their illness and the potential benefits versus oral therapy (e.g. not having to remember to take daily medication). This approach to the early use of LAIs has the potential of leading to better patient outcomes while increasing peace of mind for the clinician given the ability to recognize noncompliance more objectively.
Acknowledgement
The authors would like to acknowledge the editorial assistance of Richard S. Perry, PharmD, in the preparation of this manuscript, which was supported by Alkermes, Inc., Waltham, MA.
Declaration of conflict of interest
Dr Stevens is a paid consultant to Alkermes, Inc. Ms Zummo is a full‐time employee of Alkermes.
References
- 1. McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 2008; 30: 67–76. [DOI] [PubMed] [Google Scholar]
- 2. Saha S, Chant D, Welham J et al A systematic review of the prevalence of schizophrenia. PLoS Med 2005; 2: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leucht S, Tardy M, Komossa K et al Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta‐analysis. Lancet 2012; 379: 2063–2071. [DOI] [PubMed] [Google Scholar]
- 4. Panish J, Karve S, Candrilli SD, Dirani R. Association between adherence to and persistence with atypical antipsychotics and psychiatric relapse among US Medicaid‐enrolled patients with schizophrenia. J Pharm Health Serv Res 2013; 4: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second‐generation vs. first‐generation antipsychotics in first‐episode psychosis: a systematic review and meta‐analysis. Int J Neuropsychopharmacol 2013; 16: 1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvarez‐Jiménez M, Parker AG, Hetrick SE, McGorry PD, Gleeson JF. Preventing the second episode: a systematic review and meta‐analysis of psychosocial and pharmacological trials in first‐episode psychosis. Schizophr Bull 2011; 37: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev 2011; (6): CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mihalopoulos C, Harris M, Henry L, Harrigan S, McGorry P. Is early intervention in psychosis cost‐effective over the long term? Schizophr Bull 2009; 35: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serretti A, Mandelli L, Bajo E et al The socio‐economical burden of schizophrenia: a simulation of cost‐offset of early intervention program in Italy. Eur Psychiatry 2009; 24: 11–16. [DOI] [PubMed] [Google Scholar]
- 10. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004; 55: 886–891. [DOI] [PubMed] [Google Scholar]
- 11. Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995; 21: 419–429. [DOI] [PubMed] [Google Scholar]
- 12. Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull 2004; 30: 279–293. [DOI] [PubMed] [Google Scholar]
- 13. Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv 2001; 52: 805–811. [DOI] [PubMed] [Google Scholar]
- 14. Ascher‐Svanum H, Zhu B, Faries DE et al The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry 2010; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun SX, Liu GG, Christensen DB, Fu AZ. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin 2007; 23: 2305–2312. [DOI] [PubMed] [Google Scholar]
- 16. Kishimoto T, Robenzadeh A, Leucht C et al Long‐acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta‐analysis of randomized trials. Schizophr Bull 2014; 40: 192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kishimoto T, Agarwal V, Kishi T, Leucht S, Kane JM, Correll CU. Relapse prevention in schizophrenia: a systematic review and meta‐analysis of second‐generation antipsychotics versus first‐generation antipsychotics. Mol Psychiatry 2013; 18: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim B, Lee SH, Choi TK et al Effectiveness of risperidone long‐acting injection in first‐episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1231–1235. [DOI] [PubMed] [Google Scholar]
- 19. Emsley R, Chiliza B, Asmal L, Mashile M, Fusar‐Poli P. Long‐acting injectable antipsychotics in early psychosis: a literature review. Early Interv Psychiatry 2013; 7: 247–254. [DOI] [PubMed] [Google Scholar]
- 20. Brnabic AJ, Kelin K, Ascher‐Svanum H, Montgomery W, Kadziola Z, Karagianis J. Medication discontinuation with depot and oral antipsychotics in outpatients with schizophrenia: comparison of matched cohorts from a 12‐month observational study. Int J Clin Pract 2011; 65: 945–953. [DOI] [PubMed] [Google Scholar]
- 21. Shi L, Ascher‐Svanum H, Zhu B, Faries D, Montgomery W, Marder SR. Characteristics and use patterns of patients taking first‐generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatr Serv 2007; 58: 482–488. [DOI] [PubMed] [Google Scholar]
- 22. Stahl SM. Long‐acting injectable antipsychotics: shall the last be first? CNS Spectr 2014; 19: 3–5. [DOI] [PubMed] [Google Scholar]
- 23. Kim B, Lee SH, Yang YK, Park JI, Chung YC. Long‐acting injectable antipsychotics for first‐episode schizophrenia: the pros and cons. Schizophr Res Treatment 2012; 2012: 560836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deegan PE, Drake RE. Shared decision making and medication management in the recovery process. Psychiatr Serv 2006; 57: 1636–1639. [DOI] [PubMed] [Google Scholar]
- 25. Stanghellini G, Bolton D, Fulford WK. Person‐centered psychopathology of schizophrenia: building on Karl Jaspers' understanding of patient's attitude toward his illness. Schizophr Bull 2013; 39: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. SAMHSA . Ten guiding principles of recovery. Substance Abuse and Mental Health Services Administration (SAMSA) PEP12‐RECDEF, 2012.
- 27. Malm UI, Ivarsson BÅ, Allebeck P. Durability of the efficacy of integrated care in schizophrenia: a five‐year randomized controlled study. Psychiatr Serv 2014; 65: 1054–1057. [DOI] [PubMed] [Google Scholar]
- 28. Diamond RJ. Recovery from a psychiatrist's viewpoint. NEW DIRECTIONS IN SCHIZOPHRENIA ● A POSTGRADUATE MEDICINE SPECIAL REPORT, 10/2006; Spec No. 54–62. [PubMed]
- 29. Lasser RA, Schooler NR, Kujawa M, Docherty J, Weiden P. A new psychosocial tool for gaining patient understanding and acceptance of long‐acting injectable antipsychotic therapy. Psychiatry (Edgmont) 2009; 6: 22–27. [PMC free article] [PubMed] [Google Scholar]
- 30. Rossi G, Frediani S, Rossi R, Rossi A. Long‐acting antipsychotic drugs for the treatment of schizophrenia: use in daily practice from naturalistic observations. BMC Psychiatry 2012; 12: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Day JC, Bentall RP, Roberts C et al Attitudes toward antipsychotic medication: the impact of clinical variables and relationships with health professionals. Arch Gen Psychiatry 2005; 62: 717–724. [DOI] [PubMed] [Google Scholar]
- 32. Arango C, Amador X. Lessons learned about poor insight. Schizophr Bull 2011; 37: 27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gharabawi GM, Lasser RA, Bossie CA, Zhu Y, Amador X. Insight and its relationship to clinical outcomes in patients with schizophrenia or schizoaffective disorder receiving long‐acting risperidone. Int Clin Psychopharmacol 2006; 21: 233–240. [DOI] [PubMed] [Google Scholar]
- 34. Michel P, Baumstarck K, Auquier P et al Psychometric properties of the abbreviated version of the scale to assess unawareness in mental disorder in schizophrenia. BMC Psychiatry 2013; 13: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cañas F, Alptekin K, Azorin JM et al Improving treatment adherence in your patients with schizophrenia: the STAY initiative. Clin Drug Investig 2013; 33: 97–107. [DOI] [PubMed] [Google Scholar]
- 36. Kvrgic S, Cavelti M, Beck EM, Rüsch N, Vauth R. Therapeutic alliance in schizophrenia: the role of recovery orientation, self‐stigma, and insight. Psychiatry Res 2013; 209: 15–20. [DOI] [PubMed] [Google Scholar]
- 37. Johansen R, Iversen VC, Melle I, Hestad KA. Therapeutic alliance in early schizophrenia spectrum disorders: a cross‐sectional study. Ann Gen Psychiatry 2013; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Misdrahi D, Petit M, Blanc O, Bayle F, Llorca PM. The influence of therapeutic alliance and insight on medication adherence in schizophrenia. Nord J Psychiatry 2012; 66: 49–54. [DOI] [PubMed] [Google Scholar]
- 39. Wood L, Burke E, Morrison A. Individual cognitive behavioural therapy for psychosis (CBTp): a systematic review of qualitative literature. Behav Cogn Psychother 2015; 43: 285–297. [DOI] [PubMed] [Google Scholar]
- 40. Fe Bravo‐Ortiz M, Gutiérrez‐Casares JR, Rodríguez‐Morales A, García MA, Hidalgo‐Borrajo R. Influence of type of treatment on the well‐being of Spanish patients with schizophrenia and their caregivers. Int J Psychiatry Clin Pract 2011; 15: 286–295. [DOI] [PubMed] [Google Scholar]
- 41. Wykes T, Rose D, Williams P, David AS. Working alliance and its relationship to outcomes in a randomized controlled trial (RCT) of antipsychotic medication. BMC Psychiatry 2013; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Potkin S, Bera R, Zubek D, Lau G. Patient and prescriber perspectives on long‐acting injectable (LAI) antipsychotics and analysis of in‐office discussion regarding LAI treatment for schizophrenia. BMC Psychiatry 2013; 13: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gray R, Spilling R, Burgess D, Newey T. Antipsychotic long‐acting injections in clinical practice: medication management and patient choice. Br J Psychiatry Suppl 2009; 52: S51–56. [DOI] [PubMed] [Google Scholar]
- 44. Bola J, Kao D, Soydan H. Antipsychotic medication for early episode schizophrenia. Cochrane Database Syst Rev 2011; (6): CD006374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crossley NA, Constante M, McGuire P, Power P. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta‐analysis. Br J Psychiatry 2010; 196: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freudenreich O, McEvoy JP. Optimizing outcome with antipsychotic treatment in first‐episode schizophrenia: balancing efficacy and side effects. Clin Schizophr Relat Psychoses 2012; 6: 115–121. [DOI] [PubMed] [Google Scholar]
- 47. Taylor M, Cavanagh J, Hodgson R, Tiihonen J. Examining the effectiveness of antipsychotic medication in first‐episode psychosis. J Psychopharmacol 2012; 26 (5 Suppl.): 27–32. [DOI] [PubMed] [Google Scholar]
- 48. Lasser RA, Bossie CA, Zhu Y, Locklear JC, Kane JM. Long‐acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry 2007; 19: 65–71. [DOI] [PubMed] [Google Scholar]
- 49. Macfadden W, Bossie CA, Turkoz I, Haskins JT. Risperidone long‐acting therapy in stable patients with recently diagnosed schizophrenia. Int Clin Psychopharmacol 2010; 25: 75–82. [DOI] [PubMed] [Google Scholar]
- 50. Parellada E, Andrezina R, Milanova V et al Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long‐acting injectable. J Psychopharmacol 2005; 19 (5 Suppl.): 5–14. [DOI] [PubMed] [Google Scholar]
- 51. Taylor M, Ng KY. Should long‐acting (depot) antipsychotics be used in early schizophrenia? A systematic review. Aust N Z J Psychiatry 2013; 47: 624–630. [DOI] [PubMed] [Google Scholar]
- 52. Viala A, Cornic F, Vacheron MN. Treatment adherence with early prescription of long‐acting injectable antipsychotics in recent‐onset schizophrenia. Schizophr Res Treatment 2012; 2012: 368687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agid O, Arenovich T, Sajeev G et al An algorithm‐based approach to first‐episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011; 72: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 54. Geerts P, Martinez G, Schreiner A. Attitudes towards the administration of long‐acting antipsychotics: a survey of physicians and nurses. BMC Psychiatry 2013; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kirschner M, Theodoridou A, Fusar‐Poli P, Kaiser S, Jager M. Patients' and clinicians' attitude towards long acting depot antipsychotics in subjects with a first episode of psychosis. Ther Adv Psychopharmacol 2013; 3: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parellada E, Velligan DI, Emsley R, Kissling W. Long‐acting injectable antipsychotics in first‐episode schizophrenia. Schizophr Res Treatment 2012; 2012: 318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bartzokis G, Lu PH, Amar CP et al Long acting injection versus oral risperidone in first‐episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res 2011; 132: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kane JM, Rifkin A, Quitkin F, Nayak D, Ramos‐Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first‐episode schizophrenia. Arch Gen Psychiatry 1982; 39: 70–73. [DOI] [PubMed] [Google Scholar]
- 59. Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa A, Goldfinger SM. A randomized controlled trial of long‐acting injectable risperidone vs continuation on oral atypical antipsychotics for first‐episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry 2009; 70: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 60. Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa‐McMillan A. Maintenance treatment with long‐acting injectable risperidone in first‐episode schizophrenia: a randomized effectiveness study. J Clin Psychiatry 2012; 73: 1224–1233. [DOI] [PubMed] [Google Scholar]
- 61. Barrio P, Batalla A, Castellví P et al Effectiveness of long‐acting injectable risperidone versus oral antipsychotics in the treatment of recent‐onset schizophrenia: a case‐control study. Int Clin Psychopharmacol 2013; 28: 164–170. [DOI] [PubMed] [Google Scholar]
- 62. Dubois V, Peuskens J, Geerts P, Detraux J. Clinical outcomes of long‐acting risperidone in recent versus long‐term diagnosed Belgian schizophrenic patients: results from electronic Schizophrenia Treatment Adherence Registry (e‐STAR) and Trial for the Initiation and Maintenance Of REmission in Schizophrenia with risperidone (TIMORES). Early Interv Psychiatry 2014; 6: 39–49. [DOI] [PubMed] [Google Scholar]
- 63. Emsley R, Medori R, Koen L, Oosthuizen PP, Niehaus DJ, Rabinowitz J. Long‐acting injectable risperidone in the treatment of subjects with recent‐onset psychosis: a preliminary study. J Clin Psychopharmacol 2008; 28: 210–213. [DOI] [PubMed] [Google Scholar]
- 64. Emsley R, Oosthuizen P, Koen L, Niehaus DJ, Medori R, Rabinowitz J. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther 2008; 30: 2378–2386. [DOI] [PubMed] [Google Scholar]
- 65. Emsley R, Oosthuizen P, Koen L, Niehaus DJ, Medori R, Rabinowitz J. Remission in patients with first‐episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long‐acting injection. Int Clin Psychopharmacol 2008; 23: 325–331. [DOI] [PubMed] [Google Scholar]
- 66. Malla A, Chue P, Jordan G et al An exploratory open‐label randomized trial comparing risperidone long acting injectable (RLAI) with oral antipsychotic medication in the treatment of early psychosis. Clin Schizophr Relat Psychoses 2013; 17: 1–26. [DOI] [PubMed] [Google Scholar]
- 67. Napryeyenko O, Burba B, Martinez G et al Risperidone long‐acting injectable in recent‐onset schizophrenia examined with clinician and patient self‐report measures. J Clin Psychopharmacol 2010; 30: 200–202. [DOI] [PubMed] [Google Scholar]
- 68. Rabinowitz J, Napryeyenko O, Burba B et al Premorbid functioning and treatment response in recent‐onset schizophrenia: prospective study with risperidone long‐acting injectable. J Clin Psychopharmacol 2011; 31: 75–81. [DOI] [PubMed] [Google Scholar]
- 69. Olivares JM, Peuskens J, Pecenak J et al Clinical and resource‐use outcomes of risperidone long‐acting injection in recent and long‐term diagnosed schizophrenia patients: results from a multinational electronic registry. Curr Med Res Opin 2009; 25: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 70. Sliwa JK, Bossie CA, Fu DJ, Turkoz I, Alphs L. Long‐term tolerability of once‐monthly injectable paliperidone palmitate in subjects with recently diagnosed schizophrenia. Neuropsychiatr Dis Treat 2012; 8: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011; 168: 603–609. [DOI] [PubMed] [Google Scholar]
- 72. McEvoy JP. The costs of schizophrenia. J Clin Psychiatry 2007; 68 (Suppl. 14): 4–7. [PubMed] [Google Scholar]
- 73. Kane JM, Correll CU, Harvey PD, Olfson M. Clinical and cost implications of treating schizophrenia: safety, efficacy, relapse prevention, and patient outcomes. J Clin Psychiatry 2014; 75: e20. [Google Scholar]
- 74. Achilla E, McCrone P. The cost effectiveness of long‐acting/extended‐release antipsychotics for the treatment of schizophrenia: a systematic review of economic evaluations. Appl Health Econ Health Policy 2013; 11: 95–106. [DOI] [PubMed] [Google Scholar]
- 75. Bera R, Offord S, Zubek D et al Impact on healthcare resource usage and costs among Medicaid‐insured schizophrenia patients after initiation of treatment with long‐acting injectable antipsychotics. J Med Econ 2013; 16: 522–528. [DOI] [PubMed] [Google Scholar]
- 76. Crivera C, DeSouza C, Kozma CM, Dirani RD, Mao L, Macfadden W. Resource utilization in patients with schizophrenia who initiated risperidone long‐acting therapy: results from the Schizophrenia Outcomes Utilization Relapse and Clinical Evaluation (SOURCE). BMC Psychiatry 2011; 11: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kane JM, Sanchez R, Zhao J et al Hospitalisation rates in patients switched from oral anti‐psychotics to aripiprazole once‐monthly for the management of schizophrenia. J Med Econ 2013; 16: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin J, Wong B, Offord S, Mirski D. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res 2013; 40: 355–366. [DOI] [PubMed] [Google Scholar]
- 79. Offord S, Wong B, Mirski D, Baker RA, Lin J. Healthcare resource usage of schizophrenia patients initiating long‐acting injectable antipsychotics vs oral. J Med Econ 2013; 16: 231–239. [DOI] [PubMed] [Google Scholar]
- 80. Gopalakrishna G, Aggarwal A, Lauriello J. Long‐acting injectable aripiprazole: how might it fit in our tool box? Clin Schizophr Relat Psychoses 2013; 7: 87–92. [DOI] [PubMed] [Google Scholar]
- 81. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr 2013; 18 (Suppl. 1): 58–67. [DOI] [PubMed] [Google Scholar]
- 82. Spanarello S, La Ferla T. The pharmacokinetics of long‐acting antipsychotic medications. Curr Clin Pharmacol 2014; 9: 310–317. [DOI] [PubMed] [Google Scholar]
- 83. Taylor D. Psychopharmacology and adverse effects of antipsychotic long‐acting injections: a review. Br J Psychiatry Suppl 2009; 52: S13–19. [DOI] [PubMed] [Google Scholar]
- 84. Kennedy WK. When and how to use long‐acting injectable antipsychotics. Curr Psychiat 2012; 11: 40–43. [Google Scholar]
- 85. Iyer S, Banks N, Roy MA et al A qualitative study of experiences with and perceptions regarding long‐acting injectable antipsychotics: part I‐patient perspectives. Can J Psychiatry 2013; 58 (5 Suppl. 1): 14S–22S. [DOI] [PubMed] [Google Scholar]
- 86. Velligan DI, Medellin E, Draper M et al Barriers to, and strategies for, starting a long acting injection clinic in a community mental health center. Community Ment Health J 2011; 47: 654–659. [DOI] [PubMed] [Google Scholar]
- 87. Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long‐acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta‐analysis of mirror‐image studies. J Clin Psychiatry 2013; 74: 957–965. [DOI] [PubMed] [Google Scholar]
- 88. Kirson NY, Weiden PJ, Yermakov S et al Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry 2013; 74: 568–575. [DOI] [PubMed] [Google Scholar]
- 89. Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long‐acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol 2013; 66: S37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Manchanda R, Chue P, Malla A et al Long‐acting injectable antipsychotics: evidence of effectiveness and use. Can J Psychiatry 2013; 58: 5S–13S. [DOI] [PubMed] [Google Scholar]
- 91. Heres S, Lambert M, Vauth R. Treatment of early episode in patients with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry 2014; 29S2: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 92. Harrow M, Jobe TH, Faull RN. Do all schizophrenia patients need antipsychotic treatment continuously throughout their lifetime? A 20‐year longitudinal study. Psychol Med 2012; 42: 2145–2155. [DOI] [PubMed] [Google Scholar]
- 93. Moilanen J, Haapea M, Miettunen J et al Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication – a 10‐year follow‐up of the Northern Finland 1966 Birth Cohort study. Eur Psychiatry 2013; 28: 53–58. [DOI] [PubMed] [Google Scholar]


