Abstract
BACKGROUND
In the current American Joint Committee on Cancer/International Union Against Cancer staging system (seventh edition) for intrahepatic cholangiocarcinoma (ICC), tumor size was excluded, and periductal invasion was added as a new tumor classification‐defining factor. The objective of the current report was to propose a new staging system for ICC that would be better for stratifying the survival of patients based on data from the nationwide Liver Cancer Study Group of Japan database.
METHODS
Of 756 patients who underwent surgical resection for ICC between 2000 and 2005, multivariate analyses of the clinicopathologic factors of 419 patients who had complete data sets were performed to elucidate relevant factors for inclusion in a new tumor classification and staging system.
RESULTS
Overall survival data were best stratified using a cutoff value of 2 cm using a minimal P value approach to discriminate patient survival. The 5‐year survival rate of 15 patients who had ICC measuring ≤2 cm in greatest dimension without lymph node metastasis or vascular invasion was 100%, and this cohort was defined as T1. Multivariate analysis of prognostic factors for 267 patients with lymph node‐negative and metastasis‐negative (N0M0) disease indicated that the number of tumors, the presence arterial invasion, and the presence major biliary invasion were independent and significant prognostic factors. The proposed new system, which included tumor number, tumor size, arterial invasion, and major biliary invasion for tumor classification, provided good stratification of overall patient survival according to disease stage. Macroscopic periductal invasion was associated with major biliary invasion and an inferior prognosis.
CONCLUSIONS
The proposed new staging system, which includes a tumor cutoff size of 2 cm and major biliary invasion, may be useful for assigning patients to surgery. Cancer 2016;122:61–70. © 2015 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: biliary invasion, intrahepatic cholangiocarcinoma, periductal invasion, staging system, tumor size
Short abstract
The current staging system for intrahepatic cholangiocarcinoma has been subject to debate. The authors developed a new staging system for intrahepatic cholangiocarcinoma using complete nationwide data from 419 surgical patients provided by the Liver Cancer Study Group of Japan.
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver tumor after hepatocellular carcinoma (HCC). It is estimated that approximately 5% to 15% of primary liver cancers are ICCs,1 although recent studies have reported that the incidence of ICC has doubled to 2.1 per 100,000 person‐years in the United States over the past few decades.2, 3, 4 Nevertheless, because of its rarity compared with HCC, clinical studies based on a large number of case series in ICC are limited,5, 6, 7 and clinicopathologic features relevant for adequate tumor staging remain to be identified. Although it has been demonstrated that combined systemic chemotherapy improves the survival of patients with biliary cancer, including ICC, compared with best supportive care,8 achieving a cure relies on surgical resection. Therefore, an adequate staging system is essential to identify patients who would benefit from surgical resection.
An ICC staging system independent of HCC was first published in 1997 by the Liver Cancer Study Group of Japan (LCSGJ) based on the HCC staging system, in which a tumor cutoff size of 2 cm, the number of tumors, and the presence of vascular/serosal invasion were used to determine tumor (T)‐classification.9 Another attempt was made to produce an optimal ICC staging system with the T‐classification defined by tumor number and vascular invasion, excluding tumor size, on the basis of results from a multivariate analysis of prognosis in 60 surgical patients with mass‐forming ICC.10 In the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) staging system, ICC was traditionally staged with HCC under the category of primary liver cancer; ie, T‐classification was determined by tumor number, vascular invasion, a tumor cutoff size of 5 cm, and invasion to adjacent organs. In the AJCC seventh edition,11 however, an original classification for ICC was developed based on data from the US Surveillance, Epidemiology, and End Results (SEER) program.5 In that seventh edition, a tumor cutoff size of 5 cm was excluded, and periductal invasion was newly added as a factor for determining T‐classification. A multicenter study analyzing the survival of 522 patients with ICC in France confirmed the acceptable performance of the current version of tumor, lymph node, metastases (TNM) classification for ICC.12 Although international guidelines supported the seventh edition of the AJCC/UICC staging system,13 the limitations of this system were pointed out; in particular, issues classifying T4 disease or multiple tumors were highlighted. In addition, the pathologic definition of periductal invasion was not defined in the World Health Organization classification.1 The objective of the current study was to clarify the prognostic significance of the pathologic features of ICC and to propose a new staging system for ICC based on the large, nationwide LCSGJ database.
MATERIALS AND METHODS
With the cooperation of 795 institutions in Japan, patients with primary liver cancer are registered every 2 years and are followed prospectively in a nationwide survey conducted by the LCSGJ. ICC was diagnosed at each institution on the basis of imaging studies, clinical data, and/or histologic analyses. Among the 62,424 patients with liver cancer newly registered in the survey between 2000 and 2005, 2279 patients (3.7%) had a clinical diagnosis of ICC. In total, 1216 patients underwent surgical resection, resulting in a 53.3% resection rate. Of these, the prognosis was pursued in 1145 patients. The macroscopic findings in those 1145 patients included 1) mass‐forming (MF)–dominant ICC, including the MF type (n = 632); 2) the periductal infiltrating (PI) type (n = 96); 3) the intraductal growth (IG) type (n = 45); 4) the MF + PI type (n = 204); 5) the PI + IG type (n = 6); 6) the MF + IG type (n = 22); and 7) other types (n = 140). Of the 836 patients who had the MF or MF + PI type, histologic confirmation of cholangiocarcinoma was confirmed in 756 patients, excluding 20 patients with HCC, 7 patients with cystadenocarcinoma, 15 patients with combined HCC‐ICC, 1 patient with hepatoblastoma, 5 patients with other diseases, and 32 patients without histologic confirmation (Fig. 1).
Figure 1.
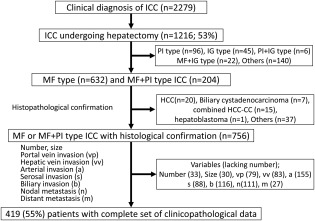
This schematic flow chart displays the population of patients with intrahepatic cholangiocarcinoma (ICC) who underwent hepatectomy between 2000 and 2005 and were registered in the nation‐wide database of the Liver Cancer Study Group of Japan. Combined HCC‐CC indicates combined hepatocellular cholangiocarcinoma; HCC, hepatocellular carcinoma; IG, intraductal growth; MF, mass forming; PI, periductal infiltrating.
Among the 756 patients who had histologically confirmed ICC, clinicopathologic data were evaluated, and 419 patients (55%) were extracted, excluding 337 patients who had incomplete information on essential clinicopathologic factors, such as tumor number (n = 33), tumor size (n = 30), portal vein invasion (n = 79), hepatic vein invasion (n = 83), arterial invasion (n = 155), serosal invasion (n = 88), biliary invasion (n = 116), lymph node metastasis (n = 111), and/or distant metastasis (n = 27). This selection was undertaken to purify the data with a view toward establishing a reliable staging system.
The definition of each pathologic factor was established based on the General Rules for the Clinical and Pathological Study of Primary Liver Cancer.9 Tumor size and number were determined based on the results of pathologic examinations. The impact of portal vein invasion (vp), hepatic vein invasion, arterial invasion, serosal invasion, and biliary invasion was evaluated according to the microscopic grades of each factor (ie, vp0 vs vp1‐vp4, vp0‐vp1 vs vp0‐vp2 vs vp3‐vp4) based on the LCSGJ classification system.9 Biliary invasion was defined as ranging from b0 to b4. Major biliary invasion was defined as b3 or b4, whereas b1 or b2 indicated minor biliary invasion. Peritoneal dissemination was included in distant metastasis. Periductal invasion was characterized by macroscopic findings of diffuse tumor infiltration along the long axis of the portal tract involving the bile duct, blood vessels, and connective tissues.9 Three hundred ten of the 419 patients (74%) underwent lymph node dissection, and lymph node metastasis was diagnosed using the resected lymph nodes.
With regard to the impact of tumor size on survival, the difference in survival for patients in each category of tumor size was evaluated using various cutoff values ranging from 1 to 7 cm in greatest dimension not only in the full cohort of 419 patients but also in 267 patients who had N0M0 disease. The correlation between the macroscopic appearance of the tumor (MF type vs MF + PI type) and the grade of biliary invasion (b0‐b4) also was examined. Univariate and multivariate analyses of survival were performed for all of the valid clinicopathologic factors.
Design of a New Staging System
Upon a review of results from these retrospective analyses, a new staging system was developed based on the following criteria: 1) TNM style should be maintained, 2) the new system should be as simple as possible, 3) each factor for determining T‐classification preferably should be estimated based on preoperative imaging studies, 4) the survival curves determined by T‐classification should be well stratified in N0M0 patients, 5) the survival curves determined by disease stage should be well stratified in all patients analyzed, 6) the distribution of patient proportions in each T‐classification and stage should be well balanced, and 7) stage IVB disease should be carefully determined because it implies that there is no indication for surgery.
Survival Curves According to Stage and Validation Analysis of the Counterpart Cohort
The overall survival of the 267 patients with N0M0 disease was stratified by T‐classification according to the preexisting staging systems, including the fourth and fifth editions of the LCSGJ system, the system published by Okabayashi et al,10 the sixth and seventh editions of the AJCC/UICC TNM classification,11 and the current new system. The overall survival of all 419 patients was stratified by stage using the same preexisting staging systems. A validation analysis was performed using the newly developed staging system on the remaining 337 patients among the 756 patients who had histopathologically confirmed ICC. Differences in survival curves were evaluated according to T‐classification and disease stage.
Statistical Analysis
Correlations between categorical variables were tested using chi‐square analysis, and continuous data were analyzed using the Mann‐Whitney U test. Survival curves for overall survival were generated using the Kaplan‐Meier method and were compared using the log‐rank test. To identify risk factors for death, multivariate regression analysis was performed with the Cox proportional hazards model using a backward elimination procedure. Probability (P) values < .05 were considered statistically significant. Data were analyzed using the SPSS version 19.0 software program (version 19.0; IBM Corp, Armonk, NY).
RESULTS
Tumor Size
The best tumor cutoff size was determined by using a minimum P value approach for the entire cohort of 419 patients and for the cohort of 267 patients with N0M0 disease. When sliding the cutoff value of tumor size from 1 cm to 7 cm, minimum P values were obtained at 2.1 cm for both the full 419‐patient cohort and the 267‐patient N0M0 cohort. The conventional cutoff value of 5 cm did not prove useful for prognostication in the study population (Fig. 2).
Figure 2.
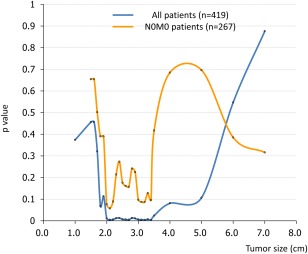
This chart illustrates the optimal cutoff value of tumor size for predicting the survival of all 419 patients and of the 267 patients who had lymph node‐negative/metastasis‐negative (N0M0) disease.
Characteristics of Tumors ≤ 2 cm
Twenty‐seven of 419 patients (6.4%) had tumors measuring ≤ 2 cm in greatest dimension. Although none of those 27 patients had distant metastasis, 7 of them (25.9%) had lymph node metastasis, 9 had portal vein invasion, and 12 had biliary invasion. Excluding the 7 patients who had lymph node metastasis, there was biliary invasion in 7 patients (35%), portal vein invasion in 5 patients (25%), hepatic vein invasion in 1 patient (5%), serosal invasion in 1 patient (5%), and arterial invasion in none of the remaining 20 patients. The 5‐year survival rate of the 15 patients without portal vein invasion was 100%, which was significantly better than that of the 5 patients with portal vein invasion (2‐year survival rate, 60%; P = .01).
Macroscopic Findings of the MF or MF + PI Type
All 419 patients were diagnosed with either MF type (n = 315) or MF + PI type (n = 104) ICC according to macroscopic findings in the specimen. Having similar sizes and numbers of tumors, the MF + PI type of ICC was more frequently associated with portal vein invasion, biliary invasion, or lymph node metastasis compared with the MF type (Table 1). The overall survival of patients who had the MF + PI type was significantly worse than that of patients who had the MF type (median survival time [MST], 43.5 vs 17.6 months; P < .001). A very high rate of biliary invasion was noted in both the MF type (45.4%) and the MF + PI type (80.8%; P < .001). The degree of biliary invasion was more advanced in patients who had the MF + PI type than in those who had the MF type, with a significantly higher rate of major biliary invasion (b3 or b4; 36.5% vs 11.7%, respectively; P < .001).
Table 1.
Correlation Between Macroscopic Type and Valid Clinicopathologic Factors in the Full Cohort of 419 Patients With Mass‐Forming–Dominant Intrahepatic Cholangiocarcinoma
| No. of Patients | |||
|---|---|---|---|
| Variable | MF Type, n = 315 | MF + PI Type, n = 104 | P |
| Tumor size, cm | |||
| ≤2 | 21 | 6 | .75 |
| >2 | 294 | 98 | |
| ≤5 | 170 | 65 | .13 |
| >5 | 145 | 39 | |
| No. of tumors | |||
| Solitary | 244 | 72 | .09 |
| Multiple | 71 | 32 | |
| Serosal invasion | |||
| Present | 201 | 47 | .10 |
| Absent | 114 | 57 | |
| Portal vein invasion | |||
| Present | 145 | 64 | .006a |
| Absent | 170 | 40 | |
| Hepatic vein invasion | |||
| Present | 86 | 33 | .39 |
| Absent | 229 | 71 | |
| Arterial invasion | |||
| Present | 21 | 12 | .11 |
| Absent | 294 | 92 | |
| Biliary invasion | |||
| b0 | 172 | 20 | < .001a |
| b1‐b4 | 143 | 84 | |
| Major biliary invasion | |||
| b0‐b2 | 278 | 66 | < .001a |
| b3, b4 | 37 | 38 | |
| Lymph node metastases | |||
| Present | 96 | 56 | < .001a |
| Absent | 219 | 48 | |
| Distant metastases | |||
| Present | 4 | 3 | .27 |
| Absent | 311 | 101 | |
Abbreviations: b0, no biliary invasion; b1, invasion of the third‐order or more peripheral branch of the bile duct; b2, invasion of the second‐order branch of the bile duct; b3, invasion of the first‐order branch of the bile duct; b4, invasion of the common hepatic duct; MF + PI type, mass‐forming plus periductal‐infiltrating type; MF type, mass‐forming type.
This P value indicates a statistically significant difference.
Univariate and Multivariate Analyses of Prognostic Factors in the Entire Cohort
Univariate analysis of the entire 419‐patient cohort revealed that tumor size, tumor number, portal vein invasion, hepatic vein invasion, arterial invasion, serosal invasion, major biliary invasion, lymph node metastasis, and distant metastasis were significant prognostic factors for survival (Table 2). Although not all types of biliary invasion were associated with worse survival, major biliary invasion was significantly associated with worse survival among all 419 patients. Multivariate analysis revealed that tumor number, lymph node metastasis, and distant metastasis were independent prognostic factors.
Table 2.
Univariate and Multivariate Analyses of Prognostic Factors for Survival in the Full Cohort of 419 Patients With Mass‐Forming–Dominant Intrahepatic Cholangiocarcinoma
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | No. of Patients | 5‐Year Survival Rate, % | MST, mo | P | HR (95% CI) | P |
| Tumor size, cm | ||||||
| ≤2 | 27 | 82.4 | ND | .011a | 2.487 (0.912‐6.780) | .075 |
| >2 | 392 | 40 | 29 | |||
| No. of tumors | ||||||
| Solitary | 316 | 53.5 | ND | < .001a | 2.570 (1.814‐3.643) | < .001a |
| Multiple | 103 | ND | 15.2 | |||
| Portal vein invasion | ||||||
| Absent | 210 | 52.1 | ND | .009a | ||
| Present | 209 | 34.2 | 25.5 | |||
| Hepatic vein invasion | ||||||
| Absent | 300 | 46.1 | 36.6 | .007a | ||
| Present | 119 | ND | 19.4 | |||
| Arterial invasion | ||||||
| Absent | 386 | 44.9 | 36.6 | .003a | ||
| Present | 33 | ND | 16.6 | |||
| Biliary invasion | ||||||
| Absent | 192 | 44.9 | 43.5 | .07 | ||
| Present | 227 | ND | 26.2 | |||
| Major biliary invasion | ||||||
| Absent | 344 | 46.5 | 36.6 | .004a | ||
| Present | 75 | ND | 20.3 | |||
| Serosal invasion | ||||||
| Absent | 258 | 48.3 | 43.5 | .001a | ||
| Present | 161 | 35.2 | 21.4 | |||
| Lymph node metastasis | ||||||
| Negative | 267 | 58.4 | ND | < .001a | 2.818 (1.992–3.987) | < .001a |
| Positive | 152 | 11.1 | 16.0 | |||
| Distant metastasis | ||||||
| Negative | 412 | 44.2 | 34.7 | < .001a | 2.940 (1.258–6.869) | .01a |
| Positive | 7 | 0 | 4.2 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; MST, medial survival time; ND, not determined.
This P value indicates a statistically significant difference.
Univariate and Multivariate Analyses of Prognostic Factors in the N0M0 Cohort
To elucidate the impact solely of T‐classification on patient survival, data on the 267 patients with N0M0 disease were analyzed. Univariate and multivariate analyses of the 267 N0M0 patients revealed that tumor number, arterial invasion, and major biliary invasion were significant independent factors for survival (Table 3). Major biliary invasion, but not all types of biliary invasion, was associated with worse survival (P = .007) (Fig. 3); whereas portal vein invasion, hepatic vein invasion, or serosal invasion were not significantly associated with survival, as evidenced by testing various cutoff values for each category.
Table 3.
Univariate and Multivariate Analyses of Prognostic Factors for Survival in the Cohort of 267 Patients With N0M0, Mass‐Forming–Dominant Intrahepatic Cholangiocarcinoma
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Variable | No. of Patients | 5‐Year Survival Rate, % | P | HR (95% CI) | P |
| Tumor size, cm | |||||
| ≤2 | 20 | 90 | .076 | ||
| >2 | 247 | 55.4 | |||
| No. of tumors | |||||
| Solitary | 219 | 63.1 | < .001a | 3.937 (2.291‐6.753) | < .001a |
| Multiple | 48 | ND | |||
| Portal vein invasion | |||||
| Absent | 160 | 63.6 | .083 | ||
| Present | 107 | 52.5 | |||
| Hepatic vein invasion | |||||
| Absent | 213 | 58.8 | .67 | ||
| Present | 54 | ND | |||
| Arterial invasion | |||||
| Absent | 253 | 59.8 | .002a | 2.791 (1.264–6.161) | .01a |
| Present | 14 | ND | |||
| Biliary invasion | |||||
| Absent | 140 | 64.4 | .085 | ||
| Present | 127 | 51.1 | |||
| Major biliary invasion | |||||
| Absent | 231 | 60.9 | .007a | 2.939 (1.551–5.566) | .001a |
| Present | 36 | ND | |||
| Serosal invasion | |||||
| Absent | 174 | 61.3 | .064 | ||
| Present | 93 | 52.7 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; ND, not determined.
This P value indicates a statistically significant difference.
Figure 3.
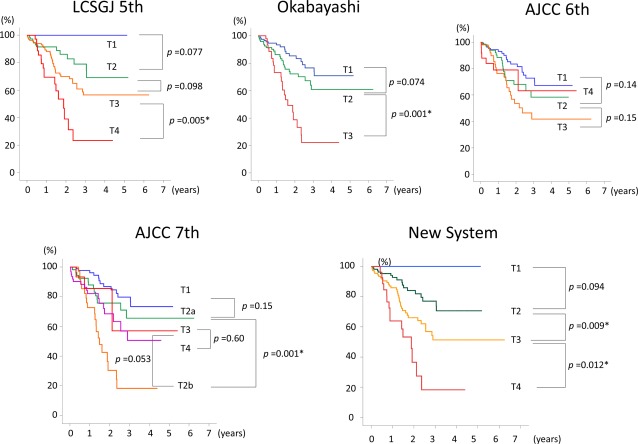
These charts compare the staging systems for intrahepatic cholangiocarcinoma based on the stratification of survival curves from the 267 patients who had N0M0 disease according to tumor classification. AJCC 6th indicates the International Union Against Cancer/American Joint Committee on Cancer classification, 6th edition; LCSGJ 5th, Liver Cancer Study Group of Japan, 5th edition; Okabayashi, the staging system published by Okabayashi et al10; AICC 7th, the International Union Against Cancer/American Joint Committee on Cancer classification, 7th edition.
A New Staging System for ICC of the Predominantly MF Type
On the basis of the results obtained from analysis of LCSGJ data described above, a new T‐classification was developed that included tumor size with a cutoff value of 2 cm, tumor number, portal vein invasion, and major biliary invasion. The style of the staging system followed the current Japanese staging system for ICC11; ie, T‐classification comprised the following factors: 1) tumor size (≤2 cm vs > 2 cm), 2) tumor number (solitary vs multiple), and 3) vascular invasion or major biliary invasion (Table 4). Vascular invasion was represented by the presence of either portal vein invasion or arterial invasion. On the basis of the above categories, no significant difference in survival was observed between 106 patients who had T1‐T3N1M0 disease and 27 patients who had T4N0M0 disease (MST, 16.6 vs 22.5 months; P = .95). No significant difference in survival was observed between 38 patients who had T4N1M0 disease and 7 patients who had M1 disease (MST, 13.4 vs 4.2 months; P = .57). Therefore, patients with T1‐T3N1M0 and T4N0M0 disease were grouped into stage IVA, whereas those with T4N1M0 and M1 disease were grouped into stage IVB (MST, 17.8 vs 12.3 months; P < .001).
Table 4.
A New Staging System for Mass‐Forming–Dominant Intrahepatic Cholangiocarcinoma
| Variable | Parameter |
|---|---|
| Criteria | |
| 1. No. of tumors | Solitary |
| 2. Size of largest tumor | ≤2 cm |
| 3. Vascular or major biliary invasion | vp0, va0, b0‐b2 |
| Tumor classification | |
| T1 | All 3 criteria are fulfilled |
| T2 | Only 2 of the 3 criteria are fulfilled |
| T3 | Only 1 of the 3 criteria is fulfilled |
| T4 | None of the 3 criteria are fulfilled |
| Stage | |
| I | T1N0M0 |
| II | T2N0M0 |
| III | T3N0M0 |
| IVA | T4N0M0 |
| T1‐T3N1M0 | |
| IVB | T4N1M0 |
| AnyTN0,N1M1 |
Abbreviations: b0‐b2, no biliary invasion or minor biliary invasion within second‐order branch of the bile duct; M, metastasis status; N, lymph node status; T, tumor classification; va0, no arterial invasion; vp0, no portal vein invasion.
Comparison With the Stratification of Overall Survival by Preexisting Staging Systems
Overall survival curves for the 267‐patient N0M0 cohort were well stratified by T‐classification using the newly proposed system (Fig. 3). In the seventh edition of the AJCC classification, patients who had T2b tumors had worse survival than those who had T3 tumors (P = .001) and T4 tumors (P = .053), suggesting worse survival for patients who had multiple ICCs (T2b) compared with those who had perforation (T3) or periductal invasion (T4).
Overall survival curves for the entire 419‐patient cohort were well stratified by disease stage using the new staging system (Fig. 4). The seventh edition of the AJCC system did not discriminate well between stages I, II, and III; however, the new staging system did discriminate well between stages I, II, and III, although the difference was marginal between stages I and II (P = .09). The fifth edition of the LCSGJ system did not discriminate well between stages IVA and IVB (P = .12); however, the new staging system did discriminate well between stages IVA and IVB (P < .001), indicating that stage IVB in the fifth edition of the LCSGJ system can be divided into 2 groups, T1‐T3N1M0 (stage IVA in the new staging system) and T4N1M0 or M1 (stage IVB in the new staging system).
Figure 4.
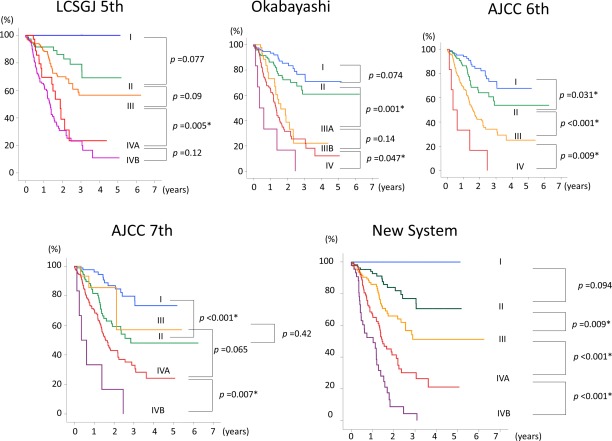
These charts compare the staging systems for intrahepatic cholangiocarcinoma based on the stratification of survival curves from the entire cohort of 419 patients according to disease stage. AJCC 6th indicates the International Union Against Cancer/American Joint Committee on Cancer classification, 6th edition; LCSGJ 5th, Liver Cancer Study Group of Japan, 5th edition; Okabayashi, the staging system published by Okabayashi et al10; AJCC 7th, the International Union Against Cancer/American Joint Committee on Cancer classification, 7th edition.
In the validation analysis, T‐classification could be identified for 198 of 337 patients (58.8%), and disease stage was available for only 134 patients (39.7%) because of a lack of clinicopathologic data. Survival curves according to T‐classification were well discriminated, whereas the survival curves for each stage were not well stratified because of the small number of patients (Fig. 5).
Figure 5.
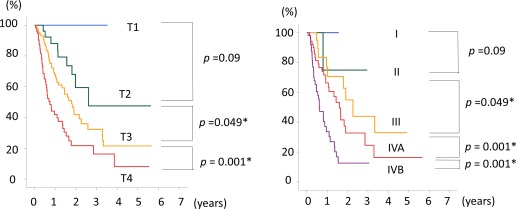
Overall survival curves are shown for the 337 patients who had incomplete clinicopathologic data stratified according to tumor (T) classification and disease stage defined using the proposed staging system.
DISCUSSION
In this nationwide survey of surgical results from patients with MF‐dominant ICC, we confirmed that a tumor cutoff size of 2 cm (and not 5 cm) in greatest dimension best differentiated the survival of patients. We observed that a cohort of patients who had ICC tumors ≤2 cm without lymph node metastasis had an extremely good prognosis (5‐year survival rate, 90%), and the 5‐year survival rate for patients without portal vein invasion was 100%. Although the multivariate analysis failed to identify tumor size as a significant prognostic factor for survival, patients who had ICC ≤2 cm without lymph node or vascular invasion could be categorized as stage I, which forecasts a very good prognosis. Second, major biliary invasion, ie, invasion of the first‐order branch of the bile duct, was an independent and significant factor for a worse prognosis for the N0M0 patient cohort in multivariate analysis (hazard ratio, 2.939; P = .001). Major biliary invasion would have a close correlation with macroscopic periductal invasion, and the current results support the addition of periductal invasion in the T‐classification according to the seventh edition of the UICC/AJCC system. However, further studies are warranted to pathologically define the concept of “periductal invasion,” because it is originally a finding of the macroscopic ICC classification.9, 14
In the UICC/AJCC seventh edition, tumor size was excluded from the T‐classification, because univariate and multivariate analyses failed to confirm the prognostic impact of tumor size on the survival of patients with a cutoff value of 2 cm or 5 cm.5 When the 2‐cm cutoff size was used, the hazard ratio for death was 1.29 in the Western series5 and 2.487 in the current study. The proportion of patients with ≤2‐cm ICC was 11% in the Western series and 6.5% in the current study. The reason why the impact of a tumor size ≤2 cm differs in Western and Eastern countries is unclear; however, it is very difficult to detect ICCs ≤2 cm because of the lack of symptoms, and surveillance is important for patients who have hepatitis C virus or other hepatic disease. It will be meaningful to reconfirm that patients who have a solitary ICC measuring ≤2 cm without vascular invasion can enjoy a 5‐year survival rate of 100% after surgical resection.
We recently observed that major biliary invasion predicted patient survival and was an independent predictor of survival in multivariate analyses of 267 patients with N0M0 ICC. Periductal invasion is not defined in the classification system.1, 11 In the AJCC/UICC seventh edition, patients with periductal invasion are classified as T4, but the overall survival of patients with T4 tumors appeared to be better than that of patients with T2b tumors (P = .053) (Fig. 4). This reversed situation may be explained by the more powerful prognostic value of the number of tumors compared with major biliary invasion (Table 2), and a microscopic definition of periductal invasion and adequate weighting of each prognostic factor are issues that remain to be resolved.
Major biliary invasion can be discussed with regard to the macroscopic MF + PI type of ICC. Among patients with MF‐dominant ICC, the presence of periductal infiltration has been considered a sign of tumor invasion along the Glissonean sheath, thereby making it a prognostic factor for worse survival. However, there is some controversy regarding whether the prognosis for patients with the MF + PI type of ICC is worse than that for those with the MF type. Yamamoto et al first described worse survival for patients with the MF + PI type of ICC than for those with the MF type,15 whereas a multicenter collective study using multivariate analysis demonstrated that macroscopic classification was not a prognostic factor.7 In the current study, the macroscopic type of ICC had a close correlation not only with biliary invasion but also with portal vein invasion and lymph node metastasis (Table 2). These results suggest strong confounding between macroscopic type and important histopathologic factors. Conversely, controversy remains regarding how to differentiate hilar bile duct cancer from ICC involving the hepatic hilum. In the current analysis, because major biliary invasion was identified as one of the important prognostic factors for patients with advanced ICC, the MF + PI type of ICC should be classified using the proposed staging system for ICC. This issue should be further discussed in the future.
The selection of appropriate factors for determining vascular or ductal invasion to complete the T‐classification appears to be complicated. Among the 5 possible candidates constituting T‐classification, portal vein invasion, hepatic vein invasion, arterial invasion, and serosal invasion are included in the fifth edition of the LCSGJ system; whereas vascular invasion, serosal invasion, and periductal invasion are included in the seventh edition of the AJCC TNM system. We examined all combinations of vascular, biliary, and serosal invasion and observed that the combination of portal vein invasion and major biliary invasion would be appropriate, because: 1) even in patients with tumors ≤2 cm, portal vein or biliary invasion can be observed in 18% and 26% of patients, respectively, so these occur more frequently than the remaining factors; 2) not only for all the entire 419‐patient cohort but also for the 267‐patient cohort with N0M0 disease, tumor numbers and major biliary invasion were significant factors predicting survival in univariate analysis; and 3) major biliary invasion may be predicted by imaging analysis as periductal invasion. Hepatic vein invasion or serosal invasion appeared to be less important for predicting survival; hence, these were excluded from the T‐classification to simplify the staging system as much as possible. The prognostic influence of the 3 criteria in the new staging system may be unequal and inferior for predicting the survival of surgical patients compared with a precisely designed nomogram.16 However, because a staging system must be simple, we adopted the style of the LCSGJ fifth edition. In addition, we included a tumor cutoff size of ≤2 cm in the criteria, because a tumor size ≤2 cm is an unambiguous condition, and patients with stage I ICC (a solitary tumor ≤ 2 cm without vascular or major biliary invasion) may have an excellent prognosis.
Patients who have ICC with lymph node metastasis may have a dismal prognosis, and surgical indications for patients with lymph node metastasis remain controversial.17, 18 In the current study, no significant difference in survival was observed between patients with T1‐T3N1M0 disease and those with T4N0M0 disease (MST, 16.6 vs 22.5 months; P = .95) or between patients with T4N1M0 disease and those with M1 disease (MST, 13.4 vs 4.2 months; P = .57). Therefore, patients with T1‐T3N1M0 and T4N0M0 disease were grouped into stage IVA, whereas those with T4N1M0 and M1 disease were grouped into stage IVB (MST, 17.8 vs 12.3 months; P < .001). Stage IVB may suggest inoperable disease, which should be carefully assessed. Uenishi et al also divided patients with N1 disease into stages IVA and IVB according to T‐classification based on results from a multicenter study that analyzed 233 patients from 9 institutions in Japan,19 and our staging system endorses their proposal.
The major limitations of the current study include the fact that we customized the classification system for patients with MF‐dominant ICC. Because the IG type of ICC is characterized by better survival compared with the MF and MF + PI types,15, 20 some modification may be necessary for other minor macroscopic types of ICC, which account for approximately 15% of all ICCs. The second drawback of this study is the insufficient validation analysis. The new T‐classification was validated using 198 of 337 patients (58.8%), and staging was validated in 134 patients (39.7%) because of a lack of clinicopathologic data. Although T‐classification defined by the new staging system demonstrated acceptable discrimination by tumor category, an international validation study will be required that includes a larger number of case series. The third problem is that the current staging system was based on the survival of patients who underwent surgical resection. The survival of patients who receive nonsurgical treatment, such as radiofrequency ablation, systemic chemotherapy, transcatheter arterial chemoembolization, and liver transplantation, should be covered by the staging system in the future.
CONCLUSION
In conclusion, the current nationwide study on the prognosis of patients with ICC revealed that a tumor cutoff size of 2 cm in greatest dimension well discriminates patient survival and that major biliary invasion and vascular invasion, represented by portal invasion, appear to be important prognostic factors for determining T‐classification in the staging system. Patients with T1‐T3N1M0 and T4N0M0 disease were included in stage IVA, whereas those with T4N1M0 and M1 disease were included in stage IVB. The results indicate that this new staging system would be useful in terms of assigning patients to surgery.
FUNDING SUPPORT
This work is supported in part by Grant‐in‐Aid for Scientific Research from the Ministry of Health and Welfare of Japan.
CONFLICT OF INTEREST DISCLOSURES
Dr. Kokudo reports research funding from Taiho Pharmaceutical, Yakult Science, and Dainippon Sumitomo Pharmaceutical outside the submitted work.
We thank Dr. Junichi Shindoh in the Department of Digestive Surgery, Toranomon Hospital, Tokyo, for his critical review of the article and Dr. Hidenori Ojima in the Pathological Department of Keio University School of Medicine for his supportive comments on the interpretation of pathologic variables.
All authors are members of The Liver Cancer Study Group of Japan.
Correction added on 4 December 2015, after first online publication: on page 64, in the section titled Macroscopic findings of the MF or MF+PI type, the 8th line has been changed to read “…MF+PI type was significantly worse than that of patients…”
REFERENCES
- 1. Nakanuma Y, Curado MP, Fanceschi S, et al. Intrahepatic cholangiocarcinoma In: Bosman FT, Carnerio F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer; 2010:217‐214. [Google Scholar]
- 2. Khan SA, Toledano MB, Taylor‐Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10:77‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472‐477. [DOI] [PubMed] [Google Scholar]
- 4. Shaib YH, EL‐Serag HB, Daliva JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case‐control study. Gastroenterology. 2005;128:620‐626. [DOI] [PubMed] [Google Scholar]
- 5. Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14‐22. [DOI] [PubMed] [Google Scholar]
- 6. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi‐institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140‐3145. [DOI] [PubMed] [Google Scholar]
- 7. Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:443‐452. [DOI] [PubMed] [Google Scholar]
- 8. Valle J, Wasan H, Palmer DH, et al; ABC‐02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 9. Liver Cancer Study Group of Japan . General Rules for the Clinical and Pathological Study of Primary Liver Cancer. First English edition. Tokyo, Japan: Kanehara & Co Ltd; 1997. [Google Scholar]
- 10. Okabayashi T, Yamamoto J, Kosuge T, et al. A new staging system for mass‐forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374‐2383. [DOI] [PubMed] [Google Scholar]
- 11. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 12. Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma. Cancer. 2011;117:2170‐2177. [DOI] [PubMed] [Google Scholar]
- 13. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268‐1289. [DOI] [PubMed] [Google Scholar]
- 14. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288‐291. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto M, Takasaki K, Yoshikawa T, Ueno K, Nakano M. Does gross appearance indicate prognosis in intrahepatic cholangiocarcinoma? J Surg Oncol. 1998;69:162‐167. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188‐1195. [DOI] [PubMed] [Google Scholar]
- 17. Inoue K, Makuuchi M, Takayama T, et al. Long‐term survival and prognostic factors in the surgical treatment of mass‐forming type cholangiocarcinoma. Surgery. 2000;127:498‐505. [DOI] [PubMed] [Google Scholar]
- 18. Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;145:411‐416. [DOI] [PubMed] [Google Scholar]
- 19. Uenishi T, Ariizumi S, Aoki T, et al. Proposal of a new staging system for mass‐forming intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:499‐508. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Shimada K, Sakamoto Y, et al. Clinicopathological characteristics of intrahepatic cholangiocellular carcinoma presenting intrahepatic bile duct growth. J Surg Oncol. 2009;99:161‐165. [DOI] [PubMed] [Google Scholar]


