Abstract
In preterm infants, poor postnatal growth is associated with adverse neurocognitive outcomes; conversely, rapid postnatal growth is supposedly harmful for future development of metabolic diseases.
Conclusion
In this systematic review, observational studies reported consistent positive associations between postnatal weight or head growth and neurocognitive outcomes; however, there was limited evidence from the few intervention studies. Evidence linking postnatal weight gain to later adiposity and other cardiovascular disease risk factors in preterm infants was also limited.
Keywords: Feeding, Growth velocity, Health, Postnatal, Preterm newborn
Key Notes.
We aimed to identify sensitive periods of postnatal growth in preterm infants associated with neurodevelopmental and metabolic outcomes.
The dissonance between findings of intervention and observational studies raises the possibility of confounding by diseases or other factors that affect both growth and cognition.
Future nutritional intervention studies in preterm and term infants should report effects on weight gain and growth, as well as later body composition and neurocognitive outcomes.
Introduction
Developmental plasticity refers to the capacity of an organism to adjust its phenotypic development in response to environmental cues to maximise its reproductive fitness, possibly through epigenetic, metabolic and/or anatomical mechanisms 1. Mismatch arises when the predicted conditions do not correspond to the actual environment and this can adversely impact long‐term health and longevity. Initial evidence that early exposure to environmental cues can alter the risk of developing metabolic diseases in humans came from associations between low birthweight and higher incidence of coronary heart disease or glucose intolerance 2. Low birthweight can result from intrauterine growth restriction and/or prematurity and, when followed by adequate postnatal nutrition, rapid (‘catch‐up’) growth typically occurs during infancy. While this confers potential advantages for preterm infants in terms of short‐term survival and later cognitive outcome, rapid catch‐up growth may increase the risk of metabolic disease later in life. The relative importance of prenatal undernutrition versus postnatal rapid growth to these outcomes is still unresolved. In contemporary birth cohorts, postnatal rapid growth occurs more often in infants who were subjected to intrauterine growth restriction, but also reflects the infant's genetic potential, and can be induced in the general population by excessive infancy nutrition, including higher protein intake. Human growth is regulated by genetic, epigenetic, nutritional and endocrine signals orchestrated in a timely fashion, and each of these mechanisms may be involved in long‐term metabolic programming 3.
There is a wealth of supportive evidence for developmental programming from experimental animal models. These have demonstrated causal effects of nutritionally induced accelerated growth, with or without low birthweight, on long‐term risks of metabolic dysfunction. Initial studies in rats over 50 years ago demonstrated the detrimental effects of excess nutrition during lactation by manipulation of litter size 4. Rats that were reared in small litters where there was little competition for the mother's milk gained more weight during lactation and remained fatter and heavier throughout life, even when weaned onto a healthy low‐fat diet, compared with animals reared in larger litters. Strikingly, raising rodent pups in large litters to reduce the plane of nutrition during lactation protects genetically prone animals from becoming obese 5. Manipulation of nutritional content during lactation has similar effects 6. When mouse or rat offspring of normally nourished mothers are suckled by dams fed a low‐protein diet, pups grow slowly and remain permanently smaller and leaner than controls. Furthermore, these animals exposed to maternal low‐protein diet during lactation are resistant to diet‐induced obesity. These findings highlight the importance of nutrition during lactation in determining long‐term risk of obesity. Studies in rodent models have also shown the association between low birthweight followed by postnatal catch‐up growth and increased risks of obesity, cardiovascular disease, insulin resistance and glucose intolerance. This has been demonstrated in a range of different models where foetal growth has been restricted including those employing maternal caloric restriction, maternal protein restriction and intrauterine artery ligation 6. Mice that were growth restricted in utero by maternal protein restriction and that then underwent accelerated growth through suckling a normally fed dam are more susceptible to diet‐induced obesity. The mechanisms underlying these programming effects remain to be fully established but may involve permanent structural changes, epigenetic changes and accelerated cellular ageing 6.
It has been suggested that avoidance of excessive rates of early growth could have a role in the prevention of cardiovascular disease 7; however, the benefits and risks of this approach must be carefully weighed in high‐risk low birthweight groups. Preterm infants are considered to be particularly susceptible to developmental programming of adverse health outcomes because of their abnormal ex‐utero growth patterns; the majority exhibit poor growth and weight gain during the initial postnatal period between preterm birth to full‐term gestational age but subsequently most show spontaneous rapid catch‐up growth back to their genetic trajectory. Some studies suggest that in preterm infants, poor early growth is associated with adverse neurodevelopmental outcomes; thus, current nutritional strategies aim to enhance their nutrient intake to promote rapid growth and brain development despite the potential long‐term metabolic costs 8. The objective of the present review was to summarise the available evidence on postnatal growth in preterm infants in relation to the potential neurodevelopmental benefits and adverse metabolic outcomes and attempt to identify critical postnatal windows during which growth might influence these outcomes.
Methods
We extracted reports published before 2003 from the review by Baird et al. 9. That extensive review included literature on term infants (AGA+SGA) and a number of outcomes not relevant to our review here (i.e. mental health, sudden infant death), so only references that referred to i) preterm infants, ii) early growth (rather than size during infancy) and iii) developmental or metabolic outcomes that were predefined were extracted for the current review. Seven relevant papers were identified, all reported neurocognitive or developmental outcomes, and these are marked in Table 1a,b.
Table 1.
Postnatal growth in a) weight (WT) and b) head circumference (HC) in preterm born infants related to later neurocognitive outcomes. Reports are ordered by study design and age at outcome assessment. Variables used for adjusted analyses: G – general; N – neonatal; S – social; IQ – maternal IQ
| Design | Timing of exposure | N | Age at outcome | Summary result | Comments | Adjustment | |
|---|---|---|---|---|---|---|---|
| (a)WT gain | |||||||
| Biasini 10 | int | b‐discharge | 61 | 3 & 12 months (GMDS) | ++ (3 months only) | Milk fortifier increased HC, WT and length gains | Nil |
| Cooke 11 | int | discharge‐6 months | 113 | 18 months (BSID) | 0 | Enriched preterm formula increased HC, WT and length gains in boys only | Nil |
| Aimone 12 | int | Discharge‐12 weeks | 39 | 18 months (BSID) | 0 | Human milk fortifier increased WT and length gains (but not HC) | G |
| Lucas 13 | int | Discharge‐9 months | 229 | 18 months (BSID) | 0 | Enriched postdischarge formula increased WT gain (but not HC) | Nil |
| Lucas 14 | Int | b‐discharge | 422 | 18 months (BSID) | 0 | Enriched preterm formula increased WT & HC gains vs. banked breast milk | Nil |
| Lucas 15 | int | b‐discharge | 360 | 7‐8 years (WISC) | ++ (Boys only*) | Enriched preterm formula increased WT & HC gains. *Only in boys who received no human milk or had the highest intakes of the trial diet. | Nil |
| O'Connor 16 | obs | in NICU | 463 | 12 months (BSID) | 0 | Nonrandomised infants on preterm formula were 500 g heavier at 12 months than breast‐fed | G,N,S (IQ) |
| Tudehopea 18 | obs | b‐12 months | 162 | 12 months (GMDS) | ++ (AGA only) | Poor WT gain in preterm‐AGA infants (n = 131) was associated with lower scores | Nil |
| Cooke 19 | obs | b‐28 day, 28 day ‐18 months | 108 | 18 months (BSID) | ++ | Preterm‐SGA infants with catch‐up WT gain did better than those who remained small | Nil |
| Belfort 20 | obs | b‐term, term‐4 months, 4‐12 months | 613 | 18 months (BSID) | ++ | Positive associations with WT gain between b‐term, and with post‐term WT gain only if proportionate to length gain | G,N,S |
| Sices 21 | obs | term‐4 months, 4‐8 months, 8‐20 months | 154 | 20 months (BSID, CP) | ++ | Poor outcomes associated with poor WT gain between term‐4 months and 8‐20 months | G,S |
| Ehrenkranz 22 | obs | b‐discharge (or 2 kg, or 120 day) | 695 | 20 months (BSID, CP) | ++ | Similar associations with HC gain | G,N,S |
| Latal‐Hajnala 23 | obs | b‐2 years | 219 | 24 months (BSID) | ++ | Infants categorised as WT above/below the 10th centile) | G,N |
| Ma 24 | obs | b‐2 years | 76 | 24 months (BSID) | 0 | Infants categorised as WT above/below the 10th centile) | G,N,S |
| Shah 25 | obs | b‐36 week gestation | ? | 24 months (BSID) | ++ | Growth failure between b‐36 wk associated with poorer psychomotor development (but not mental development) | ? |
| Nash 26 | obs | b‐18/24 months | 289 | 24 months (BSID) | ++ | Poor WT gain associated with poor outcomes, but rapid WT gain not associated with better outcomes | G,N |
| Kellehera 27 | obs | ? | 180 | 24 & 36 months (BSID) | ++ | Poor growth defined as WT <5th centile on 2 or more occasions | G,N,S |
| Powers 28 | obs | 6‐36 months | 135 | 36 months (BSID) | ++ | Similar associations with HC gain | G,N,S |
| Huang 17 | obs | b‐4to7 years | 654 | 4 to 7 years (WYCSI‐C) | 0 | Positive associations were seen in full‐term (n = 7735) but not preterm infants | G,N,S,IQ |
| Franz 29 | obs | b‐discharge disch‐5.4 years | 219 | 5.4 years (KABC, CP) | ++ (b‐discharge only) | Similar associations with HC gain | G,N,S |
| Claas 30 | obs | b‐5.5 years | 101 | 5.5 years (WPPSI, M‐ABC) | ++ | Preterm‐AGA infants who remained > ‐2 SDs had better outcomes | Nil |
| Casey 31 | obs | 4‐36 months | 985 | 8 years (WISC) | ++ | Poor WT gain was associated with poor outcomes in both SGA and AGA preterms | G,S |
| Kan 32 | obs | b‐2 years, 2‐8years | 179 | 8 years (WISC, WRAT, M‐ABC) | 0 | No association with WT gain adjusted for HC growth | G,N,S |
| Belfort 33 | obs | Term‐12 months | 905 | 8 years (WISC) | ++ | Positive association with adjusted WT gain between term‐12 months (or term‐4 months) | G,S,IQ |
| Weisglas 34 | obs | b‐3 months | 562 | 19 years (MCT‐M) | ++ | Poor outcomes in preterm‐AGA with poor WT gain and in preterm‐SGA without catch‐up | G,N,S |
| (b)HC growth | |||||||
| Biasini 10 | int | b‐discharge | 61 | 3 & 12 months (GMDS) | ++ (3 months only) | Milk fortifier increased HC, WT and length gains | Nil |
| Cooke 11 | int | discharge‐6 months | 113 | 18 months (BSID) | 0 | Enriched preterm formula increased HC, WT and length gains in boys only | Nil |
| Lucas 14 | Int | b‐discharge | 422 | 18 months (BSID) | 0 | Enriched preterm formula increased WT & HC gains vs. banked breast milk | Nil |
| Lucas 15 | int | b‐discharge | 360 | 7‐8 years (WISC) | ++ (Boys only*) | Enriched preterm formula increased WT & HC gains. *Only in boys who received no human milk or had the highest intakes of the trial diet. | Nil |
| O'Connor 16 | obs | b‐12 months | 463 | 12 months (BSID) | 0 | Nonrandomised infants on preterm formula were 500 g heavier at 12 months than breast‐fed groups | G,N,S |
| Simona 35 | obs | 0‐12 months | 48 | 12 months (BSID) | ++ | Compared catch‐up (defined as HC recovering to >5th centile) to no‐catch‐up group | Nil |
| Ehrenkranz 22 | obs | b‐discharge (or 2 kg or 200 day) | 695 | 22 months (BSID) | ++ | Positive associations also with WT gain | G,N,S |
| Forda 36 | obs | b‐2 year | 83 | 24 months (BSID) | 0 | ? | |
| Kuban 37 | obs | b‐2 year | 1200 | 24 months (BSID) | ++ | G,N | |
| Wood 38 | obs | b‐term, term‐30 months | 283 | 30 months (BSID, CP, NDI) | 0 | G,N,S | |
| Hacka 39 | obs | 8 months, 8‐20 months | 139 | 30 months (SB) | ++ (8 months only) | Positive association with HC at 8 months, but not HC growth 8‐20 months | G,N,S |
| Powers 28 | obs | 6‐12, 12‐18, 18‐24, 24‐30, 30‐36 months | 135 | 36 months (BSID, NDI) | ++ (NDI only) | Positive associations also with WT gain | G,N,S |
| Franz 29 | obs | b‐disch, disch‐5.4 years | 219 | 5.4 years (KABC, NDI) | ++ (NDI only) | Positive associations also with WT gain | G,N,S |
| Claas 30 | obs | b‐5.5 years | 101 | 5.5 years (WPPSI, M‐ABC) | ++ | Positive associations with HC growth in both SGA and AGA preterm infants | Nil |
| Stathisa 40 | obs | b‐4 months, 4‐8 months | 87 | 6 years (RWM) | ++ (b‐4 months only) | Positive association with HC growth b‐4 months, but not 4‐8 months | G,N,S |
| Brandt 41 | obs | b‐12 months | 51 | 72 months (SB) adult (MIT) | ++ | Preterm‐SGA infants. Compared catch‐up HC growth to no‐catch‐up group | G,N,S |
| Cooke 42 | obs | b‐7 years | 280 | 7 years (M‐ABC, VMI) | ++ (VMI only) | G,N | |
| Cooke 43 | obs | b‐disch, disch‐4 years, 4‐15 years | 194 | 8 years (WISC + MMI) | ++ (MMI only) | HC growth between birth discharge associated with MMI. HC at 4 and 15y was associated with IQ | G,N,S |
| Kan 32 | obs | b‐2 years, 2‐8 years | 179 | 8 years (WISC, WRAT, M‐ABC) | ++ (0‐2 years only) | HC growth only between 0 and 2y was associated with IQ, reading & spelling (but not maths & motor ability). | G,N,S |
| Belfort 33 | obs | term‐12 months | 905 | 8 years (WISC) | ++ | Positive associations with HC growth between term‐12 months (or 4‐12 months) | G,S,IQ |
‘++’ statistically significant positive association; ‘+’ nonsignificant positive trend; ‘0’ no association; ‘‐’ non‐significant inverse trend; ‘–’ significant inverse association; ‘?’ unstated adjustment factors.
Reference extracted from the review of Baird et al., 2003 9.
Cognitive tests used: BSID = Bayleys scale of infant development; CP = Assessment for cerebral palsy; GMDS = Griffiths mental development scale; KABC = Kaufman assessment battery for children; M‐ABC = Movement assessment battery for children; MCT‐M: Multicentre capacity test intermediate level; MIT = Adult Mannheimer intelligence test; MMI = Minor motor impairment assessment; NDI = Neurodevelopmental impairment; RWM = Reading, writing and maths assessments; SB = Stanford Binet Intelligence Test; VMI = Visual motor integration; WISC = Wechsler intelligence scale for children; WPPSI = Wechsler preschool and primary scale of intelligence; WRAT = Wide range achievement test; WYCSI‐C = Chinese Wechsler young children scale of intelligence.
We performed a systematic search of electronic databases (Medline (PubMed), EMBASE and Google Scholar) to identify studies published between 2003 and May 2013 reporting associations between postnatal growth and later neurocognitive or metabolic outcomes in preterm infants. MeSH terms and search words representing the following categories were combined: i) the population (e.g. preterm infants), ii) postnatal growth (e.g. early weight gain; failure to thrive) and iii) outcome related to neurocognitive development (e.g. IQ; neurological impairment) or to metabolic disease (e.g. percentage body fat). One investigator (KK) carried out the search and hand‐searched the reference lists of identified papers for further relevant papers. Abstracts were read, and if thought to be relevant, the full report was read and relevant data were extracted; 120 full papers published in 2003 or later were reviewed in this way (Fig. 1).
Figure 1.
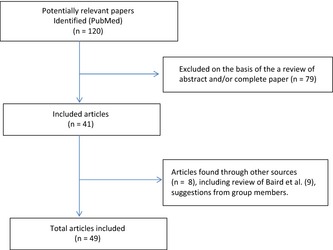
Flow chart for systematic review of literature on the effects of postnatal growth in preterm infants and later health outcomes.
Inclusion criteria:
Population
Definitions that were considered for this search were as follows: ‘preterm infants; premature infants; premmie births; low‐birthweight infants; very low‐birthweight infants; extremely low birthweights; and preterm/premature SGA/IUGR infants’. Studies of populations that also included term infants were included if the findings for the preterm infants were presented separately.
Exposure
A measure of postnatal growth velocity that could be compared between groups was identified. Care was taken to use a definition that considered gains in weight and/or head circumference between at least two time points (e.g. between birth to age six weeks) rather than only measurements of body size at one specific age. Growth was accepted if reported as an absolute velocity or as a change in SD scores; in the case of the latter, we required the growth reference used to be reported. Papers were accepted if the outcome (see below) was assessed at the same age as the second growth measurement, or later.
Outcomes
Neurocognitive development
Measures related to neurocognitive outcomes included: ‘neurodevelopment; intelligence; IQ; behaviour; motor and neurological impairment’. We applied no limit regarding the age at outcome assessment; the earliest was at the age of 12 months and the oldest was in young adults (21 years).
Metabolic
Outcomes were included related to body composition (e.g. overweight and obesity; percentage fat), insulin resistance, glucose control or cardiovascular risk factors (e.g. lipid profile; blood pressure).
Study design
Both intervention and observational studies were accepted. We considered as ‘intervention studies’ those where the intervention had an influence on postnatal growth and the study also assessed one of our review outcomes above. Some papers reported cohort analyses of interventional studies (particularly where the intervention did not influence early growth); these were labelled as ‘observational’ evidence.
Exclusion criteria
We excluded animal studies and also studies where body size at only one time point was reported. Queries about the eligibility of specific studies were resolved by discussions with MF and KO, or with all authors in cases of uncertainty. Further studies suggested by co‐authors for possible inclusion were considered, but no new eligible studies were identified in this way.
Data extraction and interpretation
KK was responsible for the data extraction, tabulation and preliminary interpretation. Only published data were considered, and no further data were sought from authors. Forty‐nine individual reports were used (34 examining early growth and later developmental outcomes and 15 examining metabolic outcomes); however, 20 (41%) of these papers were used more than once, as they reported more than one outcome of interest.
For each identified study, KK extracted information on the following: study design, timing and nature of the exposure, number of participants, and age at follow‐up measurements. All authors agreed on the generation of a summary result indicator attributed to each study to show whether it reported a positive effect/association between postnatal growth and the outcome measure (‘++’ statistically significant positive association; ‘+’ nonsignificant positive trend; ‘0’ no association; ‘‐’ nonsignificant inverse trend; and ‘—’ significant inverse association). Due to wide heterogeneity between studies in the measurement and/or categorisation of the exposure and outcome variables, quantitative summary by meta‐analysis was deemed not possible.
Results
Neurocognitive outcomes
Neurocognitive outcomes were assessed in six clinical trials that promoted faster postnatal growth using a nutritional intervention (Table 1a; Fig. 2) 10, 11, 12, 13, 14, 15. Of these, only one trial reported an overall benefit on neurodevelopment, but only at the age of three months and not at the age of 12 months 10 and one trial reported a benefit only in subgroups of boys, but not in girls at the age of 7–8 year 15. Both of these trials, and a third trial that showed no benefit at 18 months 14, tested interventions given during the prehospital discharge age period. The other three trials tested posthospital discharge interventions: one promoted faster gains in weight, length and HC in boys 11; and two promoted faster gains in weight and length, but not HC 12, 13; however, none had any benefit for neurodevelopment, assessed at the age of 18 months in all three trials.
Figure 2.
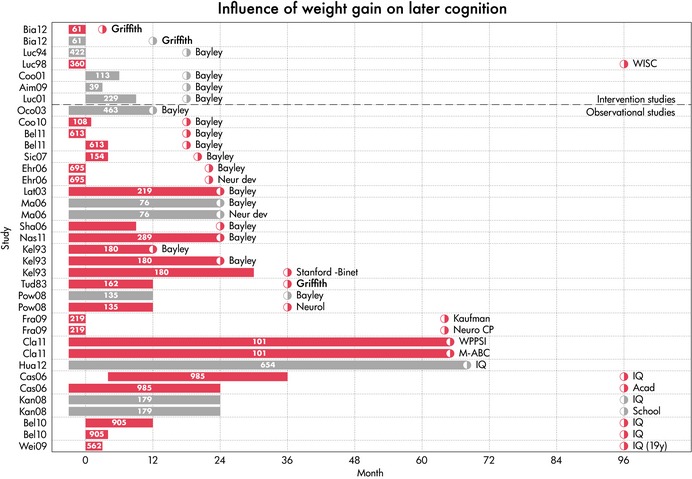
Gain‐outcome graph of weight gain and cognition. Each row indicates a study result. The label on the vertical axis is the name of the study. Age is on the horizontal axis. The bar in each row codes the period in which weight gain occurred. The half‐open circle indicates the age at which the outcome was measured, and the label describes the type of outcome. The number of children in the study is printed in the bar. A red bar indicates a significant positive association. A grey bar indicates no significant association.
In observational studies, weight gain (n = 19 studies) showed generally consistent positive associations with neurocognitive outcomes at ages ranging from 12 months to 19 year old (Table 1a; Fig. 2) 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34. Outcomes were measured using a variety of assessment tools across different studies, largely reflecting the relevance of each tool to specific age ranges. The timing of the postnatal weight gain tested as the exposure also varied widely between studies, from as early as birth‐to‐full‐term age or hospital discharge, to weight gain up to mid‐childhood. No obvious period(s) of postnatal weight gain was more consistently associated with later neurocognitive outcomes. Whilst some of these studies included adjustment for potential neonatal or social confounders, only three included adjustment for maternal IQ.
Similarly, 16 observational studies reported on the association between postnatal head growth and neurocognitive outcomes (Table 1b; Fig. 3) 16, 22, 28, 29, 30, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43. Postnatal head growth showed generally consistent positive associations with neurocognitive outcomes measured at ages ranging from 12 months old to adulthood, although the benefits for neurodevelopment outcomes were more consistent than for cognition. As for postnatal weight gain, no obvious period(s) of postnatal head growth was more consistently associated with later neurocognitive outcomes.
Figure 3.
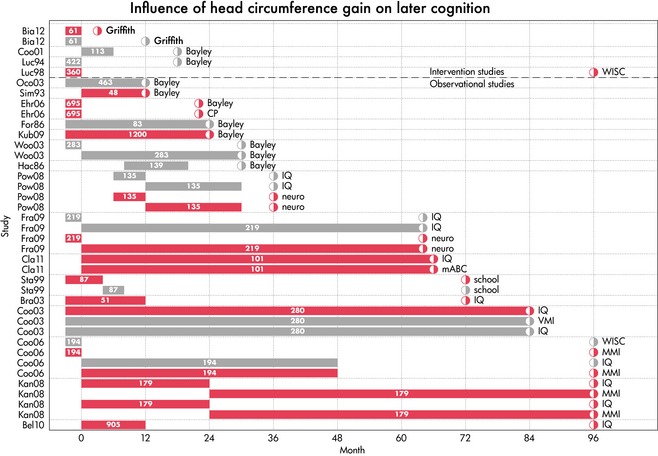
Gain‐outcome graph of head circumference and cognition. Each row indicates a study result. The label on the vertical axis is the name of the study. Age is on the horizontal axis. The bar in each row codes the period in which head circumference growth occurred. The half‐open circle indicates the age at which the outcome was measured, and the label describes the type of outcome. A red bar indicates a significant positive association. A grey bar indicates no significant association.
Percentage body fat
Percentage body fat was assessed as an outcome in three clinical trials that altered postnatal growth using a nutritional intervention (Table 2a; Fig. S1) 12, 44, 45. In all three trials, percentage body fat was assessed at the age of 12 months by DXA scans. In two trials 12, 45, the posthospital discharge intervention promoted faster weight gain, but neither had any effect on percentage body fat. In the third trial 44, surprisingly the enriched formula milk intervention given from full enteral feeding to age 12 months led to lower weight gain (the authors suggested this was due to lower milk intake, although this was not measured) and also lower percentage body fat, consistent with a positive relationship between weight gain and adiposity.
Table 2.
Postnatal weight gain in preterm born infants related to a) adiposity (% body fat); b) insulin resistance; c) other cardiovascular markers. Reports are ordered by study design and age at outcome assessment
| Design | Timing of exposure (nature) | N | Age at outcome | Summary result | Comments | |
|---|---|---|---|---|---|---|
| (a)Adiposity | ||||||
| Koo 44 | int | 41 day ‐12 months (Enriched formula) | 89 | 12 months | ++ | Intervention group surprisingly grew slower and had lower %BF |
| Aimone 12 | int | discharge‐3 months (HMF) | 39 | 12 months | 0 | Intervention increased WT and length gains, but not %BF |
| Cooke 45 | int | discharge‐6 months (Preterm formula) | 129 | 12 months | + | Intervention increased WT gain and both fat and lean mass; nonsignificant increase in %BF |
| Hernandez 46 | obs | (b‐2 weeks) b‐4 weeks (WT gain) | 26 | 24 months | (–) ++ | Surprisingly, the effect of WT gain between b‐2 weeks was opposite to that for WT gain b‐4 week |
| Ludwig‐Auser 47 | obs | b‐14 days (Energy intake) | 61 | 14.5 years | 0 | Infants with intakes >70 vs. < 70 kcal/kg/days were taller and heavier at the age of 14 years |
| Euser 48 | obs | b‐3 months, 3–12 months (WT gain) | 403 | 19 years | ++ | Stronger effect of early vs. later WT gain on BMI & %FM. Findings persisted when adjusted for height |
| Kerkhof 49 | obs | b‐term, term‐3 months (WT gain, adj. length) | 162 | 21 years | ++ | Compared rapid (>0.67 SD) vs. slow (<0.67 SD) catch‐up infancy WT gain. Stronger effect of early vs. later catch‐up |
| (b)Insulin resistance | ||||||
| Singhal 50 | int | b‐4 weeks (Preterm formula) | 216 | 15 years | ++ | Higher nutrient diet increased fasting 32‐33 proinsulin levels at 13–16 years (adjusted for BMI) |
| Fewtrell 51 | obs | b‐18 months, 18 months‐9 to 12 years (WT gain) | 385 | 10 years | ++ (18 months ‐9 to 12 year only) | WT gain 18 months ‐9/12 years associated with higher fasting, split, proinsulin and 30 minutes insulin (adjusted for HT & WT) |
| Finken 52 | obs | b‐3 months (WT gain) | 346 | 19 years | ++ | WT gain b‐3 months associated with higher fasting insulin resistance |
| Kerkhof 49 | obs | b‐term, term‐3 months (WT gain, adj. length) | 162 | 21 years | 0 | Insulin sensitivity was not associated with WT gain during any period between b‐12 months |
| Hovi 53 | obs | b‐term (WT gain) | 100 | 22 years | ++ (only in SGA, not AGA) | WT gain b‐term associated with higher fasting and 2 hour insulin levels; only in SGA subgroup (n = 31) (adjusted for BMI) |
| (c)CVD risk factors | ||||||
| Bracewell 54 | obs | b‐term, term‐30 months, term‐6 years (WT gain) | 241 | 6 years | 0 (BP) | WT gain in any period was unrelated to BP at 6 year (adjusted for BMI) |
| Belfort 33 | obs | term‐12 months (WT gain) | 666 | 6 years | ++ (BP) | WT gain term‐12 months associated with modestly higher BP (in adjusted model, SBP was 0.7 mm Hg higher vs. slower growing infants). |
| Ludwig‐Auser 47 | obs | b‐14 days (Energy intake) | 61 | 14 years | 0 (BP) | Infants with intakes >70 vs. < 70 kcal/kg/day were taller and heavier at the age of 14 years |
| Keijzer‐Veen 55 | obs | b‐5 years (WT gain) | 588 | 19 years | ++ (BP) | Also positive association with childhood height growth |
| Mortaz 57 | obs | b‐discharge; 18 months (WT gain) | 412 | 11 years | 0 (Lipids) | WT gain to 18 months was unrelated to various cholesterol metabolism parameters (adjusted for current size) |
| Kerkhof 49 | obs | term‐3 months, 9–12 months (WT gain) | 162 | 21 years | ++ (Lipids) – (BP) | WT gain term‐3 months –> higher TChol & LDL. WT gain 9‐12 months –> higher TChol, LDL & ApoB. No associations with BP |
| Singhal 58 | obs | b‐4 weeks (WT gain) | 216 | 15 years | ++ (FMD) | FMD was 4% lower in adolescents with high vs. low WT gain (adjusted for HT & WT) |
| Finken 56 | obs | 3–12 months (WT gain) | 346 | 19 years | ++ (CIMT) – (Lipids) | WT gain 3‐12 months –> greater CIMT (but not when adjusted for height). No associations with lipid profile |
‘++’ statistically significant positive association; ‘+’ nonsignificant positive trend; ‘0’ no association; ‘‐’ nonsignificant inverse trend; ‘–’ significant inverse association.
Four observational studies reported on the association between postnatal weight gain and percentage body fat (Table 2a; Fig. S1) 46, 47, 48, 49. The two larger studies reported positive associations with percentage body fat in young adults 48, 49; both studies reported stronger effects of weight gain during earlier (between birth to three months corrected age) versus later infancy (between 3–12 months). The two smaller studies reported no or variable associations between postnatal weight gain and percentage body fat at the ages of two year and 14 year 46, 47.
Insulin resistance
Insulin resistance was assessed in only one clinical trial that promoted faster postnatal growth using a nutritional intervention (Table 2b; Fig. S2) 50. The nutrient‐enhanced preterm formula milk given in the first four weeks of postnatal life increased postnatal weight gain and also increased fasting 32–33 split proinsulin levels at the age of 15 year, independent of BMI, although levels were similar to those in healthy term‐born individuals.
Of the four observational studies 49, 51, 52, 53, three reported positive associations between postnatal weight gain and insulin resistance at the ages of 10 to 22 year (Table 2b; Fig. S2) 51, 52, 53. However, there were inconsistent findings between studies, including the timing of weight gain related to insulin resistance [e.g. birth to three month post‐term 52; from 18 months only 51], and whether associations were only seen in subgroups [within preterm‐SGA but not preterm‐AGA infants 53]. Furthermore, only one observational study showed that the association with insulin resistance was independent of body composition 53.
Other cardiovascular risk factors
Associations between postnatal weight gain and various later CVD risk factors were reported by eight observational studies; most had sample sizes >160 and had long follow‐up, to ages ranging from six to 21 year old (Table 2c; Fig. S3) 33, 47, 49, 54, 55, 56, 57, 58. Positive associations were variably and/or sparsely reported with arterial blood pressure (two of five studies); total cholesterol levels (one of three studies); flow mediated dilatation (one study); and carotid artery intima‐media thickness (one of one study). Furthermore, most of the studies that reported positive associations did not include adjustment for body size at the time of outcome assessment.
Discussion
This systematic review of growth in preterm infants in relation to later outcomes found only a few intervention studies and a greater number of observational studies with information on later neurocognitive, adiposity and insulin resistance/cardiovascular risk factor outcomes. In relation to neurocognitive outcomes, intervention studies that aimed to promote postnatal growth in preterm infants, whether during hospital stay or postdischarge, produced little consistent evidence for beneficial effects of faster early growth. The observational studies reviewed showed generally consistent positive associations between postnatal weight gain and head growth (n = 18 and 15 studies, respectively) and neurocognitive outcomes at ages ranging from one year to adulthood, with no obvious period(s) of weight gain or head growth more consistently associated with later outcomes. The dissonance between findings of intervention and observational studies raises the possibility of confounding in the latter by other factors, such as neonatal morbidities that could directly affect both growth and neurocognitive outcomes, although the paucity of intervention studies limits conclusions.
In relation to later adiposity, our review of intervention studies to promote postnatal growth in preterm infants found no studies that assessed adiposity at ages older than one year; of three trials, two promoted weight gain without increasing percentage body fat, while in the third, the enriched formula milk intervention led to lower weight gain, perhaps as a result of lower milk intake. Among observational studies with data on later adiposity, the two larger studies found that greater weight gain from birth to three months corrected age was associated with a higher percentage body fat in young adulthood, with a weaker effect of weight gain from age 3–12 months; smaller studies reported no or variable associations between postnatal weight gain and percentage body fat at the ages of two year and 14 year. For insulin resistance, one intervention study that promoted faster postnatal growth led to normalised fasting 32–33 split proinsulin levels at the age of 15 year relative to children born at term, whereas those who grew more slowly in hospital had reduced proinsulin levels in adolescence. Findings were inconsistent among the four observational studies and only one reported an association between faster growth and later insulin resistance independent of body composition. No study in preterm infants examined relations between nutritional intervention to promote growth and other cardiovascular risk factors. While observational studies variably reported some positive associations between faster early growth and arterial blood pressure, total cholesterol levels, flow mediated dilatation and carotid intima‐media thickness, most did not include adjustment for body size at the time of outcome assessment.
An important limitation of this review was the inability to perform quantitative summaries and to consider effect sizes arising from the paucity of studies, the variability in which these were reported and the inconsistency of adjustment for generally agreed potentially confounding influences, most notably current body size. A further limitation is that the data available did not allow us to consider growth conditional on the infant's size at birth; this is important as a previous systematic review found that larger size at birth is associated with a higher risk of later obesity 59, and postnatal growth patterns differ in small and large infants 60. Potential sources of bias widely prevalent in the studies considered included: insufficient description of participants, high attrition rates in both the intervention and observational studies, and inadequate consideration of confounding factors. Publication bias may be a further source of error and was not formally analysed in our review due to the absence of quantitative summaries.
It is important to recognise that these findings in preterm infants may not be applicable to term‐SGA infants; focused studies in this group are needed to determine whether developmental adaptations induced before birth in term‐SGA infants result in their responses to nutritional interventions to promote growth being different to those of preterm infants. In this respect, a previous systematic review concluded that term infants who grow rapidly during infancy are at increased risk of subsequent obesity, but did not consider neurocognitive outcomes 59.
Prematurity has a high and increasing prevalence worldwide. It is widely recognised that the growth of preterm infants is frequently suboptimal, with many being discharged at lower percentiles for weight, head circumference and length than those at which they were born 61. Moreover, preterm infants are particularly susceptible to developmental programming of adverse neurodevelopmental, body composition and metabolic outcomes. Despite some evidence that the prevalence of childhood overweight and obesity is no longer rising in a number of populations the prevalence remains at an unacceptably high level. This high prevalence has major implications for the global burden of ill health and disease and preterm infants are an important group in which to optimise early nutrition and later outcomes. Having common outcome measures for infant nutrition trials in both preterm and term infants would lead to a strengthened evidence base for nutritional interventions to improve growth and long‐term outcomes during this important period of development. A recent systematic review found that early parenteral nutrition in preterm infants improves short‐term growth outcomes 62. Among very preterm infants nutrition is often suboptimal 63, and while there is yet no evidence linking progressive advancement of enteral milk feeds to risk of adverse short‐term outcomes, such as necrotising enterocolitis and late‐onset infection, the need for further trials is recognised 64.
In summary, only a few predischarge intervention studies to promote growth in preterm infants have information on later neurocognitive, adiposity and insulin resistance/cardiovascular risk factor outcomes. While there is abundant and consistent evidence from observational studies linking faster postnatal growth to better neurocognitive outcomes in preterm infants, these studies show no obvious windows of association and there is a high risk of confounding by other factors/disease processes that affect both growth and cognition. Where associations are reported in observational studies linking faster postnatal growth to adverse cardiovascular risk markers in preterm infants, the findings have often not been adjusted for body size at the time of the outcome measurements. Moreover, comparisons were often not made to the normal ranges of these outcome parameters in unselected populations. Further research is needed to determine the optimal growth in preterm infants to achieve neurocognitive benefits while minimising the longer‐term risk of chronic disease. We therefore strongly support the recommendation of the COMMENT initiative 65 that all nutritional intervention studies in preterm and term infants should report effects on weight gain and growth, as well as later body composition and neurocognitive outcomes.
Author Contributions
KK and KKO had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in study concept and design. KK participated in acquisition of data. KK participated in statistical analysis. All authors participated in analysis and interpretation of data. All authors participated in drafting of the manuscript. All authors participated in critical revision of the manuscript.
Conflict of Interest Disclosures
All authors have completed and submitted the journal's Form for Disclosure of Potential Conflicts of Interest and none were reported.
Funding/Support
The expert group received funding from the ILSI Europe Metabolic Imprinting Task Force (please see acknowledgements for further information). Industry members of this task force are listed on the ILSI Europe website at www.ilsi.eu. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007‐2013), project EarlyNutrition under grant agreement no 289346.
Role of Sponsor
The funding organisations had no role in the design, collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Figure S1 Gain‐outcome graph of weight gain and adiposity.
Figure S2 Gain‐outcome graph of weight gain and insulin levels.
Figure S3 Gain‐outcome graph of weight gain and CVD risk.
Acknowledgements
This work was conducted by an expert group of the European branch of the International Life Sciences Institute (ILSI Europe). The Metabolic Imprinting Task Force members of ILSI Europe nominated renowned scientists based on their expertise within the chosen scientific area of the study to create the expert advisory group. Expert groups comprise of at least 50% scientists from academia and the public sector and up to 50% industry scientists. The expert group carried out the work, that is collecting/analysing data/information and writing the scientific paper independently of the task force. Experts are not paid for the time spent on this work; however, the nonindustry members within the expert group received travel support from the Metabolic Imprinting Task Force to attend meetings to discuss the review. Industry members of this task force are listed on the ILSI Europe website at www.ilsi.eu and the institute has a transparency policy whereby all of this information is freely available on the website. The authors would like to thank Dr Florence Rochat who was a former member of this expert group for their active contribution to this work. This publication was coordinated by Dr Pratima Rao Jasti and Dr Jackie Whyte, Scientific Project Managers at ILSI Europe. For further information about ILSI Europe, please email info@ilsieurope.be or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies.
References
- 1. Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab 2010; 21: 199–205. [DOI] [PubMed] [Google Scholar]
- 2. Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 2000; 108(Suppl. 3): 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc 2007; 66: 423–34. [DOI] [PubMed] [Google Scholar]
- 4. Widdowson EM, McCance RA. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond B Biol Sci 1963; 158: 329–42. [DOI] [PubMed] [Google Scholar]
- 5. Patterson CM, Bouret SG, Park S, Irani BG, Dunn‐Meynell AA, Levin BE. Large litter rearing enhances leptin sensitivity and protects selectively bred diet‐induced obese rats from becoming obese. Endocrinology 2010; 151: 4270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tarry‐Adkins JL, Ozanne SE. Mechanisms of early life programming: current knowledge and future directions. Am J Clin Nutr 2011; 94: 1765S–71S. [DOI] [PubMed] [Google Scholar]
- 7. Lanigan J, Singhal A. Early nutrition and long‐term health: a practical approach. Proc Nutr Soc 2009; 68: 422–9. [DOI] [PubMed] [Google Scholar]
- 8. Embleton ND. Early nutrition and later outcomes in preterm infants. World Rev Nutr Diet 2013; 106: 26–32. [DOI] [PubMed] [Google Scholar]
- 9. Baird J, Lucas P, Kleijnen J, Fisher D, Roberts H, Law C. Defining optimal infant growth for lifetime health: a systematic review of lay and scientific literature. Available from URL http://www.mrc.soton.ac.uk/systematic-review/2005.
- 10. Biasini A, Marvulli L, Neri E, China M, Stella M, Monti F. Growth and neurological outcome in ELBW preterms fed with human milk and extra‐protein supplementation as routine practice: do we need further evidence? J Matern Fetal Neonatal Med 2012; 25(Suppl. 4): 72–4. [DOI] [PubMed] [Google Scholar]
- 11. Cooke RJ, Embleton ND, Griffin IJ, Wells JC, McCormick KP. Feeding preterm infants after hospital discharge: growth and development at 18 months of age. Pediatr Res 2001; 49: 719–22. [DOI] [PubMed] [Google Scholar]
- 12. Aimone A, Rovet J, Ward W, Jefferies A, Campbell DM, Asztalos E, et al. Growth and body composition of human milk‐fed premature infants provided with extra energy and nutrients early after hospital discharge: 1‐year follow‐up. J Pediatr Gastroenterol Nutr 2009; 49: 456–66. [DOI] [PubMed] [Google Scholar]
- 13. Lucas A, Fewtrell MS, Morley R, Singhal A, Abbott RA, Isaacs E, et al. Randomized trial of nutrient‐enriched formula versus standard formula for postdischarge preterm infants. Pediatrics 2001; 108: 703–11. [DOI] [PubMed] [Google Scholar]
- 14. Lucas A, Morley R, Cole TJ, Gore SM. A randomised multicentre study of human milk versus formula and later development in preterm infants. Arch Dis Child Fetal Neonatal Ed 1994; 70: F141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ 1998; 317: 1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Connor DL, Jacobs J, Hall R, Adamkin D, Auestad N, Castillo M, et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J Pediatr Gastroenterol Nutr 2003; 37: 437–46. [DOI] [PubMed] [Google Scholar]
- 17. Huang C, Martorell R, Ren A, Li Z. Cognition and behavioural development in early childhood: the role of birth weight and postnatal growth. Int J Epidemiol 2013; 42: 160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tudehope DI, Burns Y, O'Callaghan M, Mohay H, Silcock A. The relationship between intrauterine and postnatal growth on the subsequent psychomotor development of very low birthweight (VLBW) infants. Aust Paediatr J 1983; 19: 3–8. [DOI] [PubMed] [Google Scholar]
- 19. Cooke RJ. Postnatal growth and development in the preterm and small for gestational age infant. Nestle Nutr Workshop Ser Pediatr Program 2010; 65: 85–95; discussion 6‐8. [DOI] [PubMed] [Google Scholar]
- 20. Belfort MB, Rifas‐Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 2011; 128: e899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sices L, Wilson‐Costello D, Minich N, Friedman H, Hack M. Postdischarge growth failure among extremely low birth weight infants: correlates and consequences. Paediatr Child Health 2007; 12: 22–8. [PMC free article] [PubMed] [Google Scholar]
- 22. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006; 117: 1253–61. [DOI] [PubMed] [Google Scholar]
- 23. Latal‐Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr 2003; 143: 163–70. [DOI] [PubMed] [Google Scholar]
- 24. Ma TH, Kim KA, Ko SY. Catch‐up growth and development of very low birth weight infants. Korean J Paediatr 2006; 34: 29–33. [Google Scholar]
- 25. Shah PS, Wong KY, Merko S, Bishara R, Dunn M, Asztalos E, et al. Postnatal growth failure in preterm infants: ascertainment and relation to long‐term outcome. J Perinat Med 2006; 34: 484–9. [DOI] [PubMed] [Google Scholar]
- 26. Nash A, Dunn M, Asztalos E, Corey M, Mulvihill‐Jory B, O'Connor DL. Pattern of growth of very low birth weight preterm infants, assessed using the WHO Growth Standards, is associated with neurodevelopment. Appl Physiol Nutr Metab 2011; 36: 562–9. [DOI] [PubMed] [Google Scholar]
- 27. Kelleher KJ, Casey PH, Bradley RH, Pope SK, Whiteside L, Barrett KW, et al. Risk factors and outcomes for failure to thrive in low birth weight preterm infants. Pediatrics 1993; 91: 941–8. [PubMed] [Google Scholar]
- 28. Powers GC, Ramamurthy R, Schoolfield J, Matula K. Postdischarge growth and development in a predominantly Hispanic, very low birth weight population. Pediatrics 2008; 122: 1258–65. [DOI] [PubMed] [Google Scholar]
- 29. Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009; 123: e101–9. [DOI] [PubMed] [Google Scholar]
- 30. Claas MJ, de Vries LS, Koopman C, Uniken Venema MM, Eijsermans MJ, Bruinse HW, et al. Postnatal growth of preterm born children </= 750 g at birth. Early Hum Dev 2011; 87: 495–507. [DOI] [PubMed] [Google Scholar]
- 31. Casey PH, Whiteside‐Mansell L, Barrett K, Bradley RH, Gargus R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school‐age outcomes: an 8‐year longitudinal evaluation. Pediatrics 2006; 118: 1078–86. [DOI] [PubMed] [Google Scholar]
- 32. Kan E, Roberts G, Anderson PJ, Doyle LW. Victorian Infant Collaborative Study G. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev 2008; 84: 409–16. [DOI] [PubMed] [Google Scholar]
- 33. Belfort MB, Martin CR, Smith VC, Gillman MW, McCormick MC. Infant weight gain and school‐age blood pressure and cognition in former preterm infants. Pediatrics 2010; 125: e1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weisglas‐Kuperus N, Hille ET, Duivenvoorden HJ, Finken MJ, Wit JM, van Buuren S, et al. Intelligence of very preterm or very low birthweight infants in young adulthood. Arch Dis Child Fetal Neonatal Ed 2009; 94: F196–200. [DOI] [PubMed] [Google Scholar]
- 35. Simon NP, Brady NR, Stafford RL. Catch‐up head growth and motor performance in very‐low‐birthweight infants. Clin Pediatr (Phila) 1993; 32: 405–11. [DOI] [PubMed] [Google Scholar]
- 36. Ford G, Rickards A, Kitchen WH, Ryan MM, Lissenden JV. Relationship of growth and psychoneurologic status of 2‐year‐old children of birthweight 500‐999 g. Early Hum Dev 1986; 13: 329–37. [DOI] [PubMed] [Google Scholar]
- 37. Kuban KC, Allred EN, O'Shea TM, Paneth N, Westra S, Miller C, et al. Developmental correlates of head circumference at birth and two years in a cohort of extremely low gestational age newborns. J Pediatr 2009; 155: 344–9 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, et al. The EPICure study: growth and associated problems in children born at 25 weeks of gestational age or less. Arch Dis Child Fetal Neonatal Ed 2003; 88: F492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hack M, Breslau N. Very low birth weight infants: effects of brain growth during infancy on intelligence quotient at 3 years of age. Pediatrics 1986; 77: 196–202. [PubMed] [Google Scholar]
- 40. Stathis SL, O'Callaghan M, Harvey J, Rogers Y. Head circumference in ELBW babies is associated with learning difficulties and cognition but not ADHD in the school‐aged child. Dev Med Child Neurol 1999; 41: 375–80. [DOI] [PubMed] [Google Scholar]
- 41. Brandt I, Sticker EJ, Lentze MJ. Catch‐up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. J Pediatr 2003; 142: 463–8. [DOI] [PubMed] [Google Scholar]
- 42. Cooke RW, Foulder‐Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child 2003; 88: 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cooke RW. Are there critical periods for brain growth in children born preterm? Arch Dis Child Fetal Neonatal Ed 2006; 91: F17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koo WW, Hockman EM. Posthospital discharge feeding for preterm infants: effects of standard compared with enriched milk formula on growth, bone mass, and body composition. Am J Clin Nutr 2006; 84: 1357–64. [DOI] [PubMed] [Google Scholar]
- 45. Cooke RJ, McCormick K, Griffin IJ, Embleton N, Faulkner K, Wells JC, et al. Feeding preterm infants after hospital discharge: effect of diet on body composition. Pediatr Res 1999; 46: 461–4. [DOI] [PubMed] [Google Scholar]
- 46. Hernandez MI, Rossel K, Pena V, Cavada G, Avila A, Iniguez G, et al. Leptin and IGF‐I/II during the first weeks of life determine body composition at 2 years in infants born with very low birth weight. J Pediatr Endocrinol Metab 2012; 25: 951–5. [DOI] [PubMed] [Google Scholar]
- 47. Ludwig‐Auser H, Sherar LB, Erlandson MC, Baxter‐Jones AD, Jackowski SA, Arnold C, et al. Influence of nutrition provision during the first two weeks of life in premature infants on adolescent body composition and blood pressure. Zhongguo Dang Dai Er Ke Za Zhi 2013; 15: 161–70. [PubMed] [Google Scholar]
- 48. Euser AM, Finken MJ, Keijzer‐Veen MG, Hille ET, Wit JM, Dekker FW, et al. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr 2005; 81: 480–7. [DOI] [PubMed] [Google Scholar]
- 49. Kerkhof GF, Willemsen RH, Leunissen RW, Breukhoven PE, Hokken‐Koelega AC. Health profile of young adults born preterm: negative effects of rapid weight gain in early life. J Clin Endocrinol Metab 2012; 97: 4498–506. [DOI] [PubMed] [Google Scholar]
- 50. Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 2003; 361: 1089–97. [DOI] [PubMed] [Google Scholar]
- 51. Fewtrell MS, Doherty C, Cole TJ, Stafford M, Hales CN, Lucas A. Effects of size at birth, gestational age and early growth in preterm infants on glucose and insulin concentrations at 9‐12 years. Diabetologia 2000; 43: 714–7. [DOI] [PubMed] [Google Scholar]
- 52. Finken MJ, Keijzer‐Veen MG, Dekker FW, Frolich M, Hille ET, Romijn JA, et al. Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia 2006; 49: 478–85. [DOI] [PubMed] [Google Scholar]
- 53. Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang‐Karlsson S, Makitie O, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med 2007; 356: 2053–63. [DOI] [PubMed] [Google Scholar]
- 54. Bracewell MA, Hennessy EM, Wolke D, Marlow N. The EPICure study: growth and blood pressure at 6 years of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed 2008; 93: F108–14. [DOI] [PubMed] [Google Scholar]
- 55. Keijzer‐Veen MG, Finken MJ, Nauta J, Dekker FW, Hille ET, Frolich M, et al. Is blood pressure increased 19 years after intrauterine growth restriction and preterm birth? A prospective follow‐up study in The Netherlands. Pediatrics 2005; 116: 725–31. [DOI] [PubMed] [Google Scholar]
- 56. Finken MJ, Inderson A, Van Montfoort N, Keijzer‐Veen MG, van Weert AW, Carfil N, et al. Lipid profile and carotid intima‐media thickness in a prospective cohort of very preterm subjects at age 19 years: effects of early growth and current body composition. Pediatr Res 2006; 59: 604–9. [DOI] [PubMed] [Google Scholar]
- 57. Mortaz M, Fewtrell MS, Cole TJ, Lucas A. Birth weight, subsequent growth, and cholesterol metabolism in children 8‐12 years old born preterm. Arch Dis Child 2001; 84: 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long‐term cardiovascular health? Circulation 2004; 109: 1108–13. [DOI] [PubMed] [Google Scholar]
- 59. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005; 331: 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ong KK. Catch‐up growth in small for gestational age babies: good or bad? Curr Opin Endocrinol Diabetes Obes 2007; 14: 30–4. [DOI] [PubMed] [Google Scholar]
- 61. Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta‐analysis. Pediatrics 2012; 130: e640–9. [DOI] [PubMed] [Google Scholar]
- 62. Moyses HE, Johnson MJ, Leaf AA, Cornelius VR. Early parenteral nutrition and growth outcomes in preterm infants: a systematic review and meta‐analysis. Am J Clin Nutr 2013; 97: 816–26. [DOI] [PubMed] [Google Scholar]
- 63. Westin V. Stoltz Sjostrom E, Ahlsson F, Domellof M, Norman M. Perioperative nutrition in extremely preterm infants undergoing surgical treatment for patent ductus arteriosus is suboptimal. Acta Paediatr 2014; 103: 282–8. [DOI] [PubMed] [Google Scholar]
- 64. SIFT Investigators Group . Early enteral feeding strategies for very preterm infants: current evidence from Cochrane reviews. Arch Dis Child Fetal Neonatal Ed 2013; 98: F470–2. [DOI] [PubMed] [Google Scholar]
- 65. Koletzko B, Szajewska H, Ashwell M, Shamir R, Aggett P, Baerlocher K, et al. Documentation of functional and clinical effects of infant nutrition: setting the scene for COMMENT. Ann Nutr Metab 2012; 60: 222–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Gain‐outcome graph of weight gain and adiposity.
Figure S2 Gain‐outcome graph of weight gain and insulin levels.
Figure S3 Gain‐outcome graph of weight gain and CVD risk.


