Abstract
Objective
Long‐term success of dental implants depends on healthy peri‐implant soft tissues and adequate bone levels. This prospective study aims to assess bone level changes around implants with internal conical connection and platform‐switching abutments in the posterior mandible.
Material and methods
Adult patients missing at least two teeth in the posterior mandible and with a natural tooth mesial to the implant site received two or three adjacent internal conical connection implants. After a minimum transmucosal healing period of 8 weeks, single crown restorations were cemented over platform‐switching abutments. Changes in marginal bone levels were measured in standardized periapical radiographs from surgery and loading (baseline) to 12 months post‐loading.
Results
Twenty‐four patients received 52 implants. Bone remodeling took place between surgery and loading (−0.53 ± 0.40 mm). From loading to 12 months, there was a mean bone gain of 0.12 ± 0.42 mm which occurred mainly in the first 6 months after prosthesis delivery (0.11 ± 0.36 mm) and stabilized afterward. A total of 71.7% of all implants presented bone preservation or gain. No implant was lost at 1 year and the success rate was 100%. Patient inquiry revealed high satisfaction.
Conclusion
Internal conical connection implants with platform‐switching abutments presented high success rate and enhancement or preservation of marginal bone levels after 1 year of loading.
Keywords: bone level, bone remodeling, conical connection implants, dental implant, outcome, platform switch
One predominant condition for the long‐term success of dental implants is the establishment of a healthy peri‐implant mucosa supported by stable marginal bone (Misch et al. 2008). However, after exposure to the oral environment, some degree of marginal bone resorption is well accepted for properly osseointegrated dental implants meaning the implementation of a healthy biologic width around those implants (Berglundh & Lindhe 1996). Therefore, a limit of 1.5 mm loss of bone during the first year and 0.2 mm in the following years was the maximum established to consider an implant successful (Albrektsson et al. 1986; Smith & Zarb 1989).
Other clinical factors were also related to the amount of bone resorption, namely the grade of invasiveness during surgery (Becker et al. 2009), the distance of the implant to the neighboring teeth (Esposito et al. 1993) and implants (Tarnow et al. 2000), presence of inflammation and leukocyte infiltration (Broggini et al. 2006), and the extend of modified surface extension (Bratu et al. 2009) and occlusal strains (Kim et al. 2005). Conversely, other authors reported that marginal bone loss at the implant–abutment interface is not unavoidable, as bone preservation appeared to be possible in cases with a mismatch between abutment and implant diameter (Baumgarten et al. 2005; Gardner 2005; Lazzara & Porter 2006). Subsequently, further reports on the beneficial aspects of platform switching (PS) were published along with the increasing options for restorations based on the PS concept offered by most manufacturers (Vela‐Nebot et al. 2006; Canullo & Rasperini 2007; Hurzeler et al. 2007). Additionally, some clinical studies supported the effect of the PS concept as a trend or with significant difference in comparison to matching diameter abutments (Hurzeler et al. 2007; Cappiello et al. 2008; Canullo et al. 2009, 2010; Crespi et al. 2009; Prosper et al. 2009; Trammell et al. 2009; Vigolo & Givani 2009; Enkling et al. 2011). Despite the rising evidence of a clinical relevant effect by the PS concept, the formerly presented data were of high heterogeneity, especially due to various factors such as implant insertion depth, implant design, implant microstructure and platform diameter, often different within the same PS study and could influence the outcomes to a certain extent (Al‐Nsour et al. 2012). Thus, additional clinical studies avoiding the effect of those confounders were an urgent need to substantially support the potential benefits of platform‐switched abutments (Atieh et al. 2010; Al‐Nsour et al. 2012). Recently, a prospective randomized‐controlled clinical multicenter trial reported positive results supporting the PS concept in commercially available implants with identical outer geometry and internal implant–abutment connection for both groups complied with the elimination of relevant confounders (Guerra et al. 2014). The present prospective observational study has been planned to evaluate the marginal bone level changes in implants with internal conical connection and platform switch design in the posterior mandible.
Material and methods
Study design
This pilot study was designed as a prospective two‐center (Germany and Portugal) observational study with 60‐month follow‐up (Fig. 1). The 1‐year results are reported here. The study was approved by both local ethics committees (Mainz: 837.030.11 (7572), Coimbra: 12‐CE‐2011) in accordance with the Declaration of Helsinki (2008) and followed the STROBE guidelines (Vandenbroucke et al. 2014). All patients provided written informed consent before entry into the study.
Figure 1.
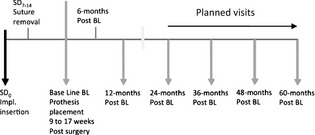
Study flowchart: schedule of visits and surgical procedures.
Inclusion and exclusion criteria
Adult male or female patients (≥18 years) presenting at least two adjacent missing teeth in the posterior mandible and an adjacent natural tooth mesial to the most proximal implant site and with opposing dentition of natural teeth or tooth/implant‐supported fixed rehabilitations were eligible for the study. Free‐end situations were allowed.
Exclusion criteria established for this study were as follows: uncontrolled systemic diseases, medications known to interfere with bone metabolism or the notification of drug or alcohol abuse. The consumption of tobacco was limited to an equivalent of 10 cigarettes per day. In addition, patients with mental or physical disorders that would handicap an adequate oral hygiene or interfere with adequate study participation excluded were as well. Local exclusion criteria were as follows: inadequate bone quality and quantity at the prospective implant sites, signs of local inflammation or unhealed extraction sockets, untreated periodontitis, history of irradiation or chemotherapy or former implant failure. Patients presenting severe bruxism or clenching habits or thin mucosal phenotypes (keratinized gingiva inferior to 4 mm) were also excluded. At the time of surgery, a lack of the primary stability or an inappropriate implant position for prosthetic rehabilitation defined further exclusion criteria.
Implants
Two to three adjacent CONELOG® SCREW‐LINE (CAMLOG Biotechnologies AG, Basel, Switzerland) implants were inserted in the selected sites. These implants have an abrasive‐blasted, acid‐etched Promote® surface that extends over the entire body of the implant up to the conical acid‐etched implant shoulder (45°) which is 0.1 and 0.15 mm height for the ∅3.8 mm and the ∅4.3 mm implants, respectively.
Implant diameter (3.8 or 4.3 mm) and length (11 or 13 mm) relied on the surgeon choice to meet the site width and height. Healing abutments, impression posts and definitive abutments were inserted following manufacturer instructions. The mismatch between abutments and implant platforms was 0.4 mm for the ∅3.8 and 0.65 mm for the ∅4.3 mm implants. All products used in this study were registered and commercially available.
Pretreatment and surgical procedures
All patients attended a pre‐surgical appointment for a basic physical examination and clinical documentation collection (standardized intraoral photographs and periapical radiographs using a customized stent). Prophylactic antibiotics were allowed according to the procedures of each center.
Surgery was performed in an outpatient facility under local anesthesia. After full thickness flap elevation, the implants were placed at bone level and respecting a minimal distance of 3.0 mm between two implants. The mesial implant was inserted at 1.5–2.0 mm distance from the neighboring natural tooth. As SmartPeg (Osstell AB, Gothenburg, Sweden) was not available at the initiation of the study, primary stability had to be assessed manually with a torque wrench and was proportionately complemented later with resonance frequency analysis (Osstell ISQ, Osstell AB). No guided bone regeneration procedures were performed.
Transmucosal healing was promoted using PS healing abutments immediately after implantation. Further standardized photographs and periapical radiographs were taken immediately postsurgery.
All patients received instructions for oral hygiene and food intake. Chlorhexidine digluconate (0.12%) rinse was prescribed three times a day until sutures were removed.
Prosthetic treatment
Conventional loading was performed after a healing phase of at least 6 weeks for class I‐III bone. To ensure that the primary‐to‐secondary bone contact conversion provided adequate stability at the time of loading (Cochran et al. 2004), implants placed into class IV bone were restored at least 12 weeks after surgery according to the instruction manual of the implant system protocol. The prosthetic abutments were fastened to the implants with 20‐Ncm torque, and 2–3 weeks later, the implants received single crowns cement retained (Fig. 1). Loading was the baseline for upcoming measurements.
Primary study objective
The primary study objective was to assess the marginal bone level changes using internal conical connection implants with platform switching in the posterior mandible.
Radiographic assessment
Standardized periapical radiographs were performed before surgery, immediately after surgery, at loading, after 6 and 12 months post‐loading. To ensure an orthographic and comparable patient position, a customized tube holder oriented at the natural dentition by occlusal fixation was utilized for each patient (Fig. 2). Further assessments were planned annually after load up to 60 months. The marginal bone level was assessed blinded to the clinical data by an independent person as the distance from the mesial or distal first visible bone contact to the implant shoulder (DIB). Each radiograph was calibrated for measurements using a known reference distance (implant diameter) and measurements were taken with an accuracy of 0.1 mm. The radiographic measurements were analyzed using the open source program ImageJ 1.44p (Schneider et al. 2012).
Figure 2.
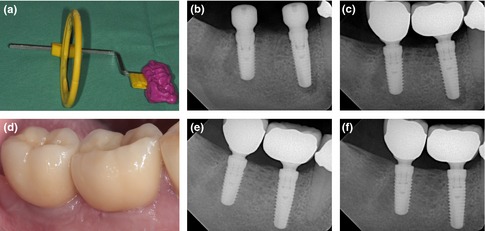
(a) Customized tube holder for standardization of the dental X‐rays. Dental X‐rays for measurement of bone level changes over time at surgery (b), begin of loading (c), at 6 months (e) and after 12 months (f). (d) Enoral photography showing healthy periimplant soft tissues at 12‐month postsurgery.
Secondary study objectives
Secondary study objectives were the determination of the success and survival rate at 1 year according to Buser et al. (2002) and the evaluation of the performance of the restorative components. Success of the restorative components was assumed, if the restorative component was intact and served the functional rehabilitation of the patient as evaluated by a questionnaire as follows. Frequency and nature of adverse events were recorded, as well as the general patient satisfaction.
The clinical peri‐implant parameters were assessed as categorical variables including plaque index (PI: 0–3), sulcus bleeding index (SBI: 0–3) and complemented by probing pocket depth in millimeter (PPD) and were recorded at loading, 6 months and 1 year after load for evaluation of the soft tissue health.
Patient satisfaction was recorded by mean of a questionnaire at each visit. The questionnaire consisted of the items: comfort, appearance, ability to chew, ability to taste and general satisfaction as categorical variables rated from very satisfied (1), satisfied (2), middle (3), unsatisfied (4) to very unsatisfied (5).
Statistical methods
Due to the single‐cohort nature of the study (pilot study), no power or sample size calculation was carried out. Descriptive statistics were reported for demographic and baseline data. Means and standard deviations were calculated for continuous variables, absolute and relative frequencies for categorical variables.
The bone level changes (BLC) were calculated as the difference in DIB measurements between the period of interest and the following, that is, between surgery and load, between load and 6 months and between 6 months and 1 year. Additionally, a functional BLC was calculated from load to 1‐year follow‐up. Mesial and distal values were considered, as well as the average of both measurements. Proximal sides of the implants were classified according to the radiographic crestal positioning of the shoulder obtained in the surgery. Implant sides with negative DIB were placed bellow the crest and cate‐gorized as subcrestal. Implants with DIB values ranging from 0 to 0.1 mm (∅ 3.8 mm implant) or 0.2 mm (∅ 4.3 mm implant) were classified as epicrestal. Implants with DIB values superior to 0.1 or 0.2 mm were classified as supracrestal. The variation of marginal bone levels over time was assessed using mixed‐effects model analysis assuming auto‐regressive covariance matrix for the repeated measures and considering both random intercepts and slopes. Center, bone type, cement used and insertion level were established as the fixed effects and the patient as the random effect. One‐way ANOVA was used to detect differences in bone level changes between the three categories. Post hoc comparisons were performed using Tukey correction.
Mesial and distal bone level changes were assessed with a paired t‐test. Comparisons between implant diameters were performed with a t‐test.
Survival analysis was applied to calculate implant success and survival rate. All statistical analysis was performed using the IBM SPSS Statistics 19 (IBM Corp., Armonk, NY, USA) with significance level set to α = 0.05.
Results
Study population: demographic data
In the period between May 2011 and Febr‐uary 2012, 24 patients (mean of 48.9 ±13.8 years) received 52 implants. Both centers recruited a similar number of patients (Portugal = 12, Germany = 12). The gender ratio was 1.4 : 1 (male = 14, female = 10). The general health status was very good: 87.5% of the patients were ASA 1 (n = 21), and 12.5% were ASA II (n = 3). A total of 21 patients (87.5%) had no use of a regular medication. Two‐thirds (66.7%) never used tobacco, four patients quit smoking upon recruitment for the study, and only four patients reported ongoing tobacco consumption of <10 cigarettes a day.
Study population: implant data
The distribution of the 52 implants per edentulous region of the posterior mandible is shown in Fig. 3. Twenty patients received two adjacent implants, whereas in four cases, three consecutive implants were inserted. Twenty‐eight implants had a diameter of 3.8 mm, and 24 implants were of a diameter of 4.3 mm. Only implants of 11 mm (n = 38) and 13 mm (n = 14) were used. Intrasurgical smoothening of the alveolar crest was performed in four cases. The majority of the cases presented D2 (n = 19, 36.5%) or D3 (n = 21, 40.4%) bone type at the particular implant site (Lekholm & Zarb 1985). The remaining 12 cases were evenly classified as D1 (six cases) and D4 (six cases). All implants had primary stability on manual testing. For 41 implants, an additional measurement of the insertion torque was taken: One implant was inserted at 40 Ncm, roughly 90% (37) had an insertion torque between 25 and 35 Ncm, and only three implants presented a torque between 15 and 20 Ncm. Wide body healing abutments were used in n = 49 implants (94.2%) and cylindrical abutments in three cases. For 20 implants, the ISQ value was reported at surgery (ISQ =75.65 ± 5.31) and loading (ISQ = 79.75 ±2.07).
Figure 3.
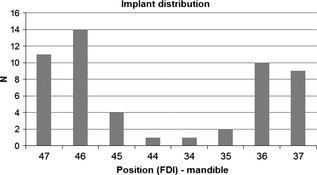
Distribution of the anatomical implant site
The postoperative healing period was uneventful. At the first postsurgery control, 44 of the implants showed no fibrin or a small line of fibrin at the implant site and eight revealed presence of fibrin. The loading phase began after a mean healing period of 11.3 ± 2.7 weeks and did not differ substantially between the two centers. Metal ceramic crowns were placed in n = 46 cases (88.5%) and full ceramic crowns in six cases (11.5%). In one center (Coimbra), the crowns were cemented with Fuji I (n = 24), while in the other center TempBond (Kerr, Italy) (n = 21), Harvard cement (Harvard Dental International GmbH, Germany) (n = 3) or Ketac™ Cem (3M Espe, USA) (n = 4) were used. The mean crown to implant ratio was 0.73 ± 0.18, ranging from 0.36 to 1.14.
Course of the study
Three patients did not complete the set of radiographs for bone level analysis due to pregnancy (radiographs at 6 and 12 months), withdrawn of consent (at 12 months) or refusal to undergo radiation exposure at the 12‐month control. Hence, 46 implants in 21 patients were traceable at the 12‐month visit and only those were considered for analysis.
Bone level analysis
The full mixed‐effects model detected no effect for the explanatory variables center (P = 0.193), bone type (P = 0.08) and cement (P = 0.565) in mean bone level changes over time and in consequence was eliminated as confounding factors for bone level analysis, allowing the analysis of the 46 implants as a single group. The random effect attributable to the patient was not significant (Wald Z = 1.93, P = 0.054).
Table 1 presents the average bone level changes mesial and distal of the implants over the course of the study. Bone loss around the implant was noticeable only between surgery and load, presenting a mean value of −0.53 ± 0.40 mm (Table 1).
Table 1.
Bone level changes (BLC) between succeeding evaluation periods for mesial and distal implant sides or according to the averaged measurements for mean values per implant
| BLC surgery–load (Mean ± SD) | BLC load – 6 months (Mean ± SD) | BLC 6–12 months (Mean ± SD) | |
|---|---|---|---|
| Mesial side | −0.55 ± 0.51 | 0.15 ± 0.41 | −0.02 ± 0.21 |
| Distal side | −0.50 ± 0.45 | 0.08 ± 0.39 | 0.03 ± 0.24 |
| Average for Implant | −0.53 ± 0.41 | 0.11 ± 0.36 | 0.00 ± 0.19 |
Within the mixed‐model context, implant insertion depth exerted a statistically significant influence on this remodeling event (F(2, 96.98) = 13.03, P < 0.05). The implants placed at subcrestal positions presented a mean bone loss of 0.36 mm superior to those placed supracrestal or at the crest level (F(2) = 4.49, P = 0.017) (Table 2). The effect of crestal positioning of the implant shoulder was more perceptible in the mesial side of the implants where the bone loss of the subcrestal implants was 0.87 ± 0.43 mm, approximately 0.5 mm higher than those placed epicrestally. In the distal side, bone level changes for the period were similar for all implants, regardless of the insertion level (F(2) = 0.43, P = 0.651).
Table 2.
Implant insertion level at surgery and bone level changes (BLC) between succeeding evaluation periods. Classification according to mesial and distal DIB measurements at surgery for the respective implant side or according to the averaged mesial and distal measurements of DIB for mean values per implant. P values for one‐way ANOVA. Post hoc analysis using Tukey correction α = 0.05
| N | DIB surgery (Mean ± SD) | BLC surgery–load (Mean ± SD) | BLC load – 6 months (Mean ± SD) | BLC 6–12 months (Mean ± SD) | |
|---|---|---|---|---|---|
| Mesial side | |||||
| Subcrestal | 19 | −0.99 ± 0.55 | −0.87 ± 0.43a | 0.02 ± 0.38 | −0.02 ± 0.21 |
| Epicrestal | 20 | 0.02 ± 0.05 | −0.39 ± 0.45 | 0.18 ± 0.44 | −0.03 ± 0.24 |
| Supracrestal | 7 | 0.71 ± 0.29 | −0.15 ± 0.37 | 0.42 ± 0.32 | −0.02 ± 0.13 |
| P < 0.05 | P = 0.075 | P = 0.978 | |||
| Distal side | |||||
| Subcrestal | 16 | −0.79 ± 0.39 | −0.58 ± 0.49 | 0.00 ± 0.42 | −0.06 ± 0.47 |
| Epicrestal | 20 | 0.01 ± 0.02 | −0.46 ± 0.38 | 0.07 ± 0.29 | 0.14 ± 0.46 |
| Supracrestal | 10 | 0.73 ± 0.31 | −0.43 ± 0.54 | 0.23 ± 0.42 | 0.09 ± 0.23 |
| P = 0.651 | P = 0.328 | P = 0.180 | |||
| Mean | |||||
| Subcrestal | 22 | −0.69 ± 0.30 | −0.69 ± 0.33b | 0.05 ± 0.34 | 0.01 ± 0.18 |
| Epicrestal | 13 | 0.01 ± 0.03 | −0.37 ± 0.40 | 0.09 ± 0.42 | −0.02 ± 0.24 |
| Supracrestal | 11 | 0.52 ± 0.23 | −0.37 ± 0.44 | 0.27 ± 0.31 | 0.01 ± 0.16 |
| P = 0.018 | P = 0.254 | P = 0.305 | |||
Statistically different from implants placed epicrestal (mean difference −0.47, P = 0.004) and the implants placed supracrestal (mean difference −0.72, P = 0.001).
Only statistically different from implants placed epicrestal (mean difference −0.32, P = 0.043).
After load, the insertion level no longer influenced the BLC and all subgroups presented homogenous changes over time, either for the mesial side (F(2, 43) = 2.17, P = 0.127) or for the distal side (F(2, 43) = 1.99, P =0.148), as seen on Fig. 4.
Figure 4.
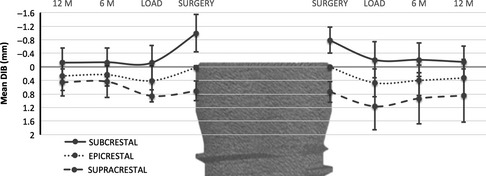
Development of bone level change. Mean DIB for the mesial (left) and distal (right) sides of the implant over the course of the study according to the level of insertion of the implant at the surgery. Values in mm.
Also, no differences between BLC of the mesial and distal sides were found from load to 6‐month period (t(45) = 1.29, P = 0.205) and from 6‐ to 12‐month period (t(45) =−1.50, P = 0.141). Consequently, mean bone levels are considered hereafter for simplification (Fig. 4).
In the first 6 months after load, the implants presented a similar positive variation in proximal bone levels, yet more noticeable for the implants placed supracrestal which presented a mean gain of 0.27 ±0.31 mm (Fig. 5). From this point onwards, bone levels stabilize until the 12‐month control (Table 2).
Figure 5.
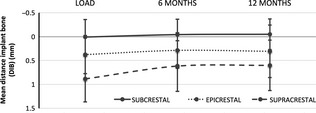
Mean bone level changes from loading (baseline) to 12‐month follow‐up according to the implant insertion level classification. Vertical axis represents the mean DIB measured at each period, and the slope of the curve reflects the bone level changes variation between two consecutive periods. Values in mm.
Overall, the mean bone level change at the implant shoulder from loading to 12‐month follow‐up was 0.12 ± 0.42 mm. No change in bone levels or bone gain was observed in 71.7% of all implants (Fig. 6) and roughly half of these presented a positive variation superior to 0.5 mm. No statistically significant differences were found between bone level changes of ∅ 3.8 mm and ∅ 4.3 mm implants t(44) = −1.18, P = 0.243.
Figure 6.
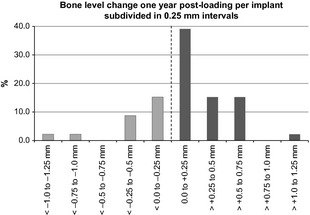
Distribution of bone level change. Mean bone level changes at 1 year post‐loading. In 71.7% of the implants, bone gain was observed.
Implant success and complications
No implant was lost during the healing period, resembling a survival and success rate of 100% after 1 year according to the success criteria of absence of complaints, peri‐implantitis, mobility and radiolucency (Buser et al. 2002). Patient questionnaire revealed a high to very high overall satisfaction.
The single adverse event noticed occurred during the insertion of the definite rehabilitation with fracture of the prosthetic screw inside of the implant connection. The event was successfully solved and the final restoration placed after repeating the prosthetic procedures.
Soft tissue health
Plaque index (PI) and SBI were examined as categorical values (0–3) and presented as bar charts (Fig. 7). The mean probing depth was <3 mm at all examinations. No statistical differences were found in the period from loading to the 12‐month visit.
Figure 7.
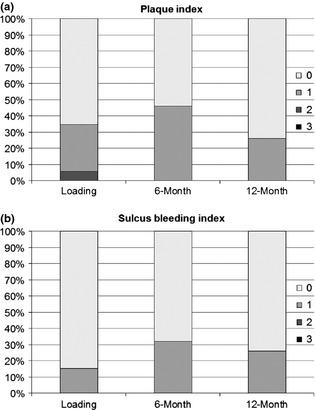
Soft Tissue Health. (a) Distribution of plaque index: Score 0 – no plaque; Score 1 – plaque only recognized by running a probe; Score 2 – plaque seen with naked eye; Score 3 – abundance of soft matter. (b) Distribution of sulcus bleeding index: Score 0 – no bleeding; Score 1 – isolated bleeding spot; Score 2 – blood, confluent red line on margin; Score 3 – heavy bleeding.
Discussion
The present study on platform‐switched and conical connection implants inserted in the posterior mandible evaluated changes in marginal bone levels after 1 year of function and some secondary objectives mentioned before related to the performance of the implant system.
Maximum survival and success was determined for the first year as all implants remained in function with healthy soft tissues and no signs of peri‐implant infection, mobility or pain/discomfort. Radiographically, no implants presented continuous radiolucency or marginal bone loss at the proximal sides of the implant superior to the 1.5–2 mm within the first year of load as proposed by Albrektsson et al. (1986) and updated by Abrahamsson & Berglundh (2009) and Smith & Zarb (1989). Results showed a mean bone gain of 0.12 ± 0.42 mm at the implant shoulder between the prosthetic delivery and the 1‐year follow‐up, preceded by a period of remodeling between surgery and load.
The trend for postsurgical bone resorption has been widely reported and occurs both in the horizontal and vertical direction (Albrektsson et al. 1986; Astrand et al. 2004; Laurell & Lundgren 2011). The results show that these tapered, self‐taping bone level implants present a mean vertical loss of −0.53 ± 0.41 mm from insertion to loading day, comparable to that reported by Guerra et al. (2014) for both platform‐switching and platform‐matching abutments. As these authors, Wang et al. (2015) also found no differences between platform‐switching and non‐platform‐switching implants until definite restoration placement, which suggests that the horizontal mismatch between the PS abutments and the implant platform is not influential for the remodeling of the aforementioned period. This supports the results of several studies that attribute initial bone loss to different events, such as surg‐ery‐induced trauma (Astrand et al. 2004; Berglundh et al. 2005), crestal location of the transition between the rough and the smooth borders of the implant (Hammerle et al. 1996; Hermann et al. 2001a,b) and vertical positioning of the interface (microgap) between the implant and abutment/restoration within the crest (Ericsson et al. 1995; Hermann et al. 2001a,b). The last aspect appears to be of particular importance as it has an impact on the magnitude of the peri‐implant inflammatory infiltrate found at the interface that is partly responsible for the resorption of the marginal bone (Broggini et al. 2006; Cochran 2006; Cochran et al. 2009a,b).
In the case of the present study, the protocol‐specified insertion depth recommends the implant shoulder to be placed at ridge level without compulsory flattening of the implant site, promoting an epicrestal positioning of the interface, in line with the consensus statements of Schwarz et al. (2014). However, the ideal vertical positioning of implants is frequently not achievable due to the inherent anatomical constrictions of the posterior mandible and the presence of a tooth proximal to the edentulous site, which causes the implant to have variable insertion depths for the proximal sides, and results in either subcrestal or supracrestal positioning of the interface. To determine the impact of the interface level on bone level changes over time, the mesial and distal sides of the implants were classified in radiographs as supra‐, epi‐ or subcrestal and analyzed accordingly. Between surgery and begin of loading, proximal sides that have been placed subcrestally presented the greatest bone loss, in average 0.32 mm superior to that of the other two groups, yet maintaining the average bone level at the crest level or below. The higher loss of the subcrestal implants is in accordance with the study by Jung et al. (2008) on platform‐switching implants inserted either epicrestal or 1 mm below or above the crest who reported significant remodeling between surgery and load, particularly for the subcrestal implants that lost more bone than those placed supracrestally, and could be a consequence of the establishment of the biologic width (Hermann et al. 2000).
Heitz‐Mayfield et al. (2013) addressed the same question using three implant systems with and without platform switching and found no differences in bone level changes among the implants placed supracrestally. At the same time, the authors reported that in the case of epicrestal positioning of the implants, systems with platform switching promoted greater bone preservation.
The course of the present study with initial bone loss and post‐loading bone gain or preservation is in line with the 5‐year multicenter prospective study of Cochran et al. (2009a,b) in which clinically significant remodeling of the marginal bone around non‐submerged implants was found before prosthesis delivery, becoming insignificant after 1 year of function, accounting to 86% of the total mean bone loss over the course of 5 years, irrespective of implant design, type of restoration or implant length.
As consequence, further investigations should address the effect of shortened healing times on bone level variation of internal connection platform‐switching implants.
After prosthesis delivery and up to the 6th month, a mean bone gain of 0.11 ± 0.36 mm was found for the implants used in this study, regardless of the initial positioning of the implant–abutment interface, which could be attributable to the integrated platform switch provided by the internal locking of the abutments into the 7.5° tapered conical connection of the implant. Load under the effect of the platform‐switching concept appeared to favor bone regain at the impl‐ant shoulder, particularly for the implants placed supracrestally, which recovered 0.27 ±0.31 mm in the period, possibly due to the biologic effect resultant from the horizontal displacement of the interface toward the center of the platform and greater separation of the inflammatory infiltrate from the marginal bone.
This is in accordance with the results presented by Guerra et al. (2014) but is inconsistent with the conclusions withdrawn by Canullo et al. (2010) on the course of a 3‐year randomized clinical trial who hypothesized that the effect of bone preservation was related to implant diameter and extent of implant/abutment mismatch rather than a biologic effect of the PS as wide diameter implants (5.5 mm) presented less resorption than ∅4.8 or 4.3 mm implants or the controls (3.8 mm). The different mismatch in ∅3.8 and 4.3 mm implants (0.4 vs 0.65 mm, respectively) used in the present study contrasts the hypothesis of those authors as no differences were detected between implants with different diameters. In fact, Canullo et al. (2012) retracts the previous hypothesis and suggests that bone resorption is mostly related to biologic width re‐establishment rather than to biomechanical factors related to implant platform diameter.
Even though a few publications report no differences on bone level changes between PS abutments and matching ones (Crespi et al. 2009; Kielbassa et al. 2009; Enkling et al. 2011; Dursun et al. 2012), the majority of the literature, supported by three systematic reviews (Atieh et al. 2010; Al‐Nsour et al. 2012; Strietzel et al. 2015), points to a positive influence of the platform‐switching concept on the preservation of marginal bone, as also verified in the present trial. Despite the heterogeneity of the studies included and the short follow‐up periods, the meta‐analysis presented by Atieh et al. and by Striet‐zel et al. revealed that platform switching induces a significantly lower marginal bone resorption than matching abutments, with the mean difference ranging from 0.37 to 0.52 mm, respectively.
Within the limitations of a single‐cohort prospective study with short observational period, the present data are further contributions to support the positive influence of the platform‐switching concept on marginal bone level preservation in the posterior mandible using an internal connection implant system. For the clinician, our data provide initial information that the positive effect on marginal bone level is irrespective of the underlying implant diameter; however, it emerged more at implants placed slightly epicrestally. From a surgical point of view, further clinical studies should address the influence of the vertical positioning (supracrestal–crestal–subcrestal) of the shoulder, whereas from a prosthetic point of view, the effect of cemented vs. screw attached rehabilitation is of high clinical interest and should be investigated in a prospective and randomized‐controlled clinical setting with high number of patients.
Conflict of interest
The authors declare that they have no conflict of interests related to this study. This study was funded by an unrestricted grant of the Camlog Foundation, Basel, Switzerland. Prof. W. Wagner and Prof. F. Guerra are members of the Camlog Foundation Board.
Moergel M, Rocha S, Messias A, Nicolau P, Guerra F, Wagner W. Radiographic evaluation of conical tapered platform‐switched implants in the posterior mandible: 1‐year results of a two‐center prospective study. Clin. Oral Impl. Res. 27, 2016, 686–693
References
- Abrahamsson, I. & Berglundh, T. (2009) Effects of different implant surfaces and designs on marginal bone‐level alterations: a review. Clinical Oral Implants Research 20(Suppl. 4): 207–215. [DOI] [PubMed] [Google Scholar]
- Albrektsson, T. , Zarb, G. , Worthington, P. & Eriksson, A.R. (1986) The long‐term efficacy of currently used dental implants: a review and proposed criteria of success. International Journal of Oral & Maxillofacial Implants 1: 11–25. [PubMed] [Google Scholar]
- Al‐Nsour, M.M. , Chan, H.L. & Wang, H.L. (2012) Effect of the platform‐switching technique on preservation of peri‐implant marginal bone: a systematic review. International Journal of Oral & Maxillofacial Implants 27: 138–145. [PubMed] [Google Scholar]
- Astrand, P. , Engquist, B. , Dahlgren, S. , Grondahl, K. , Engquist, E. & Feldmann, H. (2004) Astra tech and Branemark system implants: a 5‐year prospective study of marginal bone reactions. Clinical Oral Implants Research 15: 413–420. [DOI] [PubMed] [Google Scholar]
- Atieh, M.A. , Ibrahim, H.M. & Atieh, A.H. (2010) Platform switching for marginal bone preservation around dental implants: a systematic review and meta‐analysis. Journal of Periodontology 81: 1350–1366. [DOI] [PubMed] [Google Scholar]
- Baumgarten, H. , Cocchetto, R. , Testori, T. , Meltzer, A. & Porter, S. (2005) A new implant design for crestal bone preservation: initial observations and case report. Practical Procedures & Aesthetic Dentistry 17: 735–740. [PubMed] [Google Scholar]
- Becker, W. , Goldstein, M. , Becker, B.E. , Sennerby, L. , Kois, D. & Hujoel, P. (2009) Minimally invasive flapless implant placement: follow‐up results from a multicenter study. Journal of Periodontology 80: 347–352. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Abrahamsson, I. & Lindhe, J. (2005) Bone reactions to longstanding functional load at implants: an experimental study in dogs. Journal of Clinical Periodontology 32: 925–932. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. & Lindhe, J. (1996) Dimension of the periimplant mucosa. Biological width revisited. Journal of Clinical Periodontology 23: 971–973. [DOI] [PubMed] [Google Scholar]
- Bratu, E.A. , Tandlich, M. & Shapira, L. (2009) A rough surface implant neck with microthreads reduces the amount of marginal bone loss: a prospective clinical study. Clinical Oral Implants Research 20: 827–832. [DOI] [PubMed] [Google Scholar]
- Broggini, N. , McManus, L.M. , Hermann, J.S. , Medina, R. , Schenk, R.K. , Buser, D. & Cochran, D.L. (2006) Peri‐implant inflammation defined by the implant‐abutment interface. Journal of Dental Research 85: 473–478. [DOI] [PubMed] [Google Scholar]
- Buser, D. , Ingimarsson, S. , Dula, K. , Lussi, A. , Hirt, H.P. & Belser, U.C. (2002) Long‐term stability of osseointegrated implants in augmented bone: a 5‐year prospective study in partially edentulous patients. The International Journal of Periodontics & Restorative Dentistry 22: 109–117. [PubMed] [Google Scholar]
- Canullo, L. , Fedele, G.R. , Iannello, G. & Jepsen, S. (2010) Platform switching and marginal bone‐level alterations: the results of a randomized‐controlled trial. Clinical Oral Implants Research 21: 115–121. [DOI] [PubMed] [Google Scholar]
- Canullo, L. , Goglia, G. , Iurlaro, G. & Iannello, G. (2009) Short‐term bone level observations associated with platform switching in immediately placed and restored single maxillary implants: a preliminary report. The International Journal of Prosthodontics 22: 277–282. [PubMed] [Google Scholar]
- Canullo, L. , Iannello, G. , Penarocha, M. & Garcia, B. (2012) Impact of implant diameter on bone level changes around platform switched implants: preliminary results of 18 months follow‐up a prospective randomized match‐paired controlled trial. Clinical Oral Implants Research 23: 1142–1146. [DOI] [PubMed] [Google Scholar]
- Canullo, L. & Rasperini, G. (2007) Preservation of peri‐implant soft and hard tissues using platform switching of implants placed in immediate extraction sockets: a proof‐of‐concept study with 12‐ to 36‐month follow‐up. International Journal of Oral & Maxillofacial Implants 22: 995–1000. [PubMed] [Google Scholar]
- Cappiello, M. , Luongo, R. , Di Iorio, D. , Bugea, C. , Cocchetto, R. & Celletti, R. (2008) Evaluation of peri‐implant bone loss around platform‐switched implants. The International Journal of Periodontics & Restorative Dentistry 28: 347–355. [PubMed] [Google Scholar]
- Cochran, D.L. (2006) Peri‐implant inflammation defined by the implant‐abutment interface. Journal of Dental Research 85: 473–478. [DOI] [PubMed] [Google Scholar]
- Cochran, D.L. , Bosshardt, D.D. , Grize, L. , Higginbottom, F.L. , Jones, A.A. , Jung, R.E. , Wieland, M. & Dard, M. (2009a) Bone response to loaded implants with non‐matching implant‐abutment diameters in the canine mandible. Journal of Periodontology 80: 609–617. [DOI] [PubMed] [Google Scholar]
- Cochran, D.L. , Morton, D. & Weber, H.P. (2004) Consensus statements and recommended clinical procedures regarding loading protocols for endosseous dental implants. International Journal of Oral & Maxillofacial Implants 19(Suppl.): 109–113. [PubMed] [Google Scholar]
- Cochran, D.L. , Nummikoski, P.V. , Schoolfield, J.D. , Jones, A.A. & Oates, T.W. (2009b) A prospective multicenter 5‐year radiographic evaluation of crestal bone levels over time in 596 dental implants placed in 192 patients. Journal of Periodontology 80: 725–733. [DOI] [PubMed] [Google Scholar]
- Crespi, R. , Cappare, P. & Gherlone, E. (2009) Radiographic evaluation of marginal bone levels around platform‐switched and non‐platform‐switched implants used in an immediate loading protocol. International Journal of Oral & Maxillofacial Implants 24: 920–926. [PubMed] [Google Scholar]
- Declaration of Helsinki . (2008) World Medical Association. Available at: http://www.wma.net/en/30publications/10policies/b3/ (accessed on 13 June 2015).
- Dursun, E. , Tulunoglu, I. , Canpinar, P. , Uysal, S. , Akalin, F.A. & Tozum, T.F. (2012) Are marginal bone levels and implant stability/mobility affected by single‐stage platform switched dental implants? A comparative clinical study. Clinical Oral Implants Research 23: 1161–1167. [DOI] [PubMed] [Google Scholar]
- Enkling, N. , Johren, P. , Klimberg, V. , Bayer, S. , Mericske‐Stern, R. & Jepsen, S. (2011) Effect of platform switching on peri‐implant bone levels: a randomized clinical trial. Clinical Oral Implants Research 22: 1185–1192. [DOI] [PubMed] [Google Scholar]
- Ericsson, I. , Persson, L.G. , Berglundh, T. , Marinello, C.P. , Lindhe, J. & Klinge, B. (1995) Different types of inflammatory reactions in peri‐implant soft tissues. Journal of Clinical Periodontology 22: 255–261. [DOI] [PubMed] [Google Scholar]
- Esposito, M. , Ekestubbe, A. & Grondahl, K. (1993) Radiological evaluation of marginal bone loss at tooth surfaces facing single Branemark implants. Clinical Oral Implants Research 4: 151–157. [DOI] [PubMed] [Google Scholar]
- Gardner, D.M. (2005) Platform switching as a means to achieving implant esthetics. New York State Dental Journal 71: 34–37. [PubMed] [Google Scholar]
- Guerra, F. , Wagner, W. , Wiltfang, J. , Rocha, S. , Moergel, M. , Behrens, E. & Nicolau, P. (2014) Platform switch versus platform match in the posterior mandible ‐ 1‐year results of a multicentre randomized clinical trial. Journal of Clinical Periodontology 41: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerle, C.H. , Bragger, U. , Burgin, W. & Lang, N.P. (1996) The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clinical Oral Implants Research 7: 111–119. [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L.J. , Darby, I. , Heitz, F. & Chen, S. (2013) Preservation of crestal bone by implant design. A comparative study in minipigs. Clinical Oral Implants Research 24: 243–249. [DOI] [PubMed] [Google Scholar]
- Hermann, J.S. , Buser, D. , Schenk, R.K. , Higginbottom, F.L. & Cochran, D.L. (2000) Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clinical Oral Implants Research 11: 1–11. [DOI] [PubMed] [Google Scholar]
- Hermann, J.S. , Buser, D. , Schenk, R.K. , Schoolfield, J.D. & Cochran, D.L. (2001a) Biologic width around one‐ and two‐piece titanium implants. Clinical Oral Implants Research 12: 559–571. [DOI] [PubMed] [Google Scholar]
- Hermann, J.S. , Schoolfield, J.D. , Schenk, R.K. , Buser, D. & Cochran, D.L. (2001b) Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non‐submerged implants in the canine mandible. Journal of Periodontology 72: 1372–1383. [DOI] [PubMed] [Google Scholar]
- Hurzeler, M. , Fickl, S. , Zuhr, O. & Wachtel, H.C. (2007) Peri‐implant bone level around implants with platform‐switched abutments: preliminary data from a prospective study. Journal of Oral & Maxillofacial Surgery 65: 33–39. [DOI] [PubMed] [Google Scholar]
- Jung, R.E. , Jones, A.A. , Higginbottom, F.L. , Wilson, T.G. , Schoolfield, J. , Buser, D. , Hammerle, C.H. & Cochran, D.L. (2008) The influence of non‐matching implant and abutment diameters on radiographic crestal bone levels in dogs. Journal of Periodontology 79: 260–270. [DOI] [PubMed] [Google Scholar]
- Kielbassa, A.M. , Martinez‐de Fuentes, R. , Goldstein, M. , Arnhart, C. , Barlattani, A. , Jackowski, J. , Knauf, M. , Lorenzoni, M. , Maiorana, C. , Mericske‐Stern, R. , Rompen, E. & Sanz, M. (2009) Randomized controlled trial comparing a variable‐thread novel tapered and a standard tapered implant: interim one‐year results. Journal of Prosthetic Dentistry 101: 293–305. [DOI] [PubMed] [Google Scholar]
- Kim, Y. , Oh, T.J. , Misch, C.E. & Wang, H.L. (2005) Occlusal considerations in implant therapy: clinical guidelines with biomechanical rationale. Clinical Oral Implants Research 16: 26–35. [DOI] [PubMed] [Google Scholar]
- Laurell, L. & Lundgren, D. (2011) Marginal bone level changes at dental implants after 5 years in function: a meta‐analysis. Clinical Implant Dentistry & Related Research 13: 19–28. [DOI] [PubMed] [Google Scholar]
- Lazzara, R.J. & Porter, S.S. (2006) Platform switching: a new concept in implant dentistry for controlling postrestorative crestal bone levels. The International Journal of Periodontics & Restorative Dentistry 26: 9–17. [PubMed] [Google Scholar]
- Lekholm, U. & Zarb, G. (1985) Patient selection and preparation In: Branemark P.I., Zarb G. & Albrektsson T., eds. Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry, 199–209. Chicago, IL: Quintessence. [Google Scholar]
- Misch, C.E. , Perel, M.L. , Wang, H.L. , Sammartino, G. , Galindo‐Moreno, P. , Trisi, P. , Steigmann, M. , Rebaudi, A. , Palti, A. , Pikos, M.A. , Schwartz‐Arad, D. , Choukroun, J. , Gutierrez‐Perez, J.L. , Marenzi, G. & Valavanis, D.K. (2008) Implant success, survival, and failure: the international congress of oral implantologists (icoi) pisa consensus conference. Implant Dentistry 17: 5–15. [DOI] [PubMed] [Google Scholar]
- Prosper, L. , Redaelli, S. , Pasi, M. , Zarone, F. , Radaelli, G. & Gherlone, E.F. (2009) A randomized prospective multicenter trial evaluating the platform‐switching technique for the prevention of postrestorative crestal bone loss. International Journal of Oral & Maxillofacial Implants 24: 299–308. [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. & Eliceiri, K.W. (2012) NIH image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, F. , Alcoforado, G. , Nelson, K. , Schaer, A. , Taylor, T. , Beuer, F. & Strietzel, F.P. (2014) Impact of implant‐abutment connection, positioning of the machined collar/microgap, and platform switching on crestal bone level changes. Camlog foundation consensus report. Clinical Oral Implants Research 25: 1301–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.E. & Zarb, G.A. (1989) Criteria for success of osseointegrated endosseous implants. The Journal of Prosthetic Dentistry 62: 567–572. [DOI] [PubMed] [Google Scholar]
- Strietzel, F.P. , Neumann, K. & Hertel, M. (2015) Impact of platform switching on marginal peri‐implant bone‐level changes. A systematic review and meta‐analysis. Clinical Oral Implants Research 26: 342–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnow, D.P. , Cho, S.C. & Wallace, S.S. (2000) The effect of inter‐implant distance on the height of inter‐implant bone crest. Journal of Periodontology 71: 546–549. [DOI] [PubMed] [Google Scholar]
- Trammell, K. , Geurs, N.C. , O'Neal, S.J. , Liu, P.R. , Haigh, S.J. , McNeal, S. , Kenealy, J.N. & Reddy, M.S. (2009) A prospective, randomized, controlled comparison of platform‐switched and matched‐abutment implants in short‐span partial denture situations. The International Journal of Periodontics & Restorative Dentistry 29: 599–605. [PubMed] [Google Scholar]
- Vandenbroucke, J.P. , von Elm, E. , Altman, D.G. , Gotzsche, P.C. , Mulrow, C.D. , Pocock, S.J. , Poole, C. , Schlesselman, J.J. , Egger, M. & for the, S.I. (2014) Strengthening the reporting of observational studies in epidemiology (strobe): explanation and elaboration. International Journal of Surgery 12: 1500–1524. [DOI] [PubMed] [Google Scholar]
- Vela‐Nebot, X. , Rodriguez‐Ciurana, X. , Rodado‐Alonso, C. & Segala‐Torres, M. (2006) Benefits of an implant platform modification technique to reduce crestal bone resorption. Implant Dentistry 15: 313–320. [DOI] [PubMed] [Google Scholar]
- Vigolo, P. & Givani, A. (2009) Platform‐switched restorations on wide‐diameter implants: a 5‐year clinical prospective study. International Journal of Oral & Maxillofacial Implants 24: 103–109. [PubMed] [Google Scholar]
- Wang, Y.C. , Kan, J.Y.K. , Rungcharassaeng, K. , Roe, P. & Lozada, J.L. (2015) Marginal bone response of implants with platform switching and non‐platform switching abutments in posterior healed sites: a 1‐year prospective study. Clinical Oral Implants Research 26: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]


