Abstract
Regulatory authorities require that cell lines used in commercial production of recombinant proteins must be derived from a single cell progenitor or clone. The limiting dilution method of cell cloning required multiple rounds of low‐density cell plating and microscopic observation of a single cell in order to provide evidence of monoclonality. Other cloning methods rely on calculating statistical probability of monoclonality rather than visual microscopic observation of cells. We have combined the single cell deposition capability of the Becton Dickinson Influx™ cell sorter with the microscopic imaging capability of the SynenTec Cellavista to create a system for producing clonal production cell lines. The efficiency of single cell deposition by the Influx™ was determined to be 98% using fluorescently labeled cells. The centrifugal force required to settle the deposited cells to the bottom of the microplate well was established to be 1,126g providing a 98.1% probability that all cells will be in the focal plane of the Cellavista imaging system. The probability that a single cell was deposited by the cell sorter combined with the probability of every cell settling into the focal plane of the imager yield a combined >99% probability of documented monoclonality. © 2015 The Authors Biotechnology Progress published by Wiley Periodicals, Inc. on behalf of American Institute of Chemical Engineers Biotechnol. Prog., 31:1172–1178, 2015
Keywords: cell line development, single‐cell cloning, cell imaging, mammalian cell culture, flow cytometry
Introduction
Biopharmaceutical products are expected to be well characterized and consistent in quality to ensure patient safety and drug efficacy. The creation of stable and clonal manufacturing cell lines for biopharmaceuticals is a crucial step toward ensuring reproducible product quality. A substantial number of clones must be screened to find a commercially viable, high producing clone, typicially a time consuming, labor intensive process. Increasing pressure to bring drug products to market rapidly create the need for shortening timelines without compromising product quality.1
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) as well as the United States Food and Drug Administration (FDA) state that for recombinant products, the cell substrate expressing the desired product sequence be cloned from a single cell progenitor.2, 3 The World Health Organization (WHO) has recently expanded on the ICH statement, “the cloning procedure should be fully documented, with details of imaging techniques and/or appropriate statistics. For proteins derived from transfection with recombinant plasmid DNA technology, a single fully documented round of cloning is sufficient, provided that product homogeneity and consistent characteristics are demonstrated throughout the production process and within a defined cell age beyond the production process.”4
Limiting dilution and other low cell density plating methods that rely on statistical probabilities to claim monoclonality require multiple serial rounds of cloning to produce a clonal cell line.5, 6 Cell suspensions are not truly Poisson‐distributed due to dividing doublets or clusters of “sticky” cells which make a true single cell suspension difficult to achieve in practice. Vi‐CELL® Cell Viability Analyzer was used to estimate the percentage of single cells in ten in‐house CHO suspension lines during passaging. The percentage of single cells in these shaken cultures ranged from 70% to 93% (data not shown). Thus, although the clonality calculation for limiting dilution and other plating methods seem straightforward, they will overestimate the probability of monoclonality unless cell clusters are taken into consideration. In actuality, without a clear documented image of a single cell, a high probability of monoclonality is difficult to demonstrate.7
Flow cytometry is a process whereby cells are analyzed and sorted based on hydrodynamic focusing phenomenon and specific cellular characteristics. Fluorescence‐activated cell sorting (FACS) enables a single cell to be separated from a cell suspension with a high degree of purity. The cells are hydrodynamically focused into a single cell core stream constrained by an outer sheath fluid flow. The cells are detected in the core stream as they pass from the nozzle into air and through a focused laser beam. As cells pass through the laser beam, light is scattered around and through each cell (refracted and diffracted), which is then collected by a series of optical detectors (photomultiplier tubes). A voltage pulse is created as each cell passes through the laser such that pulse height, area, and width can be measured. Cell clusters result in an increase in pulse width reflecting the time the cells spend in the laser. The pulse width can be used to identify single cells from cell clusters during the sort process.8 The cell sorter utilizes a frequency‐tuned piston to generate uniform droplets of sheath fluid some of which contain cells. A droplet containing a desired single cell is charged by an electro‐static charging process at the time of droplet breakoff. The charged droplet is then directed into collection vessels as it falls past two highly charged deflection plates.8, 9 Fluorescence‐activated cell sorters for deposition of single cells into microculture wells have been used for cloning hybridoma cells for decades.10 More recently, high‐antibody producing CHO‐K1 cell lines have been created and cloned in 12 weeks using FACS by selecting for high intracellular fluorescent reporter expression.11
Although a cell sorter is capable of placing a single cell in a microplate well with a high degree of accuracy and efficiency, verification of a single cell progenitor is expected for regulatory purposes. Verifying that a cell line is derived from a single cell commonly involves a tedious, time‐consuming microscopic examination of all microplate wells. Automating this task with a SynenTec Cellavista cell imager allows photo‐documentation of monoclonality after cell sorting.12
In this current body of work, we have determined the single cell deposition efficiency for a Becton Dickinson (BD) Influx™ cell sorter by quantifying fluorescent beads and suspension cells labeled with a live cell fluorescent stain that were deposited onto slides in an array and 384‐well microplates.13 We have also experimentally determined the centrifugal force necessary to ensure that all cells suspended in the media are located in the focal plane at the microplate well bottom. Imaging all of the cells in a well with a Cellavista cell imager after single cell sorting provides a documented record of monoclonality which meets ICH, FDA, and WHO monoclonality standards in one round of cloning.
Materials and Methods
Cell culture
Eleven suspension GS‐CHO cell populations expressing recombinant proteins (some of which were monoclonal antibodies) were generated in house and maintained in serum‐free media. Ten GS‐CHO cell populations consisting of clonal and nonclonal stable transfectants were maintained in CD‐CHO media (Life Technologies, Carlsbad, CA) supplemented with 50 μM MSX (Sigma‐Aldrich, St. Louis, MO), 1× GSEM supplement (Sigma‐Aldrich), and 50 mg/L dextran sulfate (Sigma‐Aldrich). One nonclonal GS‐CHO cell population was maintained in ProCHO5™ media (Lonza, Walkersville, MD) supplemented with 15 μM MSX (Sigma‐Aldrich, St. Louis, MO), 1× GSEM supplement (Sigma‐Aldrich), 1× HT supplement (Life Technologies), and 50 mg/L dextran sulfate (Sigma‐Aldrich). Myeloma cells were maintained in RPMI media (Life Technologies) supplemented with 10% serum (Life Technologies). Suspension cell cultures were grown at 37°C, 6% CO2, and 120 rpm on an orbital shaking platform incubator.
Before sorting, viable cell density and cell viability were measured with a Vicell automated cell counter (Beckman Coulter, Inc. Brea, CA). Cells were centrifuged at 90g for 10 min, the media was decanted, and cells were resuspended to a concentration of 1 × 106 cells/mL in FACS buffer containing D‐PBS without Ca/Mg at pH 7.2 (Life Technologies), 0.5% recombinant human serum albumin (Sigma‐Aldrich), 5 mM EDTA (Life Technologies), and 25 mM HEPES (Calbiochem, San Diego, CA).
Flow cytometry and cell sorting
The BD Influx™ cell sorter (Becton Dickinson, Franklin Lakes, NJ) used in these experiments was equipped with small particle detection optics and electronics, an air flow‐certified HEPA filtered enclosure, exchangeable gamma‐irradiated fluidics system, accudrop technology for automatic drop delay calculation, a computerized cell deposition unit for precise droplet deposition, and sortware version 1.0.0.6. A single cell deposition efficiency of 87% was stated on the manufacturer specification sheet. Parameters adjusted on the Influx before single cell deposition sorting included; forward scatter area, side scatter area, FITC area, and PE area parameters. Forward scatter pulse width, forward scatter‐area, forward scatter‐width, forward scatter‐height, side scatter‐area, side scatter‐width, and side scatter‐height were used to exclude multiple cell containing droplets and ensure single cells were deposited. Higher acquisition rates will generally increase the likelihood that droplets will contain multiple cells; therefore, low flow rates were kept constant throughout sorting. Flow‐Check™ Fluorospheres (Beckman Coulter, Inc.) were used to perform optical alignment as well as establish sort delay and optimal settings for single cell deposition. Sheath fluid was Dulbecco's‐PBS without Ca/Mg at pH 7.2 (Life Technologies) that was filtered twice through a 0.2 μm filter. The sheath tank and sheath fluid were then autoclaved before use and allowed to come to room temperature. The sheath flow was allowed to equilibrate and form stable droplets for 2 to 4 h. A standard shutdown was performed with 70% ethanol. On the day of sorting, the autoclaved sheath was re‐connected to the instrument and allowed to equilibrate for at least 30 min before optics alignment and sort delay performance measurements.
Cell sorting efficiency quantification
The efficiency of the cell sorter for creating and sorting droplets containing a single fluorescent bead was determined using a suspension of fluorescent beads that were deposited onto glass microscope slides at a frequency of one bead/droplet by the cell sorter. Slides from 13 separate sorts over the span of 1 year were spotted with beads. Each slide had 50 droplets deposited using the automatic cell deposition unit in an array created in sortware 1.0.0.6. Each spot was microscopically examined for the presence of one or more fluorescent beads.
The efficiency of the cell sorter for depositing a single droplet/well of a 384‐well microplate was determined using a suspension of fluorescent beads deposited into an empty 384‐well microplate (Corning, Corning, NY) at a frequency of one bead/droplet/well before sorting cells. Plates from 13 sorts over the span of 1 year were spotted with beads. Random wells from each plate were microscopically examined for the presence of fluorescent beads.
The efficiency of the cell sorter for creating and sorting droplets containing a single fluorescently labeled cell was determined using suspension cells that were deposited onto glass microscope slides by the cell sorter as described above at a frequency of one cell/droplet. The sorter parameters were adjusted with fluorescent beads to sort at a frequency of one bead/droplet before sorting CTG stained cells. Slides from 18 sorts over the span of almost 2 years were spotted with fluorescent cells. Each slide had 50 droplets deposited using the automatic cell deposition unit in an array created in sortware 1.0.0.6. Each spot was microscopically examined for the presence of one or more fluorescently labeled cells.
CellTracker™ Green Labeling
GS‐CHO and myeloma suspension cells were fluorescently labeled with CellTracker™ Green, 5‐chloromethylfluorescein diacetate (CMFDA) (Life Technologies). Diffusion across the live cell membrane allows esterases to hydrolyze the nonfluorescent CMFDA to fluorescent 5‐chloromethylfluorescein, which in turn reacts with intracellular thiol‐containing proteins.14 Cells were fluorescently labeled with 20 to 50 µg/mL of CTG that was reconstituted with dimethyl sulfoxide (DMSO) (Sigma‐Aldrich). Cell suspensions at a concentration of 1 × 106 cells/ml in a sterile tube were mixed with 25 µL of DMSO‐CTG solution. Cells were incubated in a 37°C, 120 RPM shaker for 45 to 60 min. Cells were centrifuged at 90g for 10 min, the media was decanted, and cells were resuspended in 500 µL FACS buffer. The cell suspension was passed through a 12 × 75 mm polystyrene tube with 35 µm cell strainer cap (Becton Dickinson) before sorting.
Centrifugation conditions
The centrifugal force and length of time needed to bring all cells into the focal plane of the automated microscopic imaging system (well bottom) was determined using either an Allegra® X‐12 or X‐15R benchtop centrifuge (Beckman Coulter). Unstained GS‐CHO cells were sorted into 384‐well microplates containing media using the Influx™ to deposit one cell/well. The plates were then centrifuged with varying centrifugal force and time. Each centrifuged plate was scored for the presence or absence of cells on the well bottom. Wells that were determined to have no cells were again examined 24 h later for the presence or absence of cells on the well bottom. The centrifugal force and time was considered insufficient to bring all cells into the focal plane at the well bottom if a well that had been scored as empty on the day of sorting then contained cells after 24 h settling time.
In addition, GS‐CHO cells were sorted into 384‐well microplates to deposit one cell/well after which the plates were centrifuged at 1,126g for 5 min. The plates were examined microscopically for the presence of one or more cells/well noting the position of wells containing cells and empty wells. These plates were examined after 2 weeks of incubation at 37°C, 6% CO2 for cell outgrowth.
Single cell imaging
The SynenTec Cellavista Basic automated microscopic imaging system (SynenTec GmbH, Elmshorn, Germany) used for photographing wells of the 384‐well microplates contained an LED brightfield light source, a laser auto focus system, and a monochrome interlined CCD camera. The 10× microscope objective (NA 0.3, Resolution ∼920 nm ppx) was used for imaging single cells. The CCD camera provided high resolution JPEG images that had a pixel density of 2,048 × 2,048, 4.19 megapixel (1 × 1 binning) with pixel size of 0.72 µm per pixel using the 10× microscope objective. The imager had a laser autofocus system for a single autofocus in the center of each well of the plate before imaging to ensure that a cell in the well bottom focal plane is captured in the image. Well diameter definitions of 2.950 mm were set using the Cellavista Main software to include the interior of the well extending beyond the well walls, where no cells can reside. The number and sequence of exposures acquired for the single cell cloning application was predefined by the Cellavista software to include overlap of images encompassing the defined well diameter. Well images were archived by the Cellavista software, and subsequently copied onto a network server for image verification.
Statistical analysis
Probability of Single Cell per Droplet During Sorting
To account for experimental variability and the number of droplets evaluated (sample size), a one‐sided upper 95% confidence interval for the probability of more than one cell per droplet was calculated as a conservative estimate of the sorting efficiency. The data in Table 1 corresponding to “Fluorescently labeled cell deposition on slides (50 droplets/slide)” was used for this calculation. The confidence interval was calculated using the Wilson method:15
where:
Table 1.
Influx™ Sorting Efficiency of Placing One Cell or Bead per Droplet and Accuracy of Depositing One Droplet per Well
| Efficiency Evaluation Methods | Total Events (Droplets) Containing Beads or Cells | Total Events (Droplets) Containing 1 Bead or Cell | % Events with ≥2 Cells or Beads/Well or Droplet (Nonclonal) | % Events with 1 Cell or Bead/Well or Droplet (Clonal) |
|---|---|---|---|---|
| Fluorescent bead deposition on slides (50 droplets/slide) | 1,482 | 1,475 | 0.5 | 99.5 |
| Fluorescent bead deposition in 384‐well plates (50 wells/plate) | 337 | 337 | 0.0 | 100.0 |
| Fluorescently labeled cell deposition on slides (50 droplets/slide) | 2,275 | 2241 | 1.5 | 98.5 |
N is the total number of observed droplets
is the 1 − αth percentile of the standard normal distribution
1 − α is the target confidence level
is the observed proportion of cells/droplet
Probability of Cell Deposition into Focal Plane of Cellavista Imager
To account for experimental variability and the number of wells evaluated (sample size), a one‐sided upper 95% confidence interval on the probability of observing additional cells settling over time was calculated as a conservative estimate of the deposition efficiency. The probability that all cells in the well are deposited into the focal plane of the Cellavista imager was estimated by combining the results in Tables 2 and 3 at 1,126g. This confidence interval was also calculated using the Wilson method:
where:
Table 2.
Determination of Centrifugal Force Necessary to Settle Cells into the Cellavista Imager Focal Plane
| Centrifugal Force (g) | Centrifugation Time (min) | Empty Wells on Day of Sorting | Empty Wells 24‐h Postsorting | Wells with Newly Settled Cells |
|---|---|---|---|---|
| 233 | 10 | 66 | 43 | 23 |
| 524 | 10 | 80 | 53 | 27 |
| Total | 146 | 96 | 50 | |
| 931 | 5 | 37 | 37 | 0 |
| 931 | 5 | 10 | 10 | 0 |
| 931 | 5 | 68 | 66 | 2 |
| Total | 115 | 113 | 2 | |
| 1,126 | 5 | 55 | 55 | 0 |
| 1,126 | 5 | 35 | 35 | 0 |
| Total | 90 | 90 | 0 |
Table 3.
Verification that Centrifugation at 1,126g for 5 min Allows all Cells to be Captured in the Cellavista Images
| Centrifugal Force (g) | Centrifugation Time (min) | Empty Wells on Day of Sorting | Empty Wells Containing Cell Outgrowth | Percentage of Wells with Cell Outgrowth |
|---|---|---|---|---|
| 1,126 | 5 | 13 | 0 | 17 |
| 1,126 | 5 | 1 | 0 | 22 |
| 1,126 | 5 | 29 | 0 | 16 |
| 1,126 | 5 | 5 | 0 | 25 |
| Total | 48 | 0 |
N is the total number of observed wells
is the 1 − αth percentile of the standard normal distribution
1 − α is the target confidence level
is the observed proportion of cells/well
The probability of having these two events occur was determined by the probability of having greater than one cell per droplet, P(d), multiplied by the probability of a cell not settling and deposited into the focal plane for imaging, P(i):
Results and Discussion
Single cell sorting efficiency studies
The studies examining the efficiency of the Influx™ cell sorter are summarized in Table 1. These studies used a suspension of 10 µm fluorescent beads as a surrogate system for a single cell suspension. In order to determine the efficiency of the cell sorter for creating droplets containing a single fluorescent bead, a suspension of fluorescent beads were deposited onto glass microscope slides at a frequency of one bead/droplet by the cell sorter software. Droplets were deposited onto glass microscope slides at a frequency of one bead/droplet by the cell sorter and examined microscopically for accuracy. The frequency of the sorter placing only one bead/droplet was determined to be 99.5% from 1,482 droplets examined on 34 glass slides during 13 sorts. This efficiency was calculated by dividing the number of droplets containing a single bead by the total number of droplets containing any number of beads.
The second efficiency study involved programming the cell sorter to place one bead/droplet and one droplet/well into a 384‐well microplate to determine the accuracy of droplet deposition into a microplate with a well diameter of <2.950 mm. A suspension of fluorescent beads was deposited into a dry 384‐well microplate and examined microscopically for the number of beads/well. Each plate had random wells examined for the presence of one or more fluorescent beads. The frequency of the sorter placing only one droplet/well was determined to be 100% from 337 wells examined from eight microwell plates during seven sorts. This efficiency was calculated by dividing the number of wells containing a single bead by the total number of wells containing any number of beads.
The third efficiency study examined how accurately our surrogate system of fluorescent beads reflected an actual cell suspension sorted by the Influx™ cell sorter. Since cells in suspension create doublets by cell division, we wanted to ensure that multiple cell clusters were gated out. The sorter parameters were first adjusted with fluorescent beads to sort at a frequency of one bead/droplet to ensure the sorter was operating at a high level of efficiency. Suspension cells from eleven GS‐CHO cell populations and one myeloma cell line were fluorescently labeled with CellTracker™ Green and deposited onto glass microscope slides by the sorter at a frequency of one bead/droplet to determine the efficiency of the cell sorter in creating droplets containing a single cell. Figure 1 illustrates an example of dried spots containing fluorescently labeled cells from the glass slides. The droplets were microscopically examined for the presence of one or more fluorescently labeled cells. No difference was seen in deposition efficiency between cell populations. The frequency of the sorter placing only one cell/droplet was determined to be 98.5% from 2,275 droplets examined on 48 glass slides during 18 sorts. This efficiency was calculated by dividing the number of droplets containing a single cell by the total number of droplets containing any number of cells. When the Influx™ sort parameters are adjusted to operate optimally, a 99.5% efficiency of depositing one bead/droplet can be achieved before sorting fluorescently labeled cells. By using these optimal settings, the sorter had a 98.5% efficiency of depositing one cell/droplet for the cell types examined in this study. Using these observed sample data, statistical analysis showed that at the upper limit of the 95% confidence interval, no more than 2% of the droplets will contain more than one cell.
Figure 1.
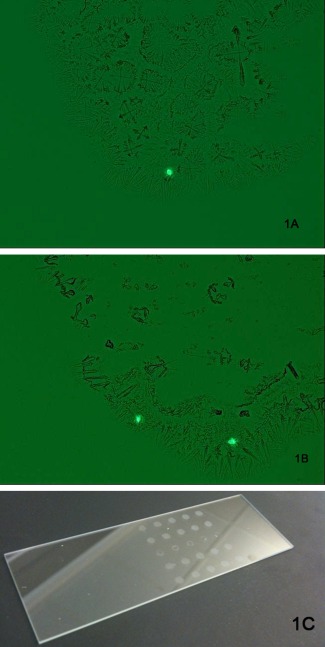
CellTracker™ Green CMFDA labeled cells in a droplet deposited by the Influx™ onto a glass slide.
Representative images of (A) one fluorescent cell and (B) two fluorescent cells from one dried droplet. Fifty droplets were deposited per slide in an array (C) and examined microscopically to determine the number of cells/droplet deposited by the sorter.
where:
N is the total number of observed droplets (n = 2,275)
is the 1 − αth percentile of the standard normal distribution (
1 − α is the target confidence level (1 − α = 0.95)
is the observed proportion of events with ≥2 cells/well from Table 1 using fluorescently labeled cells ( )
Centrifugation studies to settle cells out of suspension
Table 2 summarizes the results from the cell‐settling centrifugation studies performed on 384‐well plates containing cells sorted with the Influx™ cell sorter at one cell/drop/well. These studies determined the centrifugal force necessary to settle all cells out of suspension and deposit them onto the well bottom which is in the focal plane of the automated microscopic imaging system. After centrifugation, wells were examined by microscopy on the day of sorting and again 24 h postsorting for the presence of cells. Centrifugal force of ≤931g was found insufficient to ensure that all cells were located on the well bottom of the 384‐well plates. Increasing the length of time that the plates were centrifuged had no significant effect on bringing the cells out of suspension (data not shown). Based on these results, centrifugation at 1,126g was found to be sufficient to deposit all cells onto the well bottom. Cell outgrowth following centrifugation at 1,126g for 5 min was comparable (less than 5% difference) to cells that were not centrifuged.
Table 3 summarizes the results from observing cell outgrowth after single cell sorting with the Influx™ cell sorter at one cell/well into 384‐well plates. These plates were centrifuged 1,126g for 5 min before imaging the well bottoms with the Cellavista system. The Cellavista well images were examined for the presence or absence of cells. Wells that contained growing cells 2 weeks after sorting were compared to the images taken on the day of sorting. No empty wells on the day of sorting gave rise to growing colonies of cells after 2 weeks incubation. Cell outgrowth following 1,126g centrifugation for these experiments averaged 20%. These results reinforced the conclusion that centrifugation at 1,126g for 5 min was sufficient to deposit all cells onto the well bottom and thus be captured by the Cellavista imaging system.
When the centrifugal force is 1,126g, and the time of centrifugation is 5 min, all of the cells in suspension will settle into the focal plane of the microscopic camera for photo documentation by the Cellavista imaging system. Using these observed sample data, statistical analysis showed that at the upper limit of the 95% confidence interval, no more than 1.9% of the wells will have cells that fail to settle to the well bottom after centrifugation for 5 min at 1,126g.
where:
N is the total number of observed empty wells (n = 138)
is the 1 − αth percentile of the standard normal distribution (
1 − α is the target confidence level (1 − α = 0.95)
is the observed proportion of cells that failed to settle on the well bottom after the initial centrifugation combining all studies performed at 1,126g shown in Tables 2 and 3 ( )
Probability of monoclonality
The probability of monoclonality was estimated by combining the probability of more than one cell per droplet obtained using the Influx™ cell sorter with the probability of cells failing to deposit or settle into the Cellavista focal plane.
Using the observed sample data from Table 1 concerning the efficiency of the BD Influx cell sorter, we have shown that at the upper limit of the 95% confidence interval, no more than 2% of the droplets created by the cell sorter will contain more than one cell.
Using the combined observational sample data in Tables 2 and 3 concerning the probability that all cells in the wells will fail to settle into the camera's focal plane to be captured by photo documentation, we have shown that at the upper limit of the 95% confidence interval, no more than 1.9% of the wells will have cells that fail to settle to the well bottom after centrifugation for 5 min at 1,126g, or that no more than 1.9% of the wells will contain cells in suspension that are not in the focal plane of the imager.
In order for a production line to be derived from a nonclonal population, the cell sorter must deliver more than one cell in a drop and one or more of the cells must fail to settle into the focal plane during centrifugation. Thus, the probability of having these two events occur is: the probability of having greater than one cell per droplet, P(d), multiplied by the probability of a cell not settling into the focal plane for imaging, P(i):
0.020 × 0.019 = 0.00038 or
2.0% × 1.9% = 0.038%
This provides an overall probability of 0.038% that multiple cells will be present in a well without being captured in an image. Therefore, the combined overall probability that a cell will be clonal using single cell deposition by FACS and photo documentation imaging with verification is 99.962%, at the 95% confidence interval.
Automated microscopic single cell imaging
The Cellavista imager must have all cells in the focal plane of the microscopic camera to definitively determine the number of cells that gave rise to each colony. The 384‐well plates have cells in suspension in the media until centrifugation or settling over time brings the cells into the camera's focal plane at the well bottom. At least a 10× microscope objective is needed to be able to distinguish a single cell from a doublet on the well bottom. The Cellavista software automatically ensures that well bottom is in focus and that the images cover the entire well. The high resolution JPEG image files allow magnification for ease of cell identification as cells will appear relatively clear with a white halo as can be seen in Figure 2. The four high resolution digital images of each well are taken, stored electronically and can be used to establish proof of monoclonality.
Figure 2.
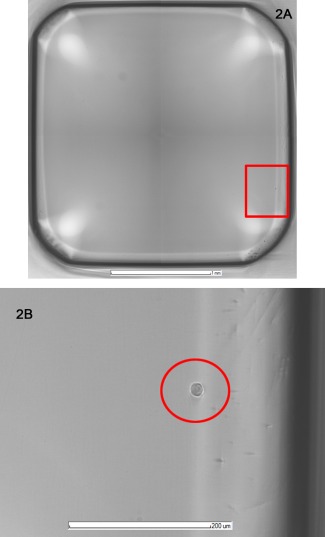
Cellavista image of one well of a 384‐well plate containing a single cell.
Cellavista software imaging parameters were 25 ms exposure time, 60% lamp intensity, 0.028 focus using 10× objective. (A) Cellavista software stitches four JPEG images together for a full well image including the well wall with at least a 3 pixel overlap of images for a well no larger than 2.950 mm diameter (data not shown). (B) Magnified image of a cell with white halo is used for identification and verification of monoclonality.
Verification of monoclonality
The well images of the 384‐well plates used for cell cloning that are photographed by the Cellavista microscopic imaging system were examined for the presence of a single cell by two trained observers. Stringent image analysis was performed visually by each observer independent of the second observer. Monoclonality analysis of the four high quality JPEG images for each well must clearly depict no more than a single cell that was obvious and in focus, cannot have large debris present that could obscure another cell, and cannot have cell‐sized debris that could be mistaken for a cell. When the well images meet these criteria, and both observers agreed from the images that only one cell was present in the well at the time of cloning, the cell line derived from this well was determined to be clonal and may be chosen as a commercial cell line.
Conclusions
The desire to bring drugs to the clinic quickly without sacrificing quality, consistency, or safety requires the use of technologically advanced methods to replace traditional methods where feasible. Demonstrating a high degree of monoclonality for production cell lines as required by regulatory agencies ensures drug product homogeneity. There are many methods available for creating monoclonal cell lines, each with its benefits and deficiencies. Here we utilize the single cell deposition capability of the Influx™ cell sorter and the monoclonality documentation of the Cellavista imager, in order to reap the benefits of combining these technologies while minimizing their individual deficits. We have established sorting and centrifugation conditions that result in high confidence of having a single cell per well while cloning. By using the visualization capability of the Cellavista imager, we can eliminate those wells containing more than one cell from proceeding forward to production, thereby resulting in >99% probability of a single‐cell progenitor for production cell lines. This cloning methodology has been implemented and successfully used for ten production cell line development campaigns. Beyond the 99.962% statistical probability of monoclonality provided by this system, the high resolution well images taken by the Cellavista are subjected to stringent image analysis performed visually by two independent observers. If the well images meet our image quality criteria, and both observers agree from the images that only one cell was present in the well at the time of cloning, the cell line derived from this well is determined to be clonal. Thus, the images create a documented record of monoclonality which meets ICH, FDA, and WHO monoclonality standards in one round of cloning.
Literature Cited
- 1. Browne SM, Al‐Rubeai M. Selection methods for high‐producing mammalian cell lines. Trends Biotechnol 2007; 25:425–432. [DOI] [PubMed] [Google Scholar]
- 2. International Conference on Harmonization . ICH Harmonised Tripartite Guideline: Derivation and Characterization of Cell Substrates used for Production of Biotechnological/Biological Products Q5D International Conference on Harmonization; 1997. Published Federal Register. 1998; 63.182:50244-50249. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5D/Step4/Q5D_Guideline.pdf. Accessed May 15, 2014. [PubMed]
- 3. Center for Biologics Evaluation and Research (CBER) U.S . Supplement to the Points to Consider in the Production and Testing of New Drugs and Biologic Produced by Recombinant DNA Technology: Nucleic Acid Characterization and Genetic Stability U.S. Food and Drug Administration; 1992. Available at: http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatory information/otherrecommendationsformanufacturers/ucm062777.pdf. Accessed May 15, 2014.
- 4. World Health Organization . Guidelines on the Quality, Safety, and Efficacy of Biotherapeutic Protein Products Prepared by Recombinant DNA Technology. Annex 3 in WHO Expert Committee on Biological Standardization Sixty‐First Report. WHO Technical Report Series, No. 978 WHO Press, World Health Organization; 2013. Available at: http://www.who.int/biologicals/expert_committee/TRS_978_61st_report.pdf. Accessed May 15, 2014.
- 5. Lefkovits I, Waldman H. Limiting Dilution Analysis of Cells in the Immune System. Cambridge, NY: Cambridge University Press; 1979. [Google Scholar]
- 6. Coller HA, Coller BS. Poisson statistical analysis of repetitive subcloning by the limiting dilution technique as a way of assessing hybridoma monoclonality. Methods Enzymol. 1986; 121:412–417. [DOI] [PubMed] [Google Scholar]
- 7. Underwood P, Bean P. Hazards of the limiting‐dilution method of cloning hybridomas. J Immunol Methods 1988; 107:119–128. [DOI] [PubMed] [Google Scholar]
- 8. Battye FL, Light A, Tarlinton DM. Single cell sorting and cloning. J Immunol Methods 2000; 243:25–32. [DOI] [PubMed] [Google Scholar]
- 9. Lee G, Hung C, Ke B, Huang G, Hwei B, Lai H. Hydrodynamic focusing for a micromachined flow cytometer. J Fluids Eng Trans ASME. 2001; 123:672–679. [Google Scholar]
- 10. Parks DR, Bryan VM, Oi VT, Herzenberg LA. Antigen‐specific identification and cloning of hybridomas with a fluorescence‐activated cell sorter. Proc Natl Acad Sci USA 1979; 76:1962–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sleiman RJ, Gray PP, McCall MN, Codamo J, Sunstrom NS. Accelerated cell line development using two‐color fluorescence activated cell sorting to select highly expressing antibody‐producing clones. Biotechnol Bioeng. 2008; 99:578–587. [DOI] [PubMed] [Google Scholar]
- 12. Lang P, Yeow K, Nichols A, Scheer A. Cellular imaging in drug discovery. Nat Rev Drug Discover. 2006; 5:343–355. [DOI] [PubMed] [Google Scholar]
- 13. Stovel R, Sweet R. Individual cell sorting. J Histochem Cytochem. 1979; 27:284–288. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y‐Z, Olson N, Mao F, Roth B, Haugland RP. New fluorescent probes for long‐term tracing of living cells. FASEB J. 1992; 6:1835. [Google Scholar]
- 15. Agresti A. Categorical Data Analysis, 2nd ed. New York: Wiley‐Interscience, 2002:16. [Google Scholar]


