Summary
Diabetes affects 10–15% of the surgical population and patients with diabetes undergoing surgery have greater complication rates, mortality rates and length of hospital stay. Modern management of the surgical patient with diabetes focuses on: thorough pre‐operative assessment and optimisation of their diabetes (as defined by a HbA1c < 69 mmol.mol−1); deciding if the patient can be managed by simple manipulation of pre‐existing treatment during a short starvation period (maximum of one missed meal) rather than use of a variable‐rate intravenous insulin infusion; and safe use of the latter when it is the only option, for example in emergency patients, patients expected not to return to a normal diet immediately postoperatively, and patients with poorly controlled diabetes. In addition, it is imperative that communication amongst healthcare professionals and between them and the patient is accurate and well informed at all times. Most patients with diabetes have many years of experience of managing their own care. The purpose of this guideline is to provide detailed guidance on the peri‐operative management of the surgical patient with diabetes that is specific to anaesthetists and to ensure that all current national guidance is concordant.
-
What other guideline statements are available on this topic?
The NHS Diabetes Guideline for the Peri‐operative Management of the Adult Patient with Diabetes 1 was published in 2011 by NHS Diabetes (now part of NHS Improving Quality), and is due to be updated in 2015.
-
Why was this guideline developed?
This guideline was developed to improve both the safety and the outcome of patients with diabetes undergoing surgical procedures.
-
How and why does this statement differ from existing guidelines?
The 2011 guideline 1 deals with the whole patient pathway, from referral for surgery from primary care to discharge and follow‐up. It is too broad in scope to be a working document for anaesthetists. This guideline summarises the existing guideline and focuses specifically on peri‐operative care.
Introduction
The demographics describing the dramatic increase in the number of patients with diabetes are well known. Patients with diabetes require surgical procedures more frequently and have longer hospital stays than those without the condition 2. The presence of diabetes or hyperglycaemia in surgical patients has been shown to lead to increased morbidity and mortality, with peri‐operative mortality rates up to 50% greater than the non‐diabetic population 2. The reasons for these adverse outcomes are multifactorial, but include: failure to identify patients with diabetes or hyperglycaemia 3, 4; multiple co‐morbidities including microvascular and macrovascular complications 5; complex polypharmacy and insulin prescribing errors 6; increased peri‐operative and postoperative infections 2, 7, 8; associated hypoglycaemia and hyperglycaemia 2; a lack of, or inadequate, institutional guidelines for management of inpatient diabetes or hyperglycaemia 2, 9; and inadequate knowledge of diabetes and hyperglycaemia management amongst staff delivering care 10.
Anaesthetists and other peri‐operative care providers should be knowledgeable and skilled in the care of patients with diabetes. Management of diabetes is a vital element in the management of surgical patients with diabetes. It is not good enough for the diabetic care to be a secondary, or sometimes forgotten, element of the peri‐operative care package.
Previous guidelines
In April 2011 NHS Diabetes (now part of NHS Improving Quality) published a document: NHS Diabetes Guideline for the Peri‐operative Management of the Adult Patient with Diabetes, in association with the Joint British Diabetes Societies (JBDS) 1 (an almost identical version, Management of Adults with Diabetes Undergoing Surgery and Elective Procedures: Improving Standards, is available at www.diabetologists-abcd.org.uk/JBDS/JBDS.htm). This comprehensive guideline provided both background information and advice to clinicians caring for patients with diabetes. Some of the recommendations in that document were due for review in the light of new evidence and, in addition, it was felt that anaesthetists and other practitioners caring for patients with diabetes in the peri‐operative period needed shorter, practical advice. The Association of Anaesthetists of Great Britain and Ireland (AAGBI) offered to co‐author this shortened guideline, in collaboration with colleagues involved with the 2011 document. The previous 2011 NHS Diabetes guidelines will also be updated in 2015.
The risks of poor diabetic control
Studies have shown that high pre‐operative and peri‐operative glucose and glycated haemoglobin (HbA1c) levels are associated with poor surgical outcomes. These findings have been seen in both elective and emergency surgery including spinal 11, vascular 12, colorectal 13, cardiac 14, 15, trauma 16, breast 17, orthopaedic 18, neurosurgical, and hepatobiliary surgery 19, 20. One study showed that the adverse outcomes include a greater than 50% increase in mortality, a 2.4‐fold increase in the incidence of postoperative respiratory infections, a doubling of surgical site infections, a threefold increase in postoperative urinary tract infections, a doubling in the incidence of myocardial infarction, and an almost twofold increase in acute kidney injury 2. Paradoxically, there are some data to show that the outcomes of patients with diabetes may not be different from, or may indeed be better than, those without diabetes if the diagnosis is known before surgery 21. The reasons for this are unknown, but may be due to increased vigilance surrounding glucose control for those with a diagnosis of diabetes.
Referral from primary care and planning surgery
The aim is to ensure that diabetes is as well controlled as possible before elective surgery and to avoid delays to surgery due to poor control. The Working Party supports the consensus advice published in the 2011 NHS Diabetes guideline that the HbA1c should be < 69 mmol.mol−1 (8.5%) for elective cases 1, and that elective surgery should be delayed if it is ≥ 69 mmol.mol−1, while control is improved. Changes to diabetes management can be made concurrently with referral to ensure the patient's diabetes is as well controlled as possible at the time of surgery. Elective surgery in patients with diabetes should be planned with the aim of minimising disruption to their self‐management.
Recommendation: Glycaemic control should be checked at the time of referral for surgery. Information about duration, type of diabetes, current treatment and complications should be made available to the secondary care team.
Surgical outpatient clinic
The adequacy of diabetes control should be assessed again at the time of listing for surgery, ideally with a recorded HbA1c < 69 mmol.mol−1 in the previous three months. If it is ≥ 69 mmol.mol−1, elective surgery should be delayed while control is improved. In a small number of cases it may not be possible to improve diabetic control pre‐operatively, particularly if the reason for surgery, such as chronic infection, is contributing to poor control, or if surgery is semi‐urgent. In these circumstances, it may be acceptable to proceed with surgery after explanation to the patient of the increased risks. Patients should be managed as a day case if the procedure is suitable and the patient fulfils the criteria for day‐case surgery management. Well‐controlled diabetes should not be a contra‐indication to day‐case surgery.
Patients with poorly controlled diabetes at the time of surgery will need close monitoring and may need to start a variable‐rate intravenous insulin infusion (VRIII).
Recommendation: Patients with diabetes should be identified early in the pre‐operative pathway.
Pre‐operative assessment
Appropriate and early pre‐operative assessment should be arranged. A pre‐operative assessment nurse may undertake the assessment with support from either an anaesthetist or a diabetes specialist nurse. It should occur sufficiently in advance of the planned surgery to ensure optimisation of glycaemic control before the date of proposed surgery. The aim is to ensure that all relevant investigations are available and checked in advance of the planned surgery, that the patient understands how to manage his/her diabetes in the peri‐operative period, and that the period of pre‐operative fasting is minimised.
Recommendation: Tests should be ordered to assess co‐morbidities in line with National Institute for Health and Care Excellence (NICE) guidance on pre‐operative testing 22. This should include urea and electrolytes and ECG for all patients with diabetes; however, a random blood glucose measurement is not indicated.
Planning admission (including day surgery)
The aim is to minimise the fasting period, ensure normoglycaemia (capillary blood glucose (CBG) 6–10 mmol.l−1) and minimise as far as possible disruption to the patient's usual routine. Ideally, the patient should be booked first on the operating list to minimise the period of fasting. If the fasting period is expected to be limited to one missed meal, the patient can be managed by modification of his/her usual diabetes medication (see below). Patients should be provided with written instructions from the pre‐operative assessment team about management of their diabetes medication on the day of surgery, the management of hypo‐ or hyperglycaemia in the peri‐operative period, and the likely effects of surgery on their diabetes control. Patients should be advised to carry a form of glucose that they can take in case of symptoms of hypoglycaemia that will not cause surgery to be cancelled, for example a clear, sugar‐containing drink (glucose tablets may be used instead, but some anaesthetists may feel they should not be taken within 6 h of the start of anaesthesia). Patients should be warned that their blood glucose control may be erratic for a few days after the procedure.
Recommendation: When possible, admission should be planned for the day of surgery, with both the patient and the ward staff aware of the planned peri‐operative diabetes care, including a plan to manage hypo‐ and hyperglycaemia. Surgery should be scheduled at the start of the theatre list to minimise disruption to the patient's glycaemic control.
Management of existing therapy
With appropriate guidance, patients with diabetes should be allowed to retain control and possession of, and continue to self‐administer, their medication. Many patients will have several years' experience and be expert in self‐medication.
The aim is to avoid hypo‐ or hyperglycaemia during the period of fasting and the time during and after the procedure, until the patient is eating and drinking normally. In people who are likely to miss one meal only, this can often be achieved by manipulating the patient's normal medication using the guidance provided in Tables 1 and 2.
Table 1.
Guideline for peri‐operative adjustment of insulin (short starvation period – no more than one missed meal)
| Insulin | Day before admission | Day of surgery | Whilst a VRIII is being useda | |
|---|---|---|---|---|
| Surgery in the morning | Surgery in the afternoon | |||
| Once daily (e.g. Lantus®, Levemir®, Tresiba®, Insulatard®, Humulin I®, Insuman®) | ||||
| Evening | Reduce dose by 20% | Check blood glucose on admission | Check blood glucose on admission | Continue at 80% of usual dose |
| Morning | Reduce dose by 20% | Reduce dose by 20%; check blood glucose on admission | Reduce dose by 20%; check blood glucose on admission | Continue at 80% of usual dose |
| Twice daily | ||||
| Biphasic or ultra‐long acting (e.g. Novomix 30®, Humulin M3®, Humalog Mix 25®, Humalog Mix 50®, Insuman® Comb 25, Insuman® Comb 50, Levemir®, Lantus®) by single injection, given twice daily | No dose change | Halve the usual morning dose; check blood glucose on admission; leave evening meal dose unchanged | Halve the usual morning dose; check blood glucose on admission; leave the evening meal dose unchanged | Stop until eating and drinking normally |
| Short‐acting (e.g. animal neutral, Novorapid®, Humulin S®, Apidra®) and intermediate‐acting (e.g. animal isophane, Insulatard®, Humulin I®, Insuman®) by separate injections, both given twice daily | No dose change | Calculate total dose of morning insulin(s); give half as intermediate‐acting only in the morning; check blood glucose on admission; leave evening meal dose unchanged | Calculate total dose of morning insulin(s); give half as intermediate‐acting only in the morning; check blood glucose on admission; leave evening meal dose unchanged | Stop until eating and drinking normally |
| Three to five injections daily | ||||
| No dose change |
Basal bolus regimens: Omit morning and lunchtime short‐acting insulins; keep basal unchangeda Premixed morning insulin: Halve morning dose and omit lunchtime dose; check blood glucose on admission |
Give usual morning insulin dose(s); omit lunchtime dose; check blood glucose on admission | Stop until eating and drinking normally | |
If the patient requires a VRIII then the long‐acting background insulin should be continued but at 80% of the dose the patient usually takes when he/she is well.
VRIII, variable‐rate intravenous insulin infusion.
Table 2.
Guideline for peri‐operative adjustment of oral hypoglycaemic agents (short starvation period – no more than one missed meal)
| Agent | Day before admission | Day of surgery | Whilst a VRIII is being used | |
|---|---|---|---|---|
| Surgery in the morning | Surgery in the afternoon | |||
| Drugs that require omission when fasting owing to risk of hypoglycaemia | ||||
| Meglitinides (e.g. repaglinide, nateglinide) | Take as normal | Omit morning dose if nil by mouth | Give morning dose if eating | Stop until eating and drinking normally |
| Sulphonylurea (e.g. glibenclamide, gliclazide, glipizide) | Take as normal | Omit morning dose (whether taking once or twice daily) | Omit (whether taking once or twice daily) | Stop until eating and drinking normally |
| Drugs that require omission when fasting owing to risk of ketoacidosis | ||||
| SGLT‐2 inhibitorsa (e.g. dapagliflozin, canagliflozin) | No dose change | Halve the usual morning dose; check blood glucose on admission; leave evening meal dose unchanged | Halve the usual morning dose; check blood glucose on admission; leave the evening meal dose unchanged | Stop until eating and drinking normally |
| Drugs that may be continued when fasting | ||||
| Acarbose | Take as normal | Omit morning dose if nil by mouth | Give morning dose if eating | Stop until eating and drinking normally |
| DPP‐IV inhibitors (e.g. sitagliptin, vildagliptin, saxagliptin, alogliptin, linagliptin) | Take as normal | Take as normal | Take as normal | Stop until eating and drinking normally |
| GLP‐1 analogues (e.g. exenatide, liraglutide, lixisenatide) | Take as normal | Take as normal | Take as normal | Take as normal |
| Metformin (procedure not requiring use of contrast mediab) | Take as normal | Take as normal | Take as normal | Stop until eating and drinking normally |
| Pioglitazone | Take as normal | Take as normal | Take as normal | Stop until eating and drinking normally |
Also omit the day after surgery.
If contrast medium is to be used or the estimated glomerular filtration rate is under 60 ml.min−1.1.73 m−2, metformin should be omitted on the day of the procedure and for the following 48 h.
VRIII, variable‐rate intravenous insulin infusion; SGLT‐2, sodium‐glucose co‐transporter‐2; DPP‐IV, dipeptidyl peptidase‐IV; GLP‐1, glucagon‐like peptide‐1.
Glycaemic control in patients with diabetes is a balance between their carbohydrate intake and utilisation (for example, exercise). It also depends on what medication they take and how those medications work. Some agents (e.g. sulphonylureas, meglitinides, insulin and to some extent, thiazolidinediones) act by lowering glucose concentrations, and doses need to be modified or the agents stopped during periods of starvation. Others work by preventing glucose levels from rising (e.g. metformin, glucagon‐like peptide‐1 analogues, dipeptidyl peptidase‐4 inhibitors); these drugs may be continued without the risk of hypoglycaemia. The time of day and the expected duration of the operation need to be considered, as will whether a VRIII will be needed. Patients with continuous subcutaneous insulin infusions only missing one meal should be advised to maintain their CBG at 6–10 mmol.l−1. If longer periods of starvation are predicted, a VRIII should be used and specialist advice sought.
Tables 1 and 2 have been designed to take all of these factors into consideration. They are a pragmatic approach to the pre‐operative management of all the available classes of agent used to manage diabetes.
Use of a variable‐rate intravenous insulin infusion
Variable‐rate intravenous insulin infusions are preferred in: patients who will miss more than one meal; those with type‐1 diabetes undergoing surgery who have not received background insulin; those with poorly controlled diabetes (defined as a HbA1c > 69 mmol.mol−1); and most patients with diabetes requiring emergency surgery. Variable‐rate intravenous insulin infusions should be administered and monitored by appropriately experienced and qualified staff. An example of a VRIII regimen is provided in Appendix 1.
Intra‐operative care and monitoring
The aim of intra‐operative care is to maintain good glycaemic control and normal electrolyte concentrations, while optimising cardiovascular function and renal perfusion. If possible, multimodal analgesia should be used along with appropriate anti‐emetic prophylaxis, to enable an early return to a normal diet and the patient's usual diabetes regimen.
Recommendation: An intra‐operative CBG range of 6–10 mmol.l−1 should be aimed for (an upper limit of 12 mmol.l−1 may be tolerated at times, e.g. if the patient has poorly controlled diabetes and is being managed by a modification of his/her normal medication without a VRIII). It should be understood by all staff that a CBG within the range of 6–10 mmol.l−1 is acceptable and that there is no requirment for a CBG of 6 mmol.l−1 to be the target. The CBG should be checked before induction of anaesthesia and monitored regularly during the procedure (at least hourly, or more frequently if the results are outside the target range). The CBG, insulin infusion rate and substrate infusion should be recorded on the anaesthetic record. Some charts use colour‐coded areas to highlight abnormal results requiring further intervention or a change of treatment (see Appendix 2).
Management of intra‐operative hyperglycaemia and hypoglycaemia
If the CBG exceeds 12 mmol.l−1 and insulin has been omitted, capillary blood ketone levels should be measured if possible (point‐of‐care devices are available). If the capillary blood ketones are > 3 mmol.l−1 or there is significant ketonuria (> 2+ on urine sticks) the patient should be treated as having diabetic ketoacidosis (DKA). Diabetic ketoacidosis is a triad of ketonaemia > 3.0 mmol.l−1, blood glucose > 11.0 mmol.l−1, and bicarbonate < 15.0 mmol.l−1 or venous pH < 7.3. Diabetic ketoacidosis is a medical emergency and specialist help should be obtained from the diabetes team.
If DKA is not present, the high blood glucose should be corrected using subcutaneous insulin (see below) or by altering the rate of the VRIII (if in use). If two subcutaneous insulin doses do not work, a VRIII should be started.
Treatment of hyperglycaemia in a patient with type‐1 diabetes
Subcutaneous rapid‐acting insulin (such as Novorapid®, Humalog® or Apidra®) should be given (up to a maximum of 6 IU), using a specific insulin syringe, assuming that 1 IU will drop the CBG by 3 mmol.l−1. Death or severe harm as a result of maladministration of insulin, including failure to use the specific insulin syringe, is a ‘Never Event’. If the patient is awake, it is important to ensure that the patient is content with proposed dose (patients may react differently to subcutaneous rapid‐acting insulin). The CBG should be checked hourly and a second dose considered only after 2 h.
Treatment of hyperglycaemia in a patient with type‐2 diabetes
Subcutaneous rapid‐acting insulin 0.1 IU.kg−1 should be given (up to a maximum of 6 IU), using a specific insulin syringe. The CBG should be checked hourly and a second dose considered only after 2 h. A VRIII should be considered if the patient remains hyperglycaemic.
Treatment of intra‐operative hypoglycaemia
For a CBG 4.0–6.0 mmol.l−1, 50 ml glucose 20% (10 g) should be given intravenously; for hypoglycaemia < 4.0 mmol.l−1 a dose of 100 ml (20 g) should be given.
Fluid management
There is a limited evidence base for the recommendation of optimal fluid management of the adult diabetic patient undergoing surgery. It is now recognised that Hartmann's solution is safe to administer to patients with diabetes and does not contribute to clinically significant hyperglycaemia 23.
Fluid management for patients requiring a VRIII
The aim is to provide glucose as a substrate to prevent proteolysis, lipolysis and ketogenesis, as well as to optimise intravascular volume status and maintain plasma electrolytes within the normal range. It is important to avoid iatrogenic hyponatraemia from the administration of hypotonic solutions. Glucose 5% solution should be avoided. Use of glucose 4% in 0.18% saline can be associated with hyponatraemia.
The substrate solution to be used should be based on the patient's current electrolyte concentrations. While there is no clear evidence that one type of balanced crystalloid fluid is better than another, half‐strength ‘normal’ saline combined with glucose is, theoretically, a reasonable compromise to achieve these aims. Thus, the initial fluid should be glucose 5% in saline 0.45% pre‐mixed with either potassium chloride 0.15% (20 mmol.l−1) or potassium chloride 0.3% (40 mmol.l−1), depending on the presence of hypokalaemia (< 3.5 mmol.l−1).
The Working Party recognises that these fluids may not be available in all institutions. It is our view that they should be made available in all areas where patients with diabetes will be managed. (Hospitals caring for children will usually have these solutions already available for general paediatric use).
Fluid should be administered at the rate that is appropriate for the patient's usual maintenance requirements – usually 25–50 ml.kg−1.day−1 (approximately 83 ml.h−1 for a 70‐kg patient) 24.
Very occasionally, the patient may develop hyponatraemia without signs of fluid overload. In these circumstances, it is acceptable to prescribe one of the following solutions as the substrate solution: glucose 5% in saline 0.9% with pre‐mixed potassium chloride 0.15% (20 mmol.l−1); or glucose 5% in saline 0.9% with pre‐mixed potassium chloride 0.3% (40 mmol.l−1). (Again, hospitals caring for children will usually have these solutions available).
Additional Hartmann's solution or another balanced isotonic crystalloid solution should be used to optimise intravascular volume status.
Fluid management for patients not requiring a VRIII
The aim is to avoid glucose‐containing solutions unless the blood glucose is low. It is important to avoid hyperchloraemic metabolic acidosis; Hartmann's solution should be administered to optimise the intravascular volume status. If the patient requires prolonged postoperative fluids (> 24 h), a VRIII should be considered and glucose 5% in saline 0.45% with pre‐mixed potassium chloride given as above.
Returning to ‘normal’ (pre‐operative) medication and diet
The postoperative blood glucose management plan, and any alterations to existing medications, should be clearly communicated to ward staff. Patients with diabetes should be involved in planning their postoperative care. If subcutaneous insulin is required in insulin‐naïve patients, or the type of insulin or the time it is to be given is to change, the specialist diabetes team should be contacted for advice.
Transferring from a VRIII back to oral treatment or subcutaneous insulin
If the patient has type‐1 diabetes and a VRIII has been used, it must be continued for 30–60 min after the patient has had their subcutaneous insulin (see below). Premature discontinuation is associated with iatrogenic DKA.
Restarting oral hypoglycaemic medication
Oral hypoglycaemic agents should be recommenced at pre‐operative doses once the patient is ready to eat and drink; withholding or reduction in sulphonylureas may be required if the food intake is likely to be reduced. Metformin should only be restarted if the estimated glomerular filtration rate exceeds 50 ml.min−1.1.73 m−2 25.
Restarting subcutaneous insulin for patients already established on insulin
Conversion to subcutaneous insulin should commence once the patient is able to eat and drink without nausea or vomiting. The pre‐surgical regimen should be restarted, but may require adjustment because the insulin requirement may change as a result of postoperative stress, infection or altered food intake. The diabetes specialist team should be consulted if the blood glucose levels are outside the acceptable range (6–12 mmol.l−1) or if a change in diabetes management is required.
The transition from intravenous to subcutaneous insulin should take place when the next meal‐related subcutaneous insulin dose is due, for example with breakfast or lunch.
For the patient on basal and bolus insulin
There should be an overlap between the end of the VRIII and the first injection of subcutaneous insulin, which should be given with a meal and the intravenous insulin and fluids discontinued 30‐60 min later.
If the patient was previously on a long‐acting insulin analogue such as Lantus®, Levemir® or Tresbia®, this should have been continued and thus the only action should be to restart his/her usual rapid‐acting insulin at the next meal as outlined above. If the basal insulin was stopped, the insulin infusion should be continued until a background insulin has been given.
For the patient on a twice‐daily, fixed‐mix regimen
The insulin should be re‐introduced before breakfast or before the evening meal, and not at any other time. The VRIII should be maintained for 30‐60 min after the subcutaneous insulin has been given.
For the patient on a continuous subcutaneous insulin infusion
The subcutaneous insulin infusion should be recommenced at the patient's normal basal rate; the VRIII should be continued until the next meal bolus has been given. The subcutaneous insulin infusions should not be re‐started at bedtime.
Resumption of normal diet
The key to successful management of the surgical patient with diabetes is resumption of his/her usual diet. This allows resumption of normal diabetes medication. Hospital discharge is only feasible once the patient has resumed eating and drinking.
Other anaesthetic considerations
Use of dexamethasone
Dexamethasone can lead to hyperglycaemia and should be used with caution in patients with diabetes 26. If it is decided that the possible benefits of earlier resumption of normal diet outweigh the disadvantages of hyperglycaemia, it should be ensured that the CBG is measured hourly for at least 4 h after administration.
Use of regional anaesthesia
Local anaesthetic techniques, when used as the sole anaesthetic, reduce the risk of postoperative nausea and vomiting. Furthermore, when used as part of the multimodal technique in general anaesthesia, they have opioid‐sparing actions, with resultant reduction of opioid‐related side‐effects. However, evidence suggests that patients with diabetes are more prone to epidural abscesses and haemodynamic instability after central neuraxial blockade (in patients with an autonomic neuropathy), and, possibly, increased risk of neuropathy after peripheral nerve blocks 27, 28, 29.
Enhanced recovery after surgery
Enhanced recovery of patients undergoing surgery utilises several strategies to promote earlier resumption of normal diet, earlier mobilisation, and reduced length of stay 30. This has particular relevance for patients with diabetes as it promotes eating and drinking (reducing the risk of iatrogenic harm from a VRIII), and by promoting earlier discharge.
Use of oral carbohydrate loading drinks
Carbohydrate loading may compromise blood glucose control and should not be used in patients with insulin‐treated diabetes who are likely to have a short period of fasting. There is some evidence that oral carbohydrate loading may be safe in patients with type‐2 diabetes 31, and if a VRIII is to be used in any case there is no reason to withhold oral carbohydrate loading drinks in any patient with diabetes.
Patients with diabetes requiring emergency surgery
There are three ways of managing diabetes in the patient requiring emergency surgery:
Modification of normal medication. This is only possible if the patient is physiologically well and is being operated on a scheduled list, for example a regular trauma list for minor hand surgery, or evacuation of retained products of conception on a regular gynaecology list.
Use of a VRIII. This should be the default technique to manage a patient undergoing emergency surgery because of the unpredictability of the starvation period caused by the erratic nature of emergency lists.
Use of a fixed‐rate intravenous insulin infusion (see below). This should only be used if the patient requires immediate surgery and has concurrent DKA.
The aim is for the patient to be taken to the operating theatre with a CBG of 6–10 mmol.l−1 (6–12 mmol.l−1 may be acceptable), without overt DKA, and having been adequately resuscitated.
Recommendation: In patients undergoing emergency surgery, the CBG should be checked regularly (hourly as a minimum whilst acutely unwell), and a VRIII established using dextrose 5% in saline 0.45% with pre‐mixed potassium chloride as the substrate.
The patient should be checked for ketonaemia (> 3.0 mmol.l−1) or significant ketonuria (> 2+ on urine sticks) if the CBG exceeds 12 mmol.l−1. If the patient has DKA and requires emergency surgery, the involvement of senior multidisciplinary input from intensivists, anaesthetists, surgeons and diabetologists should be considered to agree optimal peri‐operative management. Operating on a patient with DKA carries significant mortality and should be avoided if at all possible. The discussion should include: the requirement for surgery, because DKA can be associated with abdominal pain (negative laparotomies have been reported); the area in which the patient should be resuscitated before theatre; whether saline 0.9% with pre‐mixed potassium chloride or Hartmann's solution should be the main resuscitation fluid; whether a VRIII or a fixed‐rate infusion should be used to treat DKA in theatre; and the area in which the patient will be recovered after surgery. Recent guidelines and evidence suggest that a fixed‐rate intravenous insulin infusion is superior to a VRIII in treating DKA in a ward environment 32. However, use of a fixed‐rate infusion is associated with hypoglycaemia and requires administration of glucose 20% if the CBG is under 14 mmol.l−1.
Early involvement of the diabetes inpatient specialist team should be sought.
If possible, long‐acting insulins (Levemir, Lantus, Tresiba) should be continued in all patients at 80% of the usual dose. This is to avoid rebound hyperglycaemia when intravenous insulin is stopped.
Safety
Errors in insulin prescribing are common and insulin has been identified as one of the top five high‐risk medications in the inpatient environment 33. The wide range of preparations and devices available for insulin administration (currently > 60) increases the potential for error. One third of all inpatient medical errors leading to death within 48 h of the error involve insulin administration. Common errors include mistakes when abbreviating the word ‘units’, failure to use a specific insulin syringe, and errors when preparing insulin infusions. Use of pre‐filled syringes of insulin for infusion may reduce the risk and should be considered by Trusts.
Safe use of VRIIIs
Variable‐rate intravenous insulin infusions are over‐used in the peri‐operative setting. Their use is associated with hypoglycaemia, hyperglycaemia and ketosis on cessation, and hyponatraemia. There seems to be a significant risk of hypoglycaemia in patients with a CBG of 4–6 mmol.l−1 and on a VRIII. Patients often return to surgical wards from theatre with an intravenous insulin infusion in place but no directions for its withdrawal. Hospitals should have written guidelines for both the safe use of the VRIII and conversion from the VRIII to the usual diabetes treatment.
To ensure a steady supply of substrate it is recommended that glucose 5% in saline 0.45% with potassium chloride 0.15% or 0.3% should be administered concurrently with the VRIII, at a rate to meet the patient's maintenance fluid requirements. It is imperative that there is hourly monitoring of the CBG (to keep the CBG at 6–10 mmol.l−1), and that in patients with type‐1 diabetes, the VRIII is not discontinued until alternative subcutaneous insulin has been administered.
Metformin
A number of guidelines available for the use of metformin recommend withdrawing treatment peri‐operatively. However, evidence for this approach is lacking and there is some evidence that peri‐operative continuation of metformin is safe. Metformin is renally excreted and renal failure may lead to high plasma levels which are associated with an increased risk of lactic acidosis 34. Metformin should be withheld if there is pre‐existing renal impairment or significant risk of the patient developing acute kidney injury. Anaesthetists should be vigilant about the dangers of the use of nephrotoxic agents, including contract media 35.
Non‐steroidal anti‐inflammatory drugs (NSAIDs)
There are several additional considerations to the use of NSAIDs in patients with diabetes, including the redistribution of renal blood flow in the presence of hypovolaemia and the risk of oedema, especially if given concurrently with metformin and glitazones.
Quality control and audit
A number of bodies have published quality standards pertinent to the peri‐operative care of patients with diabetes. These include NICE, the Royal College of Anaesthetists (RCoA) and NHS Diabetes (the latter no longer exists and the Joint British Diabetes Societies Inpatient Care Group has taken over some of its roles).
National Institute for Health and Care Excellence
Quality Standard 6, Diabetes in Adults, includes an auditable standard relating to inpatient care: “People with diabetes admitted to hospital are cared for by appropriately trained staff, provided with access to a specialist diabetes team, and given the choice of self‐monitoring and managing their own insulin” 36.
Royal College of Anaesthetists
The RCoA's Guidelines for the Provision of Anaesthetic Services 37 contains much advice relating to the peri‐operative care of patients, including those with diabetes. In addition, the RCoA publication Raising the Standard – a Compendium of Audit Recipes contains details on audit and quality improvement topics pertinent to patients with diabetes 38. These focus on peri‐operative care and are suitable for use by anaesthetists wishing to assess the quality of local provision. For example, topics include availability of guidelines for, and management of, chronic medication during the peri‐operative period, and peri‐operative fasting.
NHS Diabetes
The NHS Diabetes guideline contains a number of suggested audit standards covering medication, safety, training and institutional issues 1.
National Diabetes Inpatient Audit
Whilst the audit standards suggested above may be used within an organisation, national audit projects provide a broader picture and allow benchmarking. The National Diabetes Inpatient Audit (NaDIA) is an annual snapshot audit of surgical and medical inpatients with diabetes. The majority of Trusts in England and Wales are now taking part. The audit is commissioned by the Healthcare Quality Improvement Partnership as part of the National Clinical Audit and Patient Outcomes Programme. The results are available online in the spring of each year and enable organisations to assess their performance in caring for inpatients with diabetes 39.
Included in the audit are questions relating to whether diabetes management minimises the risk of avoidable complications, harm resulting from the inpatient stay, patient experience and the change in patient feedback on the quality of care since NaDIA began. Of particular relevance to anaesthetists, NaDIA includes comparative data on the prevalence of medication errors, intravenous insulin infusions, glycaemic control and patient harm. In addition, there are considerable data from patients' feedback questionnaires concerning their experience and involvement in their own care.
Recommendation: Anaesthetists interested in peri‐operative diabetes management should access the National Diabetes Inpatient Audit and look up the results for their own organisation.
Competing interests
No external funding and no competing interests declared.
Acknowledgements
This guideline and the text are based in large part on the guidelines available from the JBDS 1. The Working Party and AAGBI are grateful to the JBDS Inpatient Care Group for permission to use its document as the basis for this guideline.
Appendix 1.
Example of a variable‐rate intravenous insulin infusion prescription. Glucose concentration is usually measured in capillary blood
| Glucose concentration; mmol.l−1 | Standard rate (use unless otherwise indicated) | Reduced rate (e.g. insulin‐sensitive patients (i.e. < 24 IU.day−1)) | Increased rate (e.g. insulin‐resistant patients (i.e. > 100 IU.day−1)) | |||
|---|---|---|---|---|---|---|
| No basal insulin | Basal insulin continued | No basal insulin | Basal insulin continued | No basal insulin | Basal insulin continued | |
| < 4 | 0.5 IU.h−1 + give 100 ml glucose 20% intravenously | STOP + give 100 ml glucose 20% intravenously | 0.2 IU.h−1 + give 100 ml glucose 20% intravenously | STOP + give 100 ml glucose 20% intravenously | 0.5 IU.h−1 + give 100 ml glucose 20% intravenously | STOP + give 100 ml glucose 20% intravenously |
| 4.1–6.0 | 0.5 IU.h−1 + consider 50 ml glucose 20% intravenously | STOP + consider 50 ml glucose 20% intravenously | 0.2 IU.h−1 + give 50 ml glucose 20% intravenously | STOP + consider 50 ml glucose 20% intravenously | 0.5 IU.h−1 + give 50 ml glucose 20% intravenously | STOP + consider 50 ml glucose 20% intravenously |
| 6.1–8.0 | 1 IU.h−1 | 1 IU.h−1 | 0.5 IU.h−1 | 0.5 IU.h−1 | 2 IU.h−1 | 2 IU.h−1 |
| 8.1–12.0 | 2 IU.h−1 | 2 IU.h−1 | 1 IU.h−1 | 1 IU.h−1 | 4 IU.h−1 | 4 IU.h−1 |
| 12.1–16.0 | 4 IU.h−1 | 4 IU.h−1 | 2 IU.h−1 | 2 IU.h−1 | 6 IU.h−1 | 6 IU.h−1 |
| 16.1–20.0 | 5 IU.h−1 | 5 IU.h−1 | 3 IU.h−1 | 3 IU.h−1 | 7 IU.h−1 | 7 IU.h−1 |
| 20.1–24.0 | 6 IU.h−1 | 6 IU.h−1 | 4 IU.h−1 | 4 IU.h−1 | 8 IU.h−1 | 8 IU.h−1 |
| > 24.1 | 8 IU.h−1 | 8 IU.h−1 | 6 IU.h−1 | 6 IU.h−1 | 10 IU.h−1 | 10 IU.h−1 |
| > 24.1 | Ensure insulin is running and that the measured blood glucose concentration is not artefactual | |||||
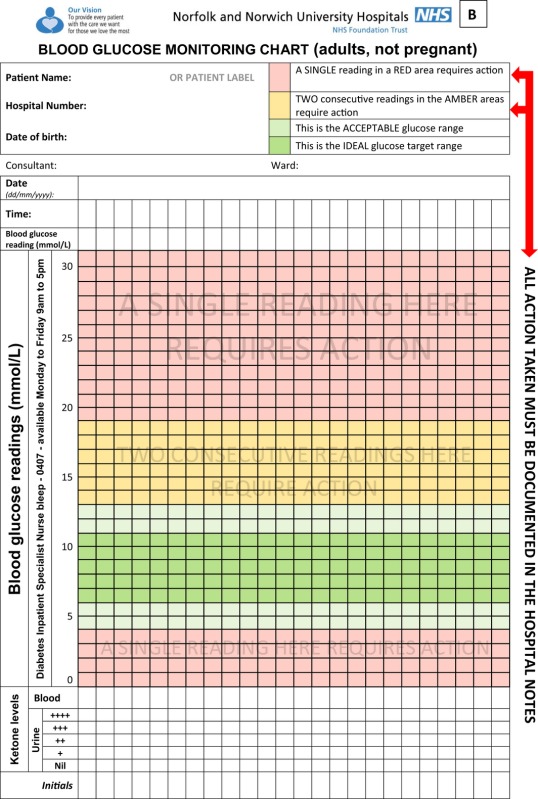
Appendix 2.
Example of a variable‐rate intravenous insulin infusion regimen observation chart. Reproduced by kind permission of the Norfolk and Norwich University Hospitals NHS Foundation Trust
This is a consensus document produced by members of a Working Party established by the Association of Anaesthetists of Great Britain and Ireland (AAGBI). It has been seen and approved by the AAGBI Board of Directors. Date of review: 2020.
References
- 1. Dhatariya K, Levy N, Kilvert A, et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabetic Medicine 2012; 29: 420–33. [DOI] [PubMed] [Google Scholar]
- 2. Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33: 1783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rayman G. Inpatient audit. Diabetes Update. https://www.diabetes.org.uk/upload/Professionals/publications/Comment_Inpatient%20audit_new.pdf (accessed 11/08/2015).
- 4. Hamblin PS, Topliss DJ, Chosich N, Lording DW, Stockigt JR. Deaths associated with diabetic ketoacidosis and hyperosmolar coma, 1973‐1988. Medical Journal of Australia 1989; 151: 441–2. [DOI] [PubMed] [Google Scholar]
- 5. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–44. [DOI] [PubMed] [Google Scholar]
- 6. National Patient Safety Agency . Insulin safety. Reducing harm associated with the unsafe use of insulin products. http://www.nrls.npsa.nhs.uk/resources/collections/10-for-2010/insulin/?entryid45=74287 (accessed 11/08/2015).
- 7. Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearce RM. Mortality and utilisation of critical care resources amongst high‐risk surgical patients in a large NHS trust. Anaesthesia 2008; 63: 695–700. [DOI] [PubMed] [Google Scholar]
- 8. Pearce RM, Harrison DA, James P, et al. Identification and characterisation of the high‐risk surgical population in the United Kingdom. Critical Care 2006; 10: R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sampson MJ, Brennan C, Dhatariya K, Jones C, Walden E. A national survey of in‐patient diabetes services in the United Kingdom. Diabetic Medicine 2007; 24: 643–9. [DOI] [PubMed] [Google Scholar]
- 10. George JT, Warriner D, McGrane DJ, et al. Lack of confidence among trainee doctors in the management of diabetes: the Trainees Own Perception of Delivery of Care (TOPDOC) Diabetes Study. Quarterly Journal of Medicine 2011; 104: 761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walid MS, Newman BF, Yelverton JC, et al. Prevalence of previously unknown elevation of glycosylated hemoglobin in spine surgery patients and impact on length of stay and total cost. Journal of Hospital Medicine 2010; 5: E10–4. [DOI] [PubMed] [Google Scholar]
- 12. O'Sullivan CJ, Hynes N, Mahendran B, et al. Haemoglobin A1c (HbA1C) in non‐diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? European Journal of Vascular and Endovascular Surgery 2006; 32: 188–97. [DOI] [PubMed] [Google Scholar]
- 13. Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. British Journal of Surgery 2009; 96: 1358–64. [DOI] [PubMed] [Google Scholar]
- 14. Halkos ME, Lattouf OM, Puskas JD, et al. Elevated preoperative hemoglobin A1c level is associated with reduced long‐term survival after coronary artery bypass surgery. Annals of Thoracic Surgery 2008; 86: 1431–7. [DOI] [PubMed] [Google Scholar]
- 15. Alserius T, Anderson RE, Hammar N, Nordqvist T, Ivert T. Elevated glycosylated haemoglobin (HbA1c) is a risk marker in coronary artery bypass surgery. Scandinavian Cardiovascular Journal 2008; 42: 392–8. [DOI] [PubMed] [Google Scholar]
- 16. Kreutziger J, Schlaepfer J, Wenzel V, Constantinescu MA. The role of admission blood glucose in outcome prediction of surviving patients with multiple injuries. Journal of Trauma – Injury, Infection and Critical Care 2009; 67: 704–8. [DOI] [PubMed] [Google Scholar]
- 17. Vilar‐Compte D, Alvarez de Iturbe I, Martin‐Onraet A, et al. Hyperglycemia as a risk factor for surgical site infections in patients undergoing mastectomy. American Journal of Infection Control 2008; 36: 192–8. [DOI] [PubMed] [Google Scholar]
- 18. Shibuya N, Humphers JM, Fluhman BL, Jupiter DC. Factors associated with nonunion, delayed union, and malunion in foot and ankle surgery in diabetic patients. Journal of Foot and Ankle Surgery 2013; 52: 207–11. [DOI] [PubMed] [Google Scholar]
- 19. Chuang SC, Lee KT, Chang WT, et al. Risk factors for wound infection after cholecystectomy. Journal of the Formosan Medical Association 2004; 103: 607–12. [PubMed] [Google Scholar]
- 20. Ambiru S, Kato A, Kimura F, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato‐biliary‐pancreatic cancer: a prospective study in a high‐volume institute in Japan. Journal of Hospital Infection 2008; 68: 230–3. [DOI] [PubMed] [Google Scholar]
- 21. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the surgical care and outcomes assessment program. Annals of Surgery 2013; 257: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence . Preoperative Tests: the Use of Routine Preoperative Tests for Elective Surgery. NICE Guideline CG3, June 2003 www.nice.org.uk/guidance/cg3 (accessed 11/08/2015).
- 23. Simpson AK, Levy N, Hall GM. Peri‐operative i.v. fluids in diabetic patients – don't forget the salt. Anaesthesia 2008; 63: 1043–5. [DOI] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence . Intravenous fluid therapy in adults in hospital. NICE guideline CG174, December 2013. www.nice.org.uk/guidance/cg174/evidence (accessed 11/08/2015). [PubMed]
- 25. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild‐to‐moderate renal insufficiency. Diabetes Care 2011; 34: 1431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid‐induced insulin resistance. Endocrinology and Metabolism Clinics of North America 2014; 43: 75–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Royal College of Anaesthetists . Major complications of central neuraxial block in the UK. Third National Audit of The Royal College of Anaesthetists, January 2009. www.rcoa.ac.uk/nap3 (accessed 11/08/2015).
- 28. Hebl JR, Kopp SL, Schroeder DR, Horlocker TT. Neurologic complications after neuraxial anesthesia or analgesia in patients with pre‐existing peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesthesia and Analgesia 2006; 103: 1294–9. [DOI] [PubMed] [Google Scholar]
- 29. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta‐analysis of 915 patients. Neurosurgical Review 2000; 23: 175–204. [DOI] [PubMed] [Google Scholar]
- 30. Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta‐analysis of randomized controlled trials. Clinical Nutrition 2010; 29: 434–40. [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson UO, Nygren J, Thorell A, et al. Pre‐operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiologica Scandinavica 2008; 52: 946–51. [DOI] [PubMed] [Google Scholar]
- 32. Joint British Diabetes Societies Inpatient Care Group . The management of diabetic ketoacidosis in adults, September 2013. http://www.diabetologists-abcd.org.uk/JBDS/JBDS_IP_DKA_Adults_Revised.pdf) (accessed 11/08/2015).
- 33. National Patient Safety Agency . The fourth report of the Patient Safety Observatory. Safety in doses: medication safety incidents in the NHS, January 2007. www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety/?entryid45=59822&q=0%c2%acsafety+in+doses%c2%ac (accessed 11/08/2015).
- 34. Duncan AI, Koch CG, Xu M, et al. Recent metformin ingestion does not increase in‐hospital morbidity or mortality after cardiac surgery. Anesthesia and Analgesia 2007; 104: 42–50. [DOI] [PubMed] [Google Scholar]
- 35. Royal College of Radiologists . Standards for Intravascular Contrast Agent Administration to Adult Patients, 2nd edn London: RCR, 2010. [Google Scholar]
- 36. National Institute for Health and Care Excellence . Diabetes in Adults. Quality Standard 6, March 2011. www.nice.org.uk/guidance/qs6 (accessed 11/08/2015).
- 37. Royal College of Anaesthetists . Guidelines for the Provision of Anaesthetic Services 2015. www.rcoa.ac.uk/GPAS2015 (accessed 11/08/2015).
- 38. Royal College of Anaesthetists . Raising the Standard: A Compendium of Audit Recipes for Continuous Quality Improvement in Anaesthesia, 3rd edn, 2012. www.rcoa.ac.uk/system/files/CSQ-ARB-2012_1.pdf (accessed 11/08/2015). [Google Scholar]
- 39. Health and Social Care Information Centre . National Diabetes Inpatient Audit. www.hscic.gov.uk/diabetesinpatientaudit (accessed 11/08/2015).