Abstract
Aim
To confirm the superiority, compared with placebo, of adding liraglutide to pre‐existing basal insulin analogue ± metformin in adults with inadequately controlled type 2 diabetes [glycated haemoglobin (HbA1c) 7.0–10.0% (53–86 mmol/mol)].
Methods
In this 26‐week, double‐blind, parallel‐group study, conducted in clinics or hospitals, 451 subjects were randomized 1 : 1 to once‐daily liraglutide 1.8 mg (dose escalated from 0.6 and 1.2 mg/day, respectively, for 1 week each; n = 226) or placebo (n = 225) added to their pre‐existing basal insulin analogue (≥20 U/day) ± metformin (≥1500 mg/day). After randomization, insulin adjustments above the pre‐study dose were not allowed. The primary endpoint was HbA1c change.
Results
After 26 weeks, HbA1c decreased more with liraglutide [−1.3% (−14.2 mmol/mol)] than with placebo [−0.1% (−1.2 mmol/mol); p < 0.0001]. More subjects on liraglutide reached HbA1c targets: <7.0% (59% vs 14%; p < 0.0001) and ≤6.5% (43% vs 4%; p < 0.0001) using slightly less insulin (35.8 IU vs 40.1 IU). Greater decreases from baseline (estimated treatment differences vs placebo; p < 0.0001) occurred in fasting plasma glucose (−1.3 mmol/l), seven‐point glucose profiles (−1.6 mmol/l), body weight (−3.1 kg) and systolic blood pressure (−5.0 mmHg). Transient gastrointestinal adverse events (nausea: 22.2% vs 3.1%) and minor hypoglycaemia (18.2% vs 12.4%) were more frequent with liraglutide than placebo, and pulse increased (4.5 beats/min) compared with placebo. No severe hypoglycaemia or pancreatitis occurred.
Conclusions
Adding liraglutide to a basal insulin analogue ± metformin significantly improved glycaemic control, body weight and systolic blood pressure compared with placebo. Typical gastrointestinal symptoms and minor hypoglycaemia were more frequent with liraglutide.
Keywords: GLP‐1 analogue, glycaemic control, incretin therapy, insulin therapy, randomised trial, weight loss therapy
Introduction
Achieving and maintaining individualized glycaemic targets, minimizing the risk of hypoglycaemia and preventing complications present challenges in the management type 2 diabetes 1. Most patients with diabetes eventually require insulin therapy 2, 3. The benefits of basal insulin combination therapy have been well documented 4, 5; however, insulin therapy increases the risk of hypoglycaemia and often leads to weight gain 6, leaving an unmet need for additional treatment options that can improve glycaemic control with greater efficacy, safety and convenience.
Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone that augments insulin secretion and suppresses glucagon secretion in a glucose‐dependent manner 7; thus, GLP‐1 receptor agonists decrease fasting plasma glucose (FPG), postprandial glucose (PPG) and body weight, with a low risk of hypoglycaemia 8. These drugs are generally well tolerated, apart from mostly transient gastrointestinal adverse events (AEs) during initiation 2. GLP‐1 receptor agonists may, therefore, fulfill the need for additional antidiabetic therapies.
There is evidence that combination therapy with GLP‐1 receptor agonists and basal insulin has benefits, including weight loss and greater patient satisfaction 9, 10, 11, 12, 13; however, the sequencing of basal insulin versus GLP‐1 receptor agonists is not well established, and different combinations of basal insulin and GLP‐1 receptor agonists have not been adequately evaluated.
Liraglutide, a once‐daily human GLP‐1 analogue approved in Europe, the USA and other countries, lowers glycated haemoglobin (HbA1c) by up to −1.5% (−16.4 mmol/mol), and induces weight loss with a low risk of hypoglycaemia. Liraglutide also improves lipid and blood pressure profiles, although slight increases in pulse rate have been reported 13, 14, 15, 16, 17, 18, 19, 20. While a large proportion of subjects achieved HbA1c targets with liraglutide, adding insulin detemir to liraglutide further reduced HbA1c in those not achieving glycaemic control with liraglutide alone 21, 22. Moreover, in a study of patients with HbA1c ≥7% (53 mmol/mol), the reverse sequence [adding liraglutide to basal insulin (degludec)] was effective in further lowering HbA1c [0.3% (3.3 mmol/mol) greater than when insulin aspart was added to the largest meal], and also in inducing weight loss, with less hypoglycaemia 23.
The aim of the present study was to investigate the efficacy, tolerability and safety, versus placebo, of adding liraglutide 1.8 mg to a stable dose of basal insulin analogue (glargine or detemir) ± metformin for 26 weeks.
Subjects and Methods
Subjects
This study included men and women (aged 18–80 years) with inadequately controlled type 2 diabetes [HbA1c 7–10% (53–86 mmol/mol)] and body mass index (BMI) 20–45 kg/m2. All subjects were treated with stable doses of basal insulin analogue (glargine or detemir; ≥20 U/day) ± metformin (≥1500 mg/day) for at least 8 weeks before enrolment (Supplemental Data, File S1).
Trial Design and Interventions
The trial was conducted in 76 office‐ or hospital‐based sites in Argentina, Canada, Finland, Germany, India, Mexico, the Netherlands, Serbia and the USA between 10 September 2012 and 22 October 2013. Protocol amendments occurring after the start of the study are described in Supplemental Data of File S1. Protocols, amendments and informed consent documents were approved by local independent ethics committees and the trial was conducted in accordance with Good Clinical Practice 24 and the Declaration of Helsinki 25.
The study was registered with ClinicalTrials.gov (identifier: NCT01617434).
The trial period was 29 weeks: 2 weeks of screening, 26 weeks of treatment and a 1‐week follow‐up period (Figure S1, File S1). Subjects were randomized 1 : 1 to receive a once‐daily dose of liraglutide 1.8 mg or placebo. Randomization was stratified according to screening HbA1c (≤8.0% vs >8.0%), metformin treatment (yes/no) and type of basal insulin analogue (insulin glargine vs insulin detemir). Liraglutide or placebo was initiated at the equivalent dose of 0.6 mg/day and the dose was increased in weekly increments of 0.6 mg to a final dose of 1.8 mg/day, which was then continued unchanged for the remainder of the study. The trial site personnel, subjects and sponsor remained blinded until trial completion.
The study medication (liraglutide or placebo) was injected subcutaneously once daily at a consistent time of day, and was added to the subject's stable pre‐study basal insulin analogue regimen ± metformin; before inclusion in the study, a subject's insulin dose had to remain stable for at least 8 weeks. For subjects with baseline HbA1c ≤8.0%, insulin dose was reduced by 20% at randomization to reduce the potential risk of hypoglycaemia. Up‐titration of insulin to no higher than the pre‐study dose was allowed during weeks 3–8. Otherwise, the insulin dose was kept at a stable, pre‐study dose level throughout the trial. Down‐titration was allowed for any subject at any time if there was a risk of hypoglycaemia (Tables S1–S3, File S1).
Assessments and Endpoints
The primary endpoint of the study was change in HbA1c from baseline to week 26. Secondary efficacy endpoints included the percentage of subjects reaching HbA1c <7 or ≤6.5%, and composite endpoints of HbA1c <7% with no weight gain and/or no hypoglycaemia. The change from baseline to week 26 in FPG (≥8 h without food and drink, except water), seven‐point self‐measured plasma glucose (SMPG) values, basal insulin dose (recorded subject diaries), body weight, BMI, blood pressure, lipids and treatment satisfaction [Diabetes Treatment Satisfaction Questionnaire (DTSQ)] 26 were also included. Safety assessments included AEs, hypoglycaemic episodes and laboratory variables including lipase and amylase levels. Hypoglycaemic episodes were classified according to the American Diabetes Association definition 27 and an additional category of minor hypoglycaemia [plasma glucose <3.1 mmol/l (56 mg/dl) or blood glucose <2.8 mmol/l (50 mg/dl)]. Confirmed hypoglycaemia was defined as minor hypoglycaemia and/or severe hypoglycaemia (a subject being unable to treat him/herself).
Statistical Analysis
The sample size was determined according to that needed to demonstrate the superiority of liraglutide vs placebo with regard to mean change in HbA1c using a significance level of 5% and a two‐sided test. The calculations were based on the assumptions that the mean difference between treatment groups was at least 0.4% and the standard deviation was 1.3%. A sample size of 223 subjects per treatment arm was needed to achieve a power of 90%.
Unless otherwise stated, efficacy endpoints were analysed using the full analysis set (all randomized subjects who received ≥1 dose of trial product and who provided at least one baseline and one post‐baseline efficacy value). Details of the statistical methods are provided in the Statistical Analyses section of file S1.
The safety analysis set included all subjects who received ≥1 dose of liraglutide or placebo. Minor and severe hypoglycaemic episodes were analysed using a negative binomial regression model.
Results
Subject Disposition
Of the 721 subjects screened, 451 were randomized to receive liraglutide (226 subjects) or placebo (225 subjects; Figure S2, File S1). One subject in the liraglutide group was randomized in error and was withdrawn before receiving the study drug. A greater percentage of subjects on liraglutide completed the study (84.5%) compared with those on placebo (77.3%). Twelve out of 225 (5.3%) subjects on liraglutide and 3 out of 225 (1.3%) on placebo withdrew because of an AE; gastrointestinal disorders were the most common AEs leading to withdrawal in the liraglutide group.
Baseline Characteristics
The baseline characteristics of the study population were balanced between the treatment groups except for gender [more women on liraglutide (46.7%) than on placebo (39.6%); Table 1].
Table 1.
Baseline demographics
| Liraglutide 1.8 mg/day | Placebo | |
|---|---|---|
| (n = 225) | (n = 225) | |
| Mean ± s.d. age, years | 59.3 ± 9.2 | 57.5 ± 11.1 |
| Mean ± s.d. duration of diabetes, years | 12.1 ± 7.1 | 12.1 ± 6.8 |
| Women/men, % | 46.7/53.3 | 39.6/60.4 |
| Mean ± s.d. weight, kg | 90.2 ± 20.0 | 91.9 ± 19.3 |
| Mean ± s.d. BMI, kg/m2 | 32.3 ± 5.6 | 32.2 ± 5.7 |
| Mean ± s.d. HbA1c | ||
| % | 8.2 ± 0.8 | 8.3 ± 0.9 |
| mmol/mol | 66.3 ± 8.8 | 67.0 ± 9.8 |
| Mean ± s.d. FPG | ||
| mmol/l | 8.3 ± 2.9 | 8.2 ± 2.9 |
| mg/dl | 149.8 ± 52.0 | 147.8 ± 52.2 |
| Metformin: no/yes, % | 8.0/92.0 | 6.7/93.3 |
| Basal insulin analogue: insulin detemir/insulin glargine, % | 33.3/66.7 | 32.0/68.0 |
| Basal insulin analogue dose* (coefficient of variation), IU | 40.5 (0.61) | 40.5 (0.52) |
BMI, body mass index; FPG, fasting plasma glucose; s.d., standard deviation.
Geometric mean.
Efficacy Endpoints
After 26 weeks of treatment, HbA1c was reduced more with liraglutide than with placebo added to a basal insulin analogue ± metformin [−1.3% (−14.2 mmol/mol) vs −0.1% (−1.2 mmol/mol)], with an estimated treatment difference (ETD) of −1.2% or −13.0 mmol/mol [95% confidence interval (CI) −1.4; −1.0% or −15.2; −10.8 mmol/mol]; p < 0.0001 (Figure 1A). A sensitivity analysis using analysis of covariance with last observation carried forward (LOCF) showed a similar ETD to that obtained by mixed model for repeated measures [−1.1% or −12.0 mmol/mol (95% CI −1.3; −1.0 or −14.2; −10.8 mmol/mol)]; p < 0.0001.
Figure 1.
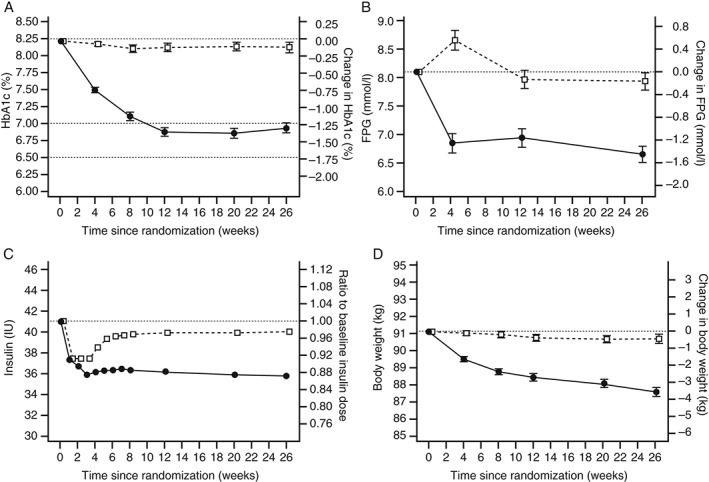
Primary and secondary outcomes from randomization to 26 weeks. Estimated mean change [standard error of the mean (s.e.m.)] in (A) glycated haemoglobin (HbA1c) and (B) fasting plasma glucose (FPG), (C) ratio of the mean basal insulin analogue dose to baseline and (D) estimated mean change (s.e.m.) body weight with liraglutide (closed circles) or placebo (open squares).
Levels of FPG decreased more from baseline to week 26 with liraglutide than with placebo [−1.4 mmol/l vs −0.2 mmol/l; ETD: −1.3 mmol/l (95% CI −1.7; −0.9); p < 0.0001 (Figure 1B)]. Additionally, the liraglutide group had greater decreases in mean glucose derived from seven‐point SMPG profiles [−2.6 mmol/l vs −1.0 mmol/l; ETD: −1.6 mmol/l (95% CI −2.0; −1.2); p < 0.0001] and estimated mean of the PPG increments of the seven‐point SMPG profiles [−0.9 mmol/l vs −0.4 mmol/l; ETD: −0.6 mmol/l (95% CI −0.9; −0.2); p < 0.0001] compared with placebo. Accordingly, more subjects achieved HbA1c targets with liraglutide than with placebo [59.2% vs 14.0% for HbA1c <7% (p < 0.0001); 42.9% vs 3.6% for HbA1c ≤6.5% (p < 0.0001); Table 2]; similar results were seen for all composite endpoints (p < 0.0001 for all).
Table 2.
Additional efficacy endpoints after 26 weeks of treatment
| Liraglutide 1.8 mg | Placebo | Estimated treatment difference or ratio (95% CI) | Estimated odds ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| (n = 225) | (n = 225) | ||||
| Systolic blood pressure, mmHg* | −5.78 | −0.76 | −5.02 | — | <0.0001 |
| (−7.45; −2.59) | |||||
| Diastolic blood pressure, mmHg* | −1.19 | −0.52 | −0.66 | — | 0.41 |
| (−2.25; 0.92) | |||||
| Pulse, beats/min | +3.20 | −1.31 | 4.51 | — | <0.0001 |
| (2.59; 6.43) | |||||
| BMI, kg/m2 * | −1.28 | −0.17 | −1.12 | — | <0.0001 |
| (−1.37; −0.86) | |||||
| Waist circumference, cm* | −3.84 | −0.88 | −2.96 | — | <0.0001 |
| (−4.00; −1.92) | |||||
| Total cholesterol, mmol/l† | 0.92 | 0.99 | 0.93 | — | 0.0002 |
| (0.89; 0.96) | |||||
| LDL cholesterol, mmol/l† | 0.90 | 1.00 | 0.91 | — | 0.0013 |
| (0.86; 0.96) | |||||
| VLDL cholesterol† | 0.89 | 0.99 | 0.91 | — | <0.01 |
| (0.84; 0.97) | |||||
| HDL cholesterol, mmol/l† | 0.97 | 0.99 | 0.98 | — | 0.16 |
| (0.95; 1.01) | |||||
| Triglycerides, mmol/l† | 0.90 | 0.99 | 0.90 | — | <0.01 |
| (0.83; 0.97) | |||||
| Free fatty acids, mmol/l† | 1.02 | 0.99 | 1.03 | — | 0.45 |
| (0.95; 1.12) | |||||
| Percentage of subjects achieving HbA1c <7% | 59.2 | 14.0 | — | 8.91 | <0.0001 |
| (5.45; 14.59) | |||||
| Percentage of subjects achieving HbA1c ≤6.5% | 42.9 | 3.6 | — | 20.12 | <0.0001 |
| (9.92; 40.84) | |||||
| Percentage of subjects achieving HbA1c <7% with no weight gain | 53.5 | 11.4 | — | 8.95 | <0.0001 |
| (5.38; 14.91) | |||||
| Percentage of subjects achieving HbA1c <7% with no hypoglycaemic episodes | 45.8 | 11.4 | — | 6.55 | <0.0001 |
| (3.96; 10.84) | |||||
| Percentage of subjects achieving HbA1c <7% with no weight gain and no hypoglycaemic episodes | 41.5 | 8.6 | 7.50 | <0.0001 | |
| (4.36; 12.92) | |||||
| Overall treatment satisfaction score‡ | 0.49 | 0.11 | 0.37 | — | <0.0001 |
| (0.20; 0.55) | |||||
| Perceived hyperglycaemia score‡ | −1.67 | −0.69 | −0.98 | — | <0.0001 |
| (−1.36; −0.60) | |||||
| Perceived hypoglycaemia score‡ | −0.17 | −0.13 | −0.04 | — | 0.83 |
| (−0.39; 0.31) |
BMI, body mass index; CI, confidence interval; n, number of subjects; VLDL, very‐low‐density lipoprotein.
Estimated means, change from baseline.
Estimated means, ratio to baseline.
Assessed by the Diabetes Treatment Satisfaction Questionnaire.
At baseline, the observed geometric mean basal insulin analogue doses for the liraglutide and placebo groups were 40.5 IU (0.46 IU/kg) and 40.5 IU (0.45 IU/kg), respectively. After 26 weeks of treatment, the ratios of the estimated mean insulin dose to baseline were 0.87 and 0.98 in the liraglutide and placebo groups, respectively. On average, subjects on liraglutide used an 11% lower basal insulin analogue dose at 26 weeks than those on placebo [estimated treatment ratio (ETR): 0.89 (95% CI 0.87; 0.92); p < 0.0001 (Figure 1C)].
From baseline to week 26, body weight was reduced more with liraglutide than with placebo [−3.5 kg vs −0.4 kg; ETD −3.1 kg (95% CI −3.9; −2.4); p < 0.0001 (Figure 1D)], which was accompanied by concomitant decreases in BMI [−1.3 kg/m2 vs −0.2 kg/m2; ETD −1.1 kg/m2 (95% CI −1.4; −0.9); p < 0.0001] and waist circumference [−3.8 cm vs −0.9 cm; ETD −3.0 cm (95% CI −4.0; −1.9); p < 0.0001 (Table 2)]. A greater reduction in systolic blood pressure was seen with liraglutide compared with placebo [−5.8 mmHg vs −0.8 mmHg; ETD −5.0 mmHg (95% CI −7.5; −2.6); p < 0.0001], but no difference was observed between the treatment groups for diastolic blood pressure (Table 2). Statistically significant reductions in total cholesterol, LDL cholesterol, very‐low‐density lipoprotein (VLDL) cholesterol and triglycerides were observed with liraglutide compared with placebo; no statistically significant differences were observed between treatments for HDL cholesterol or free fatty acid levels (Table 2).
Subjects on liraglutide reported higher overall treatment satisfaction (p < 0.0001) as assessed by the DTSQ, with lower perceived hyperglycaemia (p < 0.0001) and similar perceived hypoglycaemia compared with subjects on placebo (p = 0.83; Table 2).
Safety Endpoints
The percentage of subjects reporting AEs was greater with liraglutide than with placebo (69.3% vs 58.2%; Table 3). Gastrointestinal symptoms were the most commonly reported AEs with liraglutide (41.3% vs 16.9%), while infections and infestations were the most commonly reported AEs with placebo (30.7% vs 24.4% for the placebo and liraglutide groups, respectively). Serious AEs (SAEs) were reported by 4.9 and 3.1% of subjects in the liraglutide and placebo groups, respectively. There were significantly more confirmed hypoglycaemic episodes reported with liraglutide than with placebo, with an ETR of 2.00 (95% CI 1.03; 3.89); p = 0.04 (Table 4). The observed rates of confirmed minor hypoglycaemia were also higher for subjects on liraglutide in both HbA1c strata (Table 4). For subjects with baseline HbA1c ≤8.0%, the apparent difference versus placebo was primarily seen during the initial weeks of the trial when up‐titration of insulin was allowed (data not shown). The overall rate of hypoglycaemia according to the American Diabetes Association classifications was also higher with liraglutide than with placebo (640 vs 419 episodes per 100 patient‐years of exposure, respectively). No severe hypoglycaemic episodes were reported in either group during this trial.
Table 3.
Treatment‐emergent adverse events
| Liraglutide 1.8 mg | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 225) | (n = 225) | |||||||
| n | % | E | R | n | % | E | R | |
| Total AEs | 156 | 69.3 | 494 | 4918 | 131 | 58.2 | 370 | 3737 |
| SAEs | 11 | 4.9 | 15 | 149 | 7 | 3.1 | 10 | 101 |
| Malignant neoplasms | 3 | 1.3 | 3 | 30 | 2 | 0.9 | 3 | 30 |
| AEs reported in ≥5% of subjects | ||||||||
| Gastrointestinal disorders | 93 | 41.3 | 177 | 1762 | 38 | 16.9 | 52 | 525 |
| Nausea | 50 | 22.2 | 62 | 617 | 7 | 3.1 | 8 | 81 |
| Vomiting | 20 | 8.9 | 28 | 279 | 2 | 0.9 | 3 | 30 |
| Dyspepsia | 16 | 7.1 | 20 | 199 | 2 | 0.9 | 2 | 20 |
| Diarrhoea | 24 | 10.7 | 29 | 289 | 11 | 4.9 | 14 | 141 |
| Infections and infestations | 55 | 24.4 | 85 | 846 | 69 | 30.7 | 98 | 990 |
| Nasopharyngitis | 13 | 5.8 | 19 | 189 | 14 | 6.2 | 16 | 162 |
| Influenza | 8 | 3.6 | 11 | 110 | 26 | 7.1 | 21 | 212 |
| Musculoskeletal and connective tissue disorders | 28 | 12.4 | 36 | 358 | 37 | 16.4 | 47 | 475 |
| Back pain | 12 | 5.3 | 13 | 129 | 12 | 5.3 | 13 | 131 |
| Metabolism and nutrition disorders | 31 | 13.8 | 31 | 309 | 15 | 6.7 | 32 | 323 |
| Decreased appetite | 22 | 9.8 | 22 | 219 | 5 | 2.2 | 5 | 50 |
| Investigations | 28 | 12.4 | 36 | 358 | 17 | 7.6 | 20 | 202 |
| Lipase increased | 17 | 7.6 | 17 | 169 | 5 | 2.2 | 5 | 50 |
| Nervous system disorders | 19 | 8.4 | 23 | 229 | 23 | 10.2 | 30 | 303 |
| Headache | 8 | 3.6 | 9 | 90 | 16 | 7.1 | 20 | 202 |
%, proportion of subjects who had the specified AE; AE, adverse event; E, number of AEs; n, number of subjects with an AE (adverse event); R, event rate per 1000 patient years of exposure; SAE, serious adverse event.
Table 4.
Confirmed hypoglycaemia
| Liraglutide 1.8 mg | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 225) | (n = 225) | |||||||
| n | % | E | R | n | % | E | R | |
| All subjects* | 41 | 18.2 | 127 | 126 | 28 | 12.4 | 82 | 83 |
| Liraglutide 1.8 mg | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 94) | (n = 95) | |||||||
| n | % | E | R | n | % | E | R | |
| Baseline HbA1c ≤8.0% | 16 | 17.0 | 33 | 77 | 14 | 14.7 | 26 | 61 |
| Liraglutide 1.8 mg | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 131) | (n = 130) | |||||||
| n | % | E | R | n | % | E | R | |
| Baseline HbA1c >8.0% | 25 | 19.1 | 94 | 163 | 14 | 10.8 | 56 | 99 |
All confirmed hypoglycaemic episodes were minor as there were no severe hypoglycaemic episodes in this trial. %, proportion of subjects who had confirmed hypoglycaemic episode; E, number of hypoglycaemic episodes; n, number of subjects with a confirmed hypoglycaemic episode [minor hypoglycaemia (plasma glucose <3.1 mmol/l (56 mg/dl) or blood glucose <2.8 mmol/l (50 mg/dl)) or severe hypoglycaemia (when subject was unable to treat him/herself)]; R, event rate per 100 patient years of exposure.
Treatment ratio for rate of confirmed hypoglycaemic episodes (liraglutide/placebo): 2.00 (95% CI 1.03; 3.89); p = 0.04.
From baseline to week 26, an increase in pulse was observed with liraglutide versus placebo [+3.2 beats/min vs −1.3 beats/min; ETD: 4.5 beats/min (95% CI 2.8; 6.4); p < 0.0001 (Table 2)].
At screening, 17 (7.6%) subjects on liraglutide and 19 (8.4%) subjects on placebo had amylase levels ≥ upper limit of normal (ULN). The estimated mean amylase levels after 26 weeks of treatment were 62.68 and 55.91 U/l in subjects on liraglutide and placebo, respectively. Using a mixed model for repeated measures, the ETR to baseline of amylase levels after 26 weeks was 12% higher with liraglutide than with placebo [1.12 (95% CI 1.07; 1.18); p < 0.0001], although the mean values for amylase stayed within the normal range for both groups throughout the trial. No subject in either treatment group in this study had amylase levels ≥3 × ULN at any visit.
Similar numbers of subjects in both treatment groups had lipase levels ≥ ULN at screening: 24 (10.7%) and 26 (11.6%) subjects on liraglutide and placebo, respectively. The estimated mean lipase levels after 26 weeks of treatment were 50.68 and 35.72 U/l in subjects on liraglutide and placebo, respectively. Using a mixed model for repeated measures, the ETR to baseline of lipase levels after 26 weeks was 42% higher with liraglutide than with placebo [1.42 (95% CI 1.30; 1.55); p < 0.0001], although the mean values for lipase stayed within the normal range for both groups throughout the trial. During the trial, 7 (3.1%) subjects on liraglutide and 3 (1.3%) subjects on placebo had incidental increases in lipase levels to ≥3 × ULN; 1 subject on liraglutide had an incidental increase to ≥10 × ULN. All were transient increases. One subject on liraglutide had elevated lipase in conjunction with abdominal pain, but a computed tomography scan confirmed that there was no pancreatitis. No pancreatitis events were reported during the study.
Additionally, 4 (1.8%) subjects on liraglutide and 3 (1.3%) subjects on placebo had elevated calcitonin levels (≥20 ng/l) during this trial; all but 2 of these subjects (1 in each group) had elevated calcitonin levels (≥20 ng/l) before initiation of treatment. Mean calcitonin values stayed within the normal range for both groups throughout the trial. There were no cases of medullary thyroid carcinomas in this trial.
A blinded external independent event adjudication committee adjudicated events of neoplasms and thyroid disease requiring thyroidectomy (no events of the latter). One colon adenoma, one squamous cell carcinoma of the tongue and one pancreatic cancer (metastatic pancreatic carcinoma) were reported in 3 subjects on liraglutide (Table 3). Two basal cell carcinomas in 1 subject and one brain cancer (brain neoplasm) were reported in 2 subjects on placebo (Table 3). The pancreatic and brain cancer cases were fatal, but both the diagnoses and the deaths occurred approximately ≥2 months after the subjects had been withdrawn from the study. The subject with pancreatic cancer had been on liraglutide for 17.6 weeks before withdrawal from the trial because of unrelated SAEs, while the subject with brain cancer had been on placebo for 5.1 weeks.
No relevant adverse changes in physical examination or ECG were observed in either treatment group.
Discussion
In patients with advanced type 2 diabetes and suboptimum glycaemic control, addition of liraglutide versus placebo to stable doses of basal insulin ± metformin resulted in clinically relevant improvements in glycaemic control that were achieved using slightly lower basal insulin doses. Moreover, liraglutide reduced body weight, systolic blood pressure and selected lipids (total cholesterol, LDL cholesterol, VLDL cholesterol and triglycerides) compared with placebo. In addition, overall scores for the DTSQ improved with liraglutide compared with placebo, indicating greater patient satisfaction. Liraglutide was generally well tolerated, with the AE profile being similar to that reported in previous studies adding liraglutide to oral antidiabetic drugs 14, 15, 28 or insulin 23. Increases in gastrointestinal AEs, minor hypoglycaemia, amylase, lipase and pulse were observed with liraglutide versus placebo.
As type 2 diabetes progresses, many patients eventually require insulin therapy. Although combination of basal insulin analogues with oral antidiabetic drugs can reduce HbA1c by approximately −1.5% (−16.4 mmol/mol), with 50–60% of subjects achieving HbA1c <7% (53 mmol/mol) if insulin is properly titrated 4, 29, many patients on basal insulin regimens do not reach or maintain HbA1c targets. There is significant clinical inertia in the timely initiation or intensification of insulin therapy, partly attributable to risk and fear of associated hypoglycaemia and weight gain 6. The results of the present study suggest that adding liraglutide to basal insulin can reduce HbA1c in patients inadequately controlled by insulin therapy while simultaneously inducing weight loss. These results are consistent with recent studies suggesting that liraglutide can reverse insulin‐associated weight gain 23, 30.
With the present study design, the isolated benefits of liraglutide when added to capped basal insulin analogue ± metformin were proven. Capping the basal insulin analogue dose at a level equal to the pre‐study level is, however, also a limitation of the present study. While the subjects in this study were representative of those who would be found in real‐world experience, capping of insulin dose would not be recommended in routine clinical practice. In this respect, a treat‐to‐target trial design would be needed.
Several studies have investigated combining liraglutide and basal insulin. The improvement in HbA1c seen after 12 weeks of treatment with liraglutide + metformin was further enhanced (−0.5%; −5.5 mmol/mol) by adding insulin detemir over 52 weeks 21, 22. Moreover, intensification of insulin degludec + metformin by adding liraglutide in patients who failed to reach goal with insulin degludec alone resulted in a significantly greater reduction in HbA1c (−0.74% vs −0.39% or −8.1 mmol/mol vs −4.3 mmol/mol), greater weight loss (−2.8 kg vs +0.9 kg), and less confirmed and nocturnal confirmed hypoglycaemia compared with adding a single daily injection of prandial insulin 23.
In the present study, treatment with liraglutide added to insulin glargine or insulin detemir also provided significant and clinically relevant reductions in HbA1c and body weight, with a low incidence of hypoglycaemia, substantiating liraglutide treatment as a valid treatment option in combination with basal insulin in the management of type 2 diabetes. In further support of this notion, albeit an indirect measure, based on comparative glucose‐lowering effect across GLP‐1 receptor agonists, liraglutide ± oral antidiabetic drugs was non‐inferior to dulaglutide for HbA1c reductions 31 and met statistical superiority criteria in head‐to‐head trials against twice‐daily exenatide 18, once‐weekly exenatide 32, once‐weekly albiglutide 33 and sitagliptin 19. Head‐to‐head trials are required to investigate the comparative effectiveness of the various basal insulin analogue–GLP‐1 receptor agonist combination sequences.
Several studies in similar populations have included an active comparator rather than a placebo control. Adding either albiglutide once weekly or insulin lispro three times daily to titrated insulin glargine ± metformin and/or pioglitazone, decreased HbA1c by −0.82% (−9.0 mmol/mol) and −0.66% (−7.2 mmol/mol) after 26 weeks, respectively; weight decreased with albiglutide (−0.7 kg) but increased with insulin lispro three times daily (+0.8 kg) 12. Overall, hypoglycaemia was more frequent with insulin lispro (38% vs 25%); this included two severe episodes with insulin lispro. In patients on optimized insulin glargine and metformin, addition of either exenatide twice daily or insulin lispro three times daily resulted in similar decreases in HbA1c after 30 weeks (−1.1% or −12.0 mmol/mol) while weight decreased by −2.5 kg with exenatide twice daily but increased by +2.1 kg with lispro three times daily; hypoglycaemia was higher with insulin lispro (41% vs 30%), while nausea was greater with exenatide (47% vs 13%) 9. Lack of an active comparator is a limitation in the present study.
Additionally, the study duration of the present study was short; however, most safety and efficacy observations from the first 26 weeks of treatment in recent liraglutide phase IIIb trials were generally confirmed during subsequent 26‐week extensions 22, 28.
Long‐acting GLP‐1 receptor agonists such as liraglutide can induce tachyphylaxis with respect to the effect on delay of gastric emptying. As delayed gastric emptying seems of importance for limiting PPG elevations, this could potentially lead to a dominant effect on FPG levels and a weaker postprandial effect for long‐ versus short‐acting GLP‐1 receptor agonists 8. Nevertheless, the reduction in HbA1c observed when adding liraglutide to basal insulin reported here, which was attributed to reductions in both FPG and PPG, is the same or somewhat greater than that reported for adding the short‐acting GLP‐1 receptor agonist exenatide to basal insulin 10. This is consistent with findings that continuous subcutaneous infusion of recombinant GLP‐1 reduces mean FPG and PPG in a dose‐dependent manner 34, 35.
Insulin use can often induce weight gain. In the present study, the addition of liraglutide to basal insulin analogue resulted in a significant reduction in weight versus placebo. Several mechanisms for liraglutide‐induced weight loss have been identified, including reducing appetite 36, 37 and diminishing leptin loss 38, the latter of which may prevent pre‐diabetes in obesity.
Fewer than 20% of subjects in both groups reported a confirmed hypoglycaemic episode during the trial, and no severe hypoglycaemia was reported in either group. The incidence of confirmed hypoglycaemia, while quite low, was significantly higher with liraglutide than with placebo. The rate of confirmed hypoglycaemia was similar to that reported in trials with liraglutide added to sulphonylurea 18 and sulphonylurea ± metformin 15. The increased incidence of hypoglycaemia with liraglutide, especially in those subjects with baseline HbA1c >8% (64 mmol/mol), might have been attributable to several factors. Doses of basal insulin were not reduced at randomization for subjects with baseline HbA1c >8% (64 mmol/mol), which might have contributed to the increased hypoglycaemia with liraglutide. In addition, improvement in bedtime SMPG with liraglutide might predispose patients to more hypoglycaemia from basal insulin. Finally, basal insulin analogue titration algorithms, based solely on FPG levels, might have also played a role, as the increments of these adjustments might have been too high for some subjects.
Consistent with previous trials with liraglutide, an increase in resting pulse was observed with liraglutide. The clinical significance of this elevation is unknown, but appears to be a class effect of long‐acting GLP‐1 receptor agonists 39, 40, 41. Importantly, published meta‐analyses of the risk of major adverse cardiovascular events with GLP‐1 receptor agonists in type 2 diabetes indicated no increased risk 42, 43, 44, 45.
Elevations in lipase and amylase after week 26 were similar to those reported previously 21, 31, 46, 47. It has been reported that 10–20% of patients with type 2 diabetes not receiving a GLP‐1 receptor agonist can have spontaneous elevations of serum lipase activity 48, a finding that was also observed at baseline in this study. The mechanism underlying these elevations is not known 21, 49. A similar treatment effect has been observed with other GLP‐1 receptor agonists, suggesting a class effect 31, 46. Importantly, no pancreatitis was reported in this trial.
Based on non‐clinical findings that chronic GLP‐1 receptor agonist administration in rodents is associated with increased calcitonin and C‐cell tumour formation, monitoring of calcitonin levels has been implemented for all liraglutide trials. Throughout this trial, mean levels of calcitonin remained within the normal range for both groups, consistent with a previous study of >5000 liraglutide‐treated subjects in whom liraglutide had no clinically significant effect on calcitonin levels 50.
In summary, adding liraglutide to basal insulin therapy is a valuable, effective and safe treatment option for patients with type 2 diabetes, especially when weight loss and risk of hypoglycaemia are major considerations. Risk of hypoglycemia can be minimized by reducing the previous dose of basal insulin at the time that liraglutide is added.
Conflict of Interest
This study was funded by Novo Nordisk. A. A. is a consultant for AstraZeneca, Janssen, MannKind, and Novo Nordisk, and has received grant support from MannKind, Novo Nordisk and Sanofi. H. W. R. has received grants or research support from Amylin, AstraZeneca, Biodel, Bristol‐Myers Squibb, Lilly, Halozyme, Merck, Novartis, Novo Nordisk and Sanofi, has served on advisory boards for Amylin Pharmaceuticals, Roche Diagnostics, AstraZeneca, Biodel, Janssen, Novartis, Novo Nordisk, Sanofi and Takeda; and has served on the speakers bureau for Amylin, Merck, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Janssen, Lilly and Novo Nordisk. J. R. has served on advisory boards, received honoraria or consulting fees, or received research funding from Amylin, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Halozyme Therapeutics, Intarcia Therapeutics, Janssen, Johnson & Johnson, Lexicon, MannKind, Merck, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi and Takeda. J. T. L has received travel grants from MSD, AstraZeneca and Novo Nordisk; has served on the speakers' bureau for Amgen, AstraZeneca, BMS, Lilly, MSD, Novo Nordisk and Sanofi, and is an advisory board member for Amgen, Novo Nordisk, Medtronic and MSD. L. D. is a consultant for Novo Nordisk. K. T. and A. B. are full‐time employees of Novo Nordisk A/S. M. A. N. has served on advisory boards for, received honoraria or consulting fees from, or received research funding from Amylin, AstraZeneca, Berlin‐Chemie, Boehringer Ingelheim, Bristol‐Myers Squibb, Diartis Pharmaceuticals, Eli Lilly, GlaxoSmithKline, F. Hoffmann‐La Roche, Intarcia Therapeutics, Janssen, MannKind, Merck, MetaCure, Novartis, Novo Nordisk, Roche Pharmaceuticals, Sanofi, Takeda, Versartis and Wyeth Research. No other potential conflicts of interest relevant to this article were reported.
A. A. and K. T. planned the trial and determined the trial design. All authors analysed and interpreted data. K. T. implemented the study and oversaw the conduct of the trial. All authors participated in drafting the manuscript and all have approved the final version. M. A. N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funder, Novo Nordisk, participated in trial design and collection, review and analysis of data.
Supporting information
File S1. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial.
Acknowledgements
The authors gratefully acknowledge the contribution of the investigators and their staff and of the subjects in this trial. The authors thank Dr Leanne M. Johnson‐Huang and Dr. John Smith from Novo Nordisk for medical writing support. The authors acknowledge Watermeadow Medical, supported by Novo Nordisk, for providing editing support.
The funder, Novo Nordisk, participated in trial design and collection, review and analysis of data. Liraglutide and insulin detemir are proprietary compounds manufactured and marketed by Novo Nordisk A/S.
References
- 1. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med 2013; 368: 1613–1624. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999; 281: 2005–2012. [DOI] [PubMed] [Google Scholar]
- 4. Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26: 3080–3086. [DOI] [PubMed] [Google Scholar]
- 5. Weng J, Li Y, Xu W. Effect of intensive insulin therapy on beta‐cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel‐group trial. Lancet 2008; 371: 1753–1760. [DOI] [PubMed] [Google Scholar]
- 6. Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes 2010; 4: S11–18. [DOI] [PubMed] [Google Scholar]
- 7. Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol 2009; 297: 127–136. [DOI] [PubMed] [Google Scholar]
- 8. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 9. Diamant M, Nauck MA, Shaginian R et al. Glucagon‐like peptide‐1 receptor agonist or bolus insulin with optimized basal insulin in diabetes. Diabetes Care 2014; 37: 2763–2773. [DOI] [PubMed] [Google Scholar]
- 10. Buse JB, Bergenstal RM, Glass LC et al. Use of twice‐daily exenatide in Basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011; 154: 103–112. [DOI] [PubMed] [Google Scholar]
- 11. Lind M, Jendle J, Torffvit O, Lager I. Glucagon‐like peptide 1 (GLP‐1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes 2011; 6: 41–46. [DOI] [PubMed] [Google Scholar]
- 12. Rosenstock J, Fonseca VA, Gross JL et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care 2014; 37: 2317–2325. [DOI] [PubMed] [Google Scholar]
- 13. Riddle MC, Aronson R, Home P et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care 2013; 36: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zinman B, Gerich J, Buse J et al. Efficacy and safety of the human GLP‐1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD‐4 Met + TZD). Diabetes Care 2009; 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell‐Jones D, Vaag A, Schmitz O et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met + SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marre M, Shaw J, Brandle M et al. Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garber A, Henry R, Ratner R et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 18. Buse JB, Rosenstock J, Sesti G et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 19. Pratley RE, Nauck M, Bailey T et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet 2010; 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 20. Nauck M, Frid A, Hermansen K et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeVries JH, Bain SC, Rodbard HW et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012; 35: 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenstock J, Rodbard HW, Bain SC et al. One‐year sustained glycemic control and weight reduction in type 2 diabetes after addition of liraglutide to metformin followed by insulin detemir according to HbA1c target. J Diabetes Complications 2013; 27: 492–500. [DOI] [PubMed] [Google Scholar]
- 23. Mathieu C, Rodbard HW, Cariou B et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD‐ON). Diabetes Obes Metab 2014; 16: 636–644. [DOI] [PubMed] [Google Scholar]
- 24. International Conference on Harmonisation . ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1), step 4. 6.10.1996.
- 25. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Last amended by the 59th WMA General Assembly, Seoul: 2008. [Google Scholar]
- 26. Bradley C. Diabetes treatment satisfaction questionnaire. Change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care 1999; 22: 530–532. [DOI] [PubMed] [Google Scholar]
- 27. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 28. Pratley R, Nauck M, Bailey T et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel‐group, open‐label trial. Int J Clin Pract 2011; 65: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26‐week, randomized, parallel, treat‐to‐target trial comparing insulin detemir with NPH insulin as add‐on therapy to oral glucose‐lowering drugs in insulin‐naive people with type 2 diabetes. Diabetes Care 2006; 29: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 30. de Wit HM, Vervoort GM, Jansen HJ, de Grauw WJ, de Galan BE, Tack CJ. Liraglutide reverses pronounced insulin‐associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia 2014; 57: 1812–1819. [DOI] [PubMed] [Google Scholar]
- 31. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 32. Buse JB, Nauck M, Forst T et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet 2013; 381: 117–124. [DOI] [PubMed] [Google Scholar]
- 33. Pratley RE, Nauck MA, Barnett AH et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2: 289–297. [DOI] [PubMed] [Google Scholar]
- 34. Torekov SS, Kipnes MS, Harley RE, Holst JJ, Ehlers MR. Dose response of subcutaneous GLP‐1 infusion in patients with type 2 diabetes. Diabetes Obes Metab 2011; 13: 639–643. [DOI] [PubMed] [Google Scholar]
- 35. Torekov SS, Holst JJ, Ehlers MR. Dose response of continuous subcutaneous infusion of recombinant glucagon‐like peptide‐1 in combination with metformin and sulphonylurea over 12 weeks in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 451–456. [DOI] [PubMed] [Google Scholar]
- 36. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond) 2014; 38: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flint A, Kapitza C, Zdravkovic M. The once‐daily human GLP‐1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short‐term treatment. Diabetes Obes Metab 2013; 15: 958–962. [DOI] [PubMed] [Google Scholar]
- 38. Iepsen EW, Lundgren J, Dirksen C et al. Treatment with a GLP‐1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes (Lond) 2015; 39: 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. GlaxoSmithKline, UK . Eperzan (albiglutide) [EU summary of product characteristics]. 2014. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002735/WC500165117.pdf. Accessed 6 June 2015.
- 40. Eli Lilly and Company, Indianapolis . Trulicity (dulaglutide) [US Package Insert]. 2014. Available from URL: http://pi.lilly.com/us/trulicity‐uspi.pdf. Accessed 6 June 2015.
- 41. Amylin Pharmaceuticals, Inc., San Diego . Bydureon (exenatide) [EU summary of product characteristics]. 2011. Available from URL: http://www.astrazeneca‐us.com/cgi‐bin/az_pi.cgi?product=bydureon&country=us&popup=no. Accessed 6 June 2015.
- 42. Best JH, Hoogwerf BJ, Herman WH et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon‐like peptide 1 (GLP‐1) receptor agonist exenatide twice daily or other glucose‐lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care 2011; 34: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon‐like peptide‐1 receptor agonists on cardiovascular risk: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16: 38–47. [DOI] [PubMed] [Google Scholar]
- 44. Ratner R, Han J, Nicewarner D, Yushmanova I, Hoogwerf BJ, Shen L. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol 2011; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Food and Drug Administration Center for Drug Evaluation and Research (CDER) . Medical review: Dulaglutide (Trulicity). 2015.
- 46. Bastyr EJ, Vinik A, Owyang C, Cheng C, Shu J, Hall NC. Surveillance of lipase and amylase levels in type 2 diabetes patients assessed during a randomized clinical study: the EGO study experience. Diabetologia 2009; 52(Suppl. 1): S303–304. [Google Scholar]
- 47. Meier JJ, Rosenstock J, Hincelin‐Mery A et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care 2015; 38: 1263–1673. [DOI] [PubMed] [Google Scholar]
- 48. Steinberg WM, Nauck MA, Zinman B et al. LEADER 3‐lipase and amylase activity in subjects with type 2 diabetes: baseline data from over 9000 subjects in the LEADER trial. Pancreas 2014; 43: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide‐1 receptor agonists or dipeptidyl‐peptidase‐4 inhibitors in the outpatient setting. Endocr Pract 2012; 18: 472–477. [DOI] [PubMed] [Google Scholar]
- 50. Hegedus L, Moses AC, Zdravkovic M, Le TT, Daniels GH. GLP‐1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP‐1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96: 853–860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial.


