Abstract
Objective
Patient‐reported outcomes (PROs) provide an opportunity to collect important information relating to patient well‐being, which is often difficult for physicians to measure (e.g., quality of life, pain, fatigue, and sleep). Here we evaluate the effects of certolizumab pegol (CZP) on PROs during the 24‐week, double‐blind phase of the RAPID axial spondyloarthritis (SpA) trial, a phase 3 trial of axial SpA patients, including both ankylosing spondylitis (AS) and nonradiographic axial SpA patients.
Methods
A total of 325 patients with active axial SpA were randomized 1:1:1 to placebo, CZP 200 mg every 2 weeks, or CZP 400 mg every 4 weeks. The primary end point was the Assessment of SpondyloArthritis International Society criteria for 20% improvement in disease activity response at week 12, and has been reported previously. PROs included total back pain, nocturnal back pain, a daily pain diary, the Sleep Problems Index II (SPI) domain of the Medical Outcomes Study (MOS) Sleep Scale, fatigue, the Ankylosing Spondylitis Quality of Life (ASQOL) measure, and the Short Form 36‐item (SF‐36) health survey physical component summary (PCS), mental component summary (MCS), and domains.
Results
Patients treated with CZP reported significant improvements from week 1 for nocturnal back pain (placebo −0.6, CZP 200 mg every 2 weeks −1.9, and CZP 400 mg every 4 weeks −1.6; P < 0.001) and ASQOL (placebo −1.0, CZP 200 mg every 2 weeks −2.3, and CZP 400 mg every 4 weeks −1.9; P < 0.05) compared with placebo, while significant improvements in total back pain were seen from day 2. Patients treated with both CZP dosing regimens also had significantly greater improvements in fatigue, MOS‐SPI, SF‐36 PCS, MCS, and domains compared with placebo. Improvements were similar in both AS and nonradiographic axial SpA patients.
Conclusion
Both CZP dosing schedules rapidly improved patient well‐being, as measured by PROs, including pain, fatigue, sleep, SF‐36, and ASQOL in both AS and nonradiographic axial SpA patients.
Introduction
Axial spondyloarthritis (SpA) is a chronic inflammatory disease, primarily characterized by inflammation of the sacroiliac joints and spine, resulting in chronic back pain. Axial SpA includes patients with or without definitive radiographic changes in the sacroiliac joints. These subpopulations are known, respectively, as ankylosing spondylitis (AS) 1 and nonradiographic axial SpA 2, 3.
Box 1. Significance & Innovations.
Patient‐reported outcomes (PROs) are important measurements in randomized controlled trials, allowing assessment of health status from the patient's perspective.
Certolizumab pegol improved all PROs measured, including the Short Form 36‐item health survey, health‐related quality of life, pain, fatigue, and sleep.
Similar improvements in PROs were observed in both ankylosing spondylitis and nonradiographic axial spondyloarthritis patients.
Patient‐reported outcomes (PROs), defined as reports of patient health provided directly by the patient, can include instruments that monitor physical functioning, such as the Bath Ankylosing Spondylitis Functional Index (BASFI) and the physical functioning domain of the Short Form 36‐item (SF‐36) health survey, as well as measures of patient well‐being, such as pain, fatigue, and sleep outcomes. PROs are key tools for assessing both disease burden and treatment efficacy, since they measure disease aspects that cannot be easily scored by clinicians. Furthermore, the use of PROs has been acknowledged as important by many rheumatology societies 4, 5.
Issues associated with pain, fatigue, and sleep are important to patients with AS; in a report of AS patients questioned about issues associated with their disease, pain, fatigue, and sleep were among the 4 most commonly reported factors 6. Furthermore, in a related analysis, when patients with axial SpA were asked to identify relevant signs, symptoms, and impacts of their disease, issues such as fatigue, sleep, and pain were among the first identified 7.
Patients with AS consistently report lower health‐related quality of life (HRQOL) compared with the general population 8, 9, though the burden in the overall axial SpA population has been less well documented. Furthermore, although the efficacy of anti–tumor necrosis factor (anti‐TNF) therapies in improving the burden of PROs has been demonstrated in the treatment of AS 8, 9, there are currently no published randomized controlled trials (RCTs) considering the efficacy of anti‐TNFs in improving QOL in patients with active axial SpA, including both AS and nonradiographic axial SpA populations.
Certolizumab pegol (CZP) is a PEGylated Fc‐free anti‐TNF that has been shown to improve PROs in rheumatoid arthritis 10 and psoriatic arthritis 11. The clinical outcomes and certain composite PROs (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] and BASFI) from the RAPID axial SpA RCT have been reported previously 12. However, these composite PROs, while providing information on patients’ disease activity and physical function, do not provide any insight on the effect of anti‐TNF treatment on the individual components of patient QOL (pain, fatigue, and sleep), although these are known to be of importance to patients. Therefore, in this publication we present the effect of CZP treatment on these individual QOL components in the wider axial SpA population.
Methods
Patients and trial design
The study group consisted of 325 patients, age ≥18 years. Patient characteristics and inclusion/exclusion criteria are described elsewhere 12. The RAPID–axial SpA trial is a multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled clinical trial to evaluate the efficacy and safety of CZP in adult patients with active axial SpA. Data are presented here from the 24‐week, double‐blind, placebo‐controlled treatment period.
Patients were randomized 1:1:1 to placebo or CZP 400 mg at weeks 0, 2, and 4 (loading dose), followed by either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks. Patients receiving placebo who did not achieve an Assessment of SpondyloArthritis International Society criteria for 20% improvement (ASAS20) in disease activity response at both week 14 and 16 were re‐randomized 1:1 at week 16 to either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks, following the CZP loading dose.
Trial procedures and evaluations
The primary end point of the RAPID–axial SpA trial was an ASAS20 response at week 12; this and composite PROs (BASDAI and BASFI) are reported elsewhere 12. PROs reported here include total back pain (1 item, 0–10 numeric rating scale [NRS], where 0 = no pain and 10 = most severe pain), assessed at regular study visits and through a daily pain diary (day 0–28); nocturnal back pain (1 item, 0–10 NRS, where 0 = no pain and 10 = most severe pain); the Sleep Problems Index II domain of the Medical Outcomes Study Sleep Scale (MOS‐SPI; 9 items, 6‐point scale, where 0 = none of the time and 6 = all of the time, transformed linearly to range from 0–100); the ASQOL measure (18 items, 0–18 scale, where higher scores indicate worse HRQOL); and the SF‐36 (36 items, 8 domains, 0–100 scale, where higher scores indicate better health status) mental component summary (MCS), physical component summary (PCS), and domains.
Improvements in PROs were also measured using minimum clinically important differences (MCIDs), defined as a clinically relevant change in a patient's status. These were defined as a ≥1‐point decrease from baseline for total back pain 4, a ≥6‐point decrease for MOS‐SPI 13, a ≥2‐point decrease for ASQOL 14, and a ≥2.5‐point increase for SF‐36 PCS and MCS 15. Additional end points were the achievement of age‐ and sex‐adjusted population norms for SF‐36 MCS and PCS, defined as a score greater than the first quartile of population norms (general US population) 16.
Statistical analysis
All analyses were conducted in the Full Analysis Set (FAS). The statistical methods used here included the analysis of covariance for continuous end points and Wald tests for responder analyses, with missing data imputed by last observation carried forward and nonresponder imputation, respectively. The study was powered for the primary end point (ASAS20 response at week 12) 12.
Results
Patient disposition and baseline characteristics
A total of 325 patients were randomized and 298 (91.7%) completed to week 24. The FAS included all 218 patients randomized to CZP, and 106 of 107 patients randomized to placebo (324 patients). Overall, 178 patients (55%) were included in the AS subpopulation and 147 (45%) in the nonradiographic axial SpA subpopulation.
The treatment groups and study populations were generally well‐balanced with respect to demographics and baseline characteristics 12. At baseline, patients with axial SpA reported a significant burden of disease on HRQOL in both the physical and mental components of the SF‐36, compared to age‐ and sex‐adjusted population norms (Figure 1B).
Figure 1.
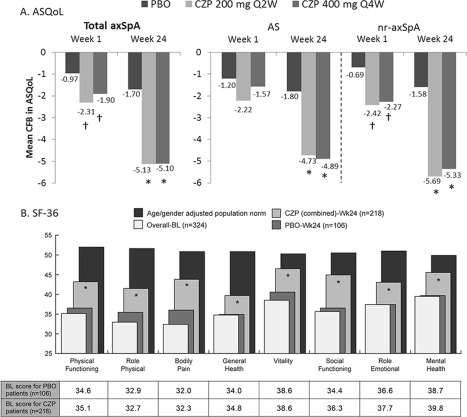
Changes in health‐related quality of life (QoL) in A, change from baseline (CFB) in ankylosing spondylitis (AS) QoL, and B, Short Form 36‐item (SF‐36) health survey domains, showing combined mean score at baseline (BL), certolizumab pegol (CZP), and placebo (PBO) mean score at week 24 and age‐ and sex‐adjusted population norms. Number of patients for PBO, CZP 200 mg every 2 weeks (Q2W), and CZP 400 mg every 4 weeks (Q4W), respectively, are total axial spondyloarthritis (axSpA): 106, 111, and 107; AS: 57, 65, and 56; and nonradiographic (nr) axSpA: 49, 46, and 51. * = P < 0.001; † = P < 0.05 CZP groups vs. PBO.
Patient‐reported outcomes
Pain, fatigue, and sleep
Improvements were observed in the CZP 200 mg every 2 weeks and CZP 400 mg every 4 weeks groups versus placebo in total back pain from week 1 to week 24 (Figure 2A) and in nocturnal back pain from week 1 (−1.9 and −1.6 versus −0.6; P < 0.001) to week 24 (−3.7 and −3.6 versus −1.3; P < 0.001).
Figure 2.
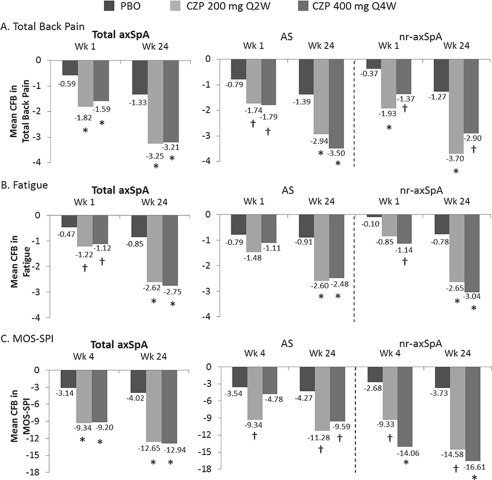
Change from baseline (CFB) in total back pain (numeric rating scale [NRS]) (A), fatigue NRS (B), and sleep problems (Medical Outcomes Study Sleep Problems Index II [MOS‐SPI]) (C). Number of patients for placebo (PBO), certolizumab pegol (CZP) 200 mg every 2 weeks (Q2W), and CZP 400 mg every 4 weeks (Q4W), respectively, are total axial spondyloarthritis (axSpA): 106, 111, and 107; AS: 57, 65, and 56; and nonradiographic (nr) axSpA: 49, 46, and 51. AS = ankylosing spondylitis; * = P < 0.001; † = P < 0.05 CZP groups vs. PBO.
A rapid response to CZP was observed in the alleviation of pain, with clinically relevant improvements observed from day 2 for both dosing regimens compared to placebo (Figure 3). Improvements were seen up to day 28, with the mean improvement by day 2 being >50% of the total improvement by day 28 (−1.4 at day 2 and −2.5 at day 28, for combined CZP doses). Treatment with CZP also led to relief in both fatigue and sleep problems compared to placebo, from week 1 (fatigue) or week 4 (sleep) to week 24 (Figures 2B and C).
Figure 3.
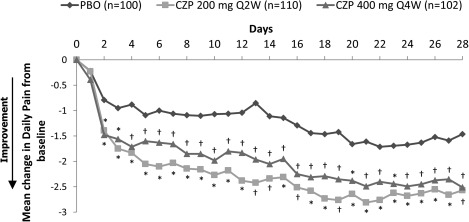
Change from baseline in daily pain. Values (no.) for placebo (PBO), certolizumab pegol (CZP) 200 mg every 2 weeks (Q2W), and CZP 400 mg every 4 weeks (Q4W), respectively, are total axial spondyloarthritis: 106, 111, and 107; ankylosing spondylitis: 57, 65, and 56; and nonradiographic axial spondyloarthritis: 49, 46, and 51. * = P < 0.001; † = P < 0.05 CZP groups vs. PBO.
At week 24, more CZP‐treated axial SpA patients reported improvements greater or equal to the MCID for total back pain and sleep compared to placebo. At week 24, the proportion of patients achieving greater or equal to the MCID for CZP 200 mg every 2 weeks and CZP 400 mg every 4 weeks versus placebo was 80.2% and 77.6% versus 35.8% (P < 0.001) for total back pain, and 57.7% and 61.7% versus 22.4% (P < 0.001) for sleep. For both outcomes, the proportion of patients achieving an MCID response was substantially higher in the combined CZP group compared with placebo from the first measurement taken (week 1 for total back pain [P < 0.05] and week 4 for MOS‐SPI [P < 0.001]).
HRQOL
Patients receiving CZP reported improvements from week 1 in ASQOL compared with those receiving placebo (Figure 1A). By week 24 the percentage of patients achieving ASQOL greater than or equal to the MCID was substantially higher for both dosing regimens than placebo (69.4% and 69.2% versus 28.3%, respectively; P < 0.001).
Relevant improvements compared to placebo (P < 0.05) were seen in all SF‐36 domains from week 4 (physical function, vitality, role physical, bodily pain, social functioning, and mental health) or week 8 (general health, role emotional) to week 24 (Figure 1B). The proportion of patients within or above the first quartile of population norms for PCS was 27% for CZP versus 6% for placebo, and 55% versus 19% for MCS, respectively. At week 24, more CZP‐treated patients reported improvements greater than or equal to the MCID for PCS (76.6% and 70.1% versus 27.4%) and MCS (53.2% and 60.7% versus 23.6%) compared to placebo (P < 0.001).
PROs in the AS and nonradiographic axial SpA subpopulations
Improvements in PROs following CZP treatment were similar across the AS and nonradiographic axial SpA subpopulations, including total back pain (Figure 2A), fatigue (Figure 2B) and ASQOL (Figure 1A). However, nonradiographic axial SpA patients showed greater improvements in MOS‐SPI compared to the AS subpopulation (Figure 2C), potentially due to the higher MOS‐SPI baseline scores observed for nonradiographic axial SpA patients (nonradiographic axial SpA 52.8 and 50.8, and AS 47.5 and 45.9, for 200 mg every 2 weeks and 400 mg every 4 weeks, respectively).
Although the proportion of CZP‐treated AS and nonradiographic axial SpA patients achieving MCID response at week 24, relative to placebo, was generally similar across PROs (data not shown), the proportion of patients achieving MOS‐SPI MCID response in the nonradiographic axial SpA group was greater than for AS (68.0% versus 52.9%, respectively, for combined CZP doses).
Discussion
In addition to previous findings regarding the clinical outcomes and composite PROs from the RAPID–axial SpA trial 12, we show here that CZP treatment, with either dose regimen, is associated with improvements in individual PROs important to patient well‐being, such as pain, fatigue, sleep, and QOL. Improvements in pain, fatigue, sleep, and HRQOL were seen from their first assessment. This rapid improvement is very important for patients, as it provides swift reassurance that their treatment is effective, aiding emotional well‐being and helping set treatment expectations, which may lead to better adherence to the treatment regimen.
Fatigue has been demonstrated to be a key symptom in the majority of AS patients, and can occur independently of functional impairments 6. Furthermore, sleep problems are an underreported issue for axial SpA patients, which are not taken into account in most commonly reported composite PRO measures. Therefore, the results presented above demonstrate the potential of CZP to improve some of the most important aspects of axial SpA from the patients’ perspective 6, 7.
In contrast to other reports of QOL in AS patients treated with anti‐TNF agents, this work reports PRO improvements in the wider axial SpA population, including AS and nonradiographic axial SpA patients. Similar improvements in all PROs were observed in both subpopulations, with the exception of sleep, where improvements were higher in nonradiographic axial SpA patients. This difference may be due to the worse baseline MOS‐SPI score in nonradiographic axial SpA, or due to differences in the demographics of the 2 subpopulations (in particular the higher proportion of females in the nonradiographic axial SpA population, since sex has previously been shown to be associated with sleep problems in AS) 17. These improvements across subpopulations were mirrored by similar improvements in the clinical 12 and magnetic resonance imaging 18 outcomes of the RAPID–axial SpA trial, suggesting that both subpopulations, despite a lack of structural damage in nonradiographic axial SpA patients, can be successfully treated with CZP, therefore alleviating highly burdensome symptoms in axial SpA patients with objective signs of inflammation.
Normalization of patients’ HRQOL is a key treatment goal in axial SpA. CZP was shown to improve patient HRQOL, with many patients reporting improvements beyond MCID levels, and some even “normalizing” their QOL. Substantial improvements were seen across all domains of the SF‐36, with mean gains in some domains (vitality and mental health) almost reaching the age‐ and sex‐adjusted population norms. However, it should be noted that the baseline impairment in these (mental) domains was less than for the SF‐36 physical domains (as expected in a physically debilitating disease such as axial SpA).
The limitations of this study include its relatively short time span (24 weeks) and the short period of the trial in which a full placebo population was present (16 weeks).
In axial SpA patients, CZP treatment (administered as either 200 mg every 2 weeks or 400 mg every 4 weeks) was shown to reduce the burden of disease on pain, fatigue, sleep, and HRQOL. Improvements were similar in both the AS and nonradiographic axial SpA subpopulations.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Sieper had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sieper, Deodhar, Coteur, Woltering.
Acquisition of data. Kivitz, van Tubergen, Deodhar, Landewé.
Analysis and interpretation of data. Sieper, Kivitz, van Tubergen, Deodhar, Coteur, Woltering, Landewé.
ROLE OF THE STUDY SPONSOR
UCB Pharma sponsored the study and the development of the manuscript. In addition to content approval by the authors, UCB Pharma signed off on the abstract following a full review to ensure that the data presented in the publication are scientifically, technically, and medically supportable and did not contain any information that has the potential to damage the intellectual property of UCB Pharma. Additionally, UCB ensured that the publication complies with applicable laws, regulations, guidelines, and good industry practice.
ACKNOWLEDGMENTS
The authors thank Marine Champsaur, UCB Pharma, Brussels, Belgium for publication coordination and Costello Medical Consulting, UK, for writing and editorial assistance, which was funded by UCB Pharma.
ClinicalTrials.gov identifier: NCT01087762.
REFERENCES
- 1. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 4. Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum 1993;36:729–40. [DOI] [PubMed] [Google Scholar]
- 5. Van Gestel AM, Prevoo ML, van 't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- 6. Ward MM. Health‐related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res 1999;12:247–55. [PubMed] [Google Scholar]
- 7. Van Tubergen A, Black P, Coteur G. Are patient‐reported outcome instruments for ankylosing spondylitis fit‐for‐purpose for the axial spondyloarthritis patient? A qualitative and psychometric analysis. Rheumatology (Oxford) E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8. Kimel M, Revicki D, Rao S, Fryback D, Feeny D, Harnam N, et al. Norms‐based assessment of patient‐reported outcomes associated with adalimumab monotherapy in patients with ankylosing spondylitis. Clin Exp Rheumatol 2011;29:624–32. [PubMed] [Google Scholar]
- 9. Martin‐Mola E, Sieper J, Leirisalo‐Repo M, Dijkmans BA, Vlahos B, Pedersen R, et al. Sustained efficacy and safety, including patient‐reported outcomes, with etanercept treatment over 5 years in patients with ankylosing spondylitis. Clin Exp Rheumatol 2010;28:238–45. [PubMed] [Google Scholar]
- 10. Strand V, Smolen JS, van Vollenhoven RF, Mease P, Burmester GR, Hiepe F, et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient‐reported outcomes from the RAPID 2 trial. Ann Rheum Dis 2011;70:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gladman D, Fleischmann R, Coteur G, Woltering F, Mease PJ. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24‐week patient‐reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken) 2014;66:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landewe R, Braun J, Deodhar A, Dougados M, Maksymowych W, Mease P, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24‐week results of a double‐blind randomised placebo‐controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spritzer H. MOS sleep scale: a manual for use and scoring. Los Angeles (CA): Rand; 2003. [Google Scholar]
- 14. Van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long‐term results from the ATLAS trial. Ann Rheum Dis 2009;68:922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strand V, Scott DL, Emery P, Kalden JR, Smolen JS, Cannon GW, et al. Physical function and health related quality of life: analysis of 2‐year data from randomized, controlled studies of leflunomide, sulfasalazine, or methotrexate in patients with active rheumatoid arthritis. J Rheumatol 2005;32:590–601. [PubMed] [Google Scholar]
- 16. Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF‐36 health survey (standard and acute forms). Lincoln (RI): QualityMetric Incorporated; 2000. [Google Scholar]
- 17. Hultgren S, Broman JE, Gudbjornsson B, Hetta J, Lindqvist U. Sleep disturbances in outpatients with ankylosing spondylitis: a questionnaire study with gender implications. Scand J Rheumatol 2000;29:365–9. [DOI] [PubMed] [Google Scholar]
- 18. Van der Heijde D, Maksymowych W, Landewe R, Stach C, Hoepken B, Fichtner A, et al. Effect of certolizumab pegol on inflammation of spine and sacroiliac joints in patients with axial spondyloarthritis: 12‐week magnetic resonance imaging results of RAPID‐axSpA. Ann Rheum Dis 2013;72 Suppl 3:515–6. [Google Scholar]


