To the Editor: Many anti‐cancer vaccination strategies have been tested in mice and humans in the attempt to eradicate leukemia cells 1. The vast majority of clinical trials are based on peptide vaccination which allows the induction of cellular response to specific tumor associated antigens 2. WT1(Wilms tumor‐1) gene is located on chromosome 11p13 and encodes a zinc finger transcription factor that plays an important role in cell growth and differentiation. WT1 was originally described as a tumor suppressor gene although many evidences demonstrated that it plays an oncogenic function in the setting of leukemia. WT1 protein represents an optimal tumor antigen since it is highly expressed in acute leukemias, myelodysplastic syndromes (MDS) and myeloproliferative neoplasms 3. By contrast, it is expressed at very low levels in normal hematopoietic progenitors. Expression of the WT1 protein is restricted to a limited set of tissues, including the gonads, uterus, kidney, and spleen.
The success of a particular peptide vaccine to elicit an immune response is influenced by many parameters, including the presence of helper T cell epitopes, processing and presentation by professional antigen presenting cells (APCs), biodistribution, peptide length, peptide affinity, and route of administration. Recently Brayer 4 and colleagues published in this journal the results of WT1 peptide vaccination in AML and MDS. The conclusion from this study and many others based on WT1 peptide vaccination is that this strategy is safe, feasible but, al least in this study, it is not able to induce a consistent and measurable WT1 specific T cell response. In the majority of the clinical trials WT1 peptide elicited CD3+ CD8+ T cells. Additional trials showed that the combination of short and long peptides induced also CD3+ CD4+ T cells. Interestingly, it was shown that long peptide elicited the strongest immunologial response against WT1. The clinical results are overall encouraging, describing several patients obtainig molecular remission, partial responses or stable disease. The main limits are the immune tolerance and immune‐evasion. Two main strategies have been tested to overcome these limits, the use of long‐sequence peptides preferentially processed by APCs in the lymph node, cincumventing some of the tolerance mechanisms, and the addition of adjuvant to stimulate APC. Here, we report the results of WT1 protein vaccination in mice.
The complete WT1 murine coding sequence cloned in an expression vector (pGEX‐4T‐1) together with GST protein has been amplified. The fusion protein GST‐WT1 has been transfected in E.Coli and purified. Thirthy C57BL/6J mice have been utilized according to the scheme represented in Fig. 1 panel A. The first group (10 mice) was vaccinated performing a first injection with 30 μg of GST‐WT1 protein + 50 μg of complete Freund adjuvant (AD) at Week 0. After 2 and 4 weeks, a second and third dose 30 μg of GST‐WT1 protein + 50 μg of AD were injected. The second group (5 mice) was vaccinated with 30 μg of GST‐WT1 protein only at week 0, 2 and 4. The third group (5 mice) was vaccinated with 30 μg of GST only plus AD at week 0, 2, and 4. The fourth group (10 mice) was treated with PBS only and used as control. After 2 additional weeks (weeks 6) 200.000 TRAMP‐C cells, a singenic prostatic cancer cell line overexpressing WT1, were injected subcutaneously in all animals. After 8 weeks from the first injection half the mice were sacrified to evaluate the immune response, both cytotoxic and humoral and the tumor burden, while half of them were sacrified after 15 weeks to evaluate immune response, tumor borden, and organ toxicity.
Figure 1.
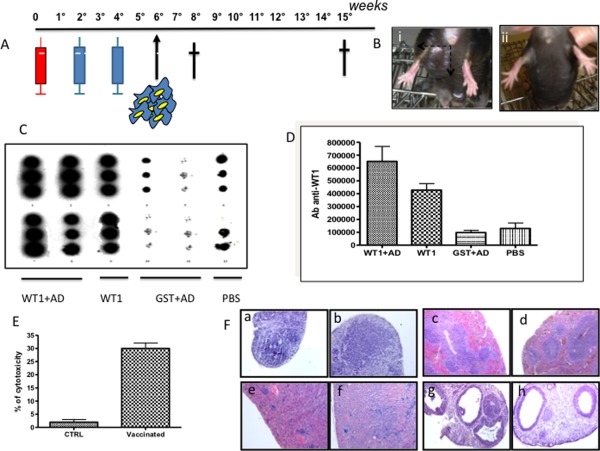
Panel A: Scheme of vaccination. Panel B: Response in terms of tumor bourden in vaccinated mice (ii) in which the tumor is undetectable compared to control mice (i) which developed a measurable tumor mass of 1.5 cm after 8 weeks from the first vaccination. Panel C: Dot blot analysis for the detection of specific antibodies against WT1. The analysis has been performed after 8 weeks from the first vaccination. Panel D: Quantification of the dot blot results. Panel E: 51Cr release test for the evaluation of cytotoxicity. Panel F: Evaluation of organ toxicity before and after vaccination, respectively, in lymphonode (a,b), spleen (c,d), kidney (e, f), and ovary (g,h).
Dot blot analysis on mice serum showed the presence of IgG antibodies againt WT1 after vaccination with GST‐WT1 protein + AD and GST‐WT1 protein alone. By contrast, the antibodies were not present after injection of GST +AD and PBS. (Fig. 1 panel C and D). Furthermore, cytotoxicity of T cells was evaluated by 51Cr release test. In mice injected with GST‐WT1 protein + AD the level of cytotoxixity was 30% ± 2 compared to 2% ± 0.5 (background level) in control mice. Finally, we examined the toxixity in organs which physiologically express WT1 al low levels: lymphonode, spleen, ovary, and kidney in vaccinated mice and controls. No toxicity was observed (Fig. 1 panel F). Hemocromocytometric analysis as well as BM smears (data not shown) excluded any kind of hematological toxicity. The mean Hb level was 13.9 gr/dL in vaccinated mice and 14.2 gr/dL in controls (P > 0.05), the median WBC count was 5135/μl in vaccinated mice and 6357//μl in controls (P > 0.05), the median platelet count was 1128000/μl in vaccinated mice and 1020000/μl in controls (P > 0.05). In conclusion, vaccination with WT1 protein induces a significant cytotoxic response and a potent antibody response. This results, at least in mice, in a significant reduction of the tumor borden. The median reduction of the volume of the tumor after 8 weeks of vacciantion is 62%. (Fig. 1 panel B). This strategy may allow to overcome some of the limits associated with peptide vaccination including the restriction of the HLA typing of the patient and the prevalent T CD8+ response. This strategy allows to exploit the whole reactive potential of the immune system, both cytotoxic and humoral.
Paolo Nicoli,1 Chiara Calabrese,1 Rosa Maria Pellegrino,1 Valentina Rosso,1 Enrico Bracco,2 Elisabetta Signorino,1 Sonia Carturan,1 Jessica Petiti,1 Daniela Gallo,1 Valentina Gaidano,1 Marco De Gobbi,1 Antonella Roetto,1 Giuseppe Saglio,1 and Daniela Cilloni1* 1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy; 2Department of Oncology, University of Turin, Turin, Italy
Conflict of interest: Nothing to report.
References
- 1. Oka Y, Tsuboi, A , Kawakami M, et al. Development of WT1 peptide cancer vaccine against hematopoietic malignancies and solid cancers. Curr Med Chem 2006;13:2345–2352. [DOI] [PubMed] [Google Scholar]
- 2. Oka Y, Tsuboi, A , Taguchi T, et al. Induction of WT1 (Wilms’ tumor gene)‐specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cilloni D, Gottardi, E , De Micheli D, et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002;16:2115–2121. [DOI] [PubMed] [Google Scholar]
- 4. Brayer J, Lancet, JE , Powers J, et al. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am J Hematol, in press. doi: 10.1002/ajh.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]


