Abstract
Objective
The efficacy and safety of abatacept in patients with juvenile idiopathic arthritis (JIA) who experienced an inadequate response to disease‐modifying antirheumatic drugs were previously established in a phase III study that included a 4‐month open‐label lead‐in period, a 6‐month double‐blind withdrawal period, and a long‐term extension (LTE) phase. The aim of this study was to present the safety, efficacy, and patient‐reported outcomes of abatacept treatment (10 mg/kg every 4 weeks) during the LTE phase, for up to 7 years of followup.
Methods
Patients enrolled in the phase III trial could enter the open‐label LTE phase if they had not achieved a response to treatment at month 4 or if they had received abatacept or placebo during the double‐blind period.
Results
One hundred fifty‐three (80.5%) of 190 patients entered the LTE phase, and 69 patients (36.3%) completed it. The overall incidence rate (events per 100 patient‐years) of adverse events decreased during the LTE phase (433.61 events during the short‐term phase [combined lead‐in and double‐blind periods] versus 132.39 events during the LTE phase). Similar results were observed for serious adverse events (6.82 versus 5.60), serious infections (1.13 versus 1.72), malignancies (1.12 versus 0), and autoimmune events (2.26 versus 1.18). American College of Rheumatology (ACR) Pediatric 30 (Pedi 30) responses, Pedi 70 responses, and clinically inactive disease status were maintained throughout the LTE phase in patients who continued to receive therapy. Improvements in the Child Health Questionnaire physical and psychosocial summary scores were maintained over time.
Conclusion
Long‐term abatacept treatment for up to 7 years was associated with consistent safety, sustained efficacy, and quality‐of‐life benefits in patients with JIA.
Juvenile idiopathic arthritis (JIA) is one of the most common chronic diseases of childhood and the most prevalent of the pediatric rheumatic illnesses 1, 2, 3. The outcome for patients with JIA can be described in terms of persistent synovitis and joint damage, with diminished daily function and quality of life (QOL); nearly 50% of children with JIA will have recurrent or persistent disease and will enter adulthood with active arthritis and ongoing joint destruction 1. Importantly, the associated disability and pain can have a negative impact on physical and psychological health 4, 5. Furthermore, JIA is associated with a significant burden on caregivers 6.
Treatment with biologic agents has led to improved clinical outcomes in terms of reductions in disease activity and inflammation, with concurrent improvements in function and health‐related QOL (HRQOL), as evaluated using patient‐reported outcomes (PROs) 7, 8, 9, 10, 11, 12, 13, 14, 15. The chronic nature and childhood onset of JIA mean that many patients will continue to receive therapy with biologic agents for extended periods of time. Furthermore, the possible increased risk of certain adverse events (AEs) associated with biologic agent treatment must be monitored. As such, it is important to evaluate the safety and tolerability, and the sustainability of clinical efficacy and QOL benefits, of long‐term treatment.
Abatacept is a biologic agent that selectively modulates T cell costimulation. It is currently approved in the US and the European Union for the treatment of moderately to severely active polyarticular JIA in patients ages 6 years or older 16. Abatacept selectively modulates the CD28:CD80/86 costimulatory signal required for full T cell activation 17. The efficacy and safety of abatacept in patients with JIA who had an inadequate response to at least 1 disease‐modifying antirheumatic drug (DMARD), including anti–tumor necrosis factor (anti‐TNF) antagonists, were previously examined in a phase III study, which included a 4‐month open‐label lead‐in phase and a 6‐month double‐blind period, followed by a long‐term extension (LTE) phase 12.
The efficacy of abatacept was confirmed during the 6‐month double‐blind period, during which placebo‐treated patients were 3 times more likely to experience a flare compared with patients who continued to receive abatacept. Furthermore, the frequency of AEs did not differ between the abatacept and placebo groups. Improvements in QOL and PROs, such as pain, activity limitation, and sleep, were also observed in patients treated with abatacept compared with those treated with placebo 18. Safety and efficacy results from ≥21 months of the LTE period demonstrated that long‐term abatacept treatment continued to be well tolerated and was associated with clinically significant and durable efficacy, including in patients who were not responders (according to the American College of Rheumatology [ACR] Pediatric 30 [Pedi 30] criteria for improvement) by month 4 of therapy 19. The aim of this study was to assess the long‐term safety, clinical efficacy, QOL, and PROs in patients exposed to abatacept for ∼30 additional months, for up to 7 years of total followup.
PATIENTS AND METHODS
Patient population and study design
The phase III study (ClinicalTrials.gov identifier NCT00095173) included patients with JIA ages 6–17 years from 43 pediatric rheumatology centers in Europe, Latin America, and the US belonging to the Pediatric Rheumatology Collaborative Study Group or the Paediatric Rheumatology International Trials Organisation 20. To be eligible, patients were required to have had an inadequate response or intolerance to at least 1 DMARD (including TNF‐blocking agents), active disease at the time of enrollment (at least 2 joints with active arthritis and 2 joints with limited range of motion), and a history of at least 5 joints with active arthritis (swollen joints or joints with a limited range of motion and pain or tenderness).
The study design was previously described 19. Briefly, the study consisted of a 4‐month open‐label lead‐in phase, during which all patients received open‐label abatacept, followed by a 6‐month double‐blind withdrawal phase, during which patients who had achieved an ACR Pedi 30 response at the end of the 4‐month lead‐in period were assigned randomly to receive either abatacept or placebo. These patients could then enter the open‐label LTE phase. Patients were also eligible to enter the LTE directly if they completed the open‐label lead‐in phase without achieving an ACR Pedi 30 response or if they had experienced a flare during the double‐blind withdrawal period while receiving abatacept or placebo. The study ended after the last patient completed 5 years of followup during the LTE phase.
During the open‐label LTE phase, all patients received abatacept by intravenous infusion at a dose of 10 mg/kg, up to a maximum dose of 1,000 mg. The dosages of oral corticosteroids (maximum 10 mg/day prednisone equivalent or 0.2 mg/kg/day, whichever was less) and methotrexate (≤30 mg/m2 body surface area/week up to a maximum allowed dosage of 40 mg/m2 body surface area/week if required) could be adjusted during the LTE phase. The use of nonsteroidal antiinflammatory drugs or analgesics and the addition of hydroxychloroquine, sulfasalazine, or azathioprine were permitted during the LTE phase. Up to 2 intraarticular injections of corticosteroids per year were permitted, and any injected joint was considered “active” at all subsequent visits.
This study was conducted in accordance with Good Clinical Practice and with the ethics principles of the Declaration of Helsinki. The protocol, amendments, and patient informed consent form received approval by the relevant institutional review board or ethics committee at each participating site.
Assessments performed
The primary objective of the LTE period was to assess the safety of continued long‐term abatacept treatment. Safety data were recorded at each study visit up to a maximum of 7 years of treatment, representing the maximum exposure time in the study. Acute infusion‐related events were defined as those reported within 1 hour of the start of infusion. The definitions of a serious adverse event (SAE) included a fatal or life‐threatening AE, an AE requiring hospitalization or prolonged hospitalization, an AE resulting in persistent or significant disability or incapacity, cancer, a congenital anomaly/birth defect, overdose, development of drug dependency or drug abuse, or an important medical event.
The secondary objective was to assess the efficacy of abatacept treatment. Clinical efficacy and QOL evaluations were performed quarterly during the LTE phase and collected until day 1,765 (∼5.5 years). Improvements in the signs and symptoms of JIA were evaluated in terms of the proportions of patients achieving ACR Pedi 30, Pedi 50, Pedi 70, and Pedi 90 responses 21 or clinically inactive disease status (as determined using the study‐specific definition of no joints with active disease, a physician's global assessment disease activity score of ≤10 [range 0–100], and an erythrocyte sedimentation rate [ESR] of ≤20 mm/hour). The ACR Pedi responses were calculated by comparing the differences between the last visit and the baseline visit at the start of the phase III study, prior to the first abatacept infusion.
In this study, ACR Pedi responses were evaluated using the ESR as the laboratory measure of acute inflammation in the core set of measures of improvement and response in JIA 21. The Child Health Questionnaire (CHQ) was used to evaluate physical, emotional, and social aspects of HRQOL in the patients; raw scores were transformed to a scale of 0–100, with higher scores indicating better QOL. Physical summary and psychosocial summary scores of the CHQ were normalized using data from healthy children in the US; mean ± SD scores of 50 ± 10 were considered within the normal range for healthy children 22, 23.
Disability was assessed using the Childhood Health Assessment Questionnaire (C‐HAQ) disability index, which is a component of the criteria for an ACR Pedi response 23. Pain was assessed using the parent's global assessment score. Parents were asked how much pain due to illness their child had experienced in the past week; responses were measured using an anchored 100‐mm visual analog scale, where 0 indicates no pain and 100 indicates very severe pain. The impact of JIA on sleep quality was evaluated using the Children's Sleep Habits Questionnaire (CSHQ) 24, 25; total scores range from 0 to 100, with higher scores indicating more sleep problems. The CSHQ consists of 33 items, assessing the following 8 domains of sleep behavior: bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night awakenings, parasomnias, sleep‐disordered breathing, and daytime sleepiness. The questionnaire has been validated in children ages 4–10 years, with a total score of ≥41 identifying children with sleep disorders.
Participation in daily activities for both patients and parents was assessed using a questionnaire in which parents were asked the following 3 questions (with responses scored as the number of days during the past 30 days): On how many days did your child's JIA keep him/her from attending school (excluding vacation/holidays)? On how many days did your child's JIA keep you from doing your usual activities? On how many days did you pay for child care for your son/daughter with JIA in order for you to engage in your usual activities?
To evaluate immunogenicity, serum samples were obtained from the patients prior to dosing (day 1) and on the scheduled assessment days and assayed for the induction of abatacept‐specific antibodies, using enzyme‐linked immunosorbent assays to detect antibodies to the whole abatacept molecule (CTLA‐4 and immunoglobulin portion) or the CTLA‐4 region alone (CTLA‐4 “tip”).
Statistical analysis
The analyses were based on data derived from the 153 patients who entered the LTE phase and received at least 1 infusion of abatacept; the data for these patients were pooled and represent the open‐label lead‐in, double‐blind randomized, and open‐label LTE periods. Safety data are presented as incidence rates (IRs) with 95% confidence intervals (95% CIs), which were calculated as the number of events per 100 patient‐years of exposure (number of patients with event/exposure [person‐years] × 100). Patient‐years of exposure were censored at the time of the first occurrence of an AE. Safety analyses were based on data obtained up to 56 days after administration of the last abatacept dose.
Clinical efficacy and QOL analyses were based on data derived from patients for whom data were available at each time point (as‐observed) and are presented for 3 subgroups: patients who received abatacept during the 6‐month double‐blind withdrawal period (double‐blind abatacept group), patients who received placebo during the 6‐month double‐blind withdrawal period (double‐blind placebo group), and patients who did not achieve an ACR Pedi 30 response during the initial 4‐month open‐label lead‐in period and who entered the LTE phase directly (initial nonresponder group). ACR Pedi responses over time during the LTE phase were combined from day 1 of the LTE for the double‐blind abatacept and double‐blind placebo groups, because all patients were receiving abatacept at this stage. No formal statistical testing was performed during the LTE phase.
RESULTS
Patient disposition
Of the 190 patients enrolled in the phase III trial, 153 (80.5%) entered the LTE phase. One hundred seventeen patients (58 in the abatacept group and 59 in the placebo group) entered the LTE phase following completion of the double‐blind withdrawal period or after developing disease flare during this period, and 36 patients who completed the lead‐in phase but did not achieve an ACR Pedi 30 response entered the LTE phase directly. Of the 37 children who did not enter the LTE phase, 20 discontinued participation during the open‐label lead‐in period (lack of efficacy, n = 17; AEs, n = 1; lost to followup, n = 1; other, n = 1); 11 patients who were nonresponders during the lead‐in period did not enter the LTE phase and instead left the study (the reasons were not collected); 1 patient who was a responder during the lead‐in period left the study before the start of the double‐blind period (withdrawal of consent); and 5 patients left the study during the double‐blind period (in the abatacept arm, lack of efficacy [n = 1] and withdrawal of consent [n = 1]; in the placebo arm, lack of efficacy [n = 3]). Of the 153 patients who entered the LTE phase, 69 completed it, and 84 patients discontinued treatment, including 24 who discontinued due to lack of efficacy and 6 who discontinued because of AEs (see Figure 1 for a complete listing of all discontinuations).
Figure 1.
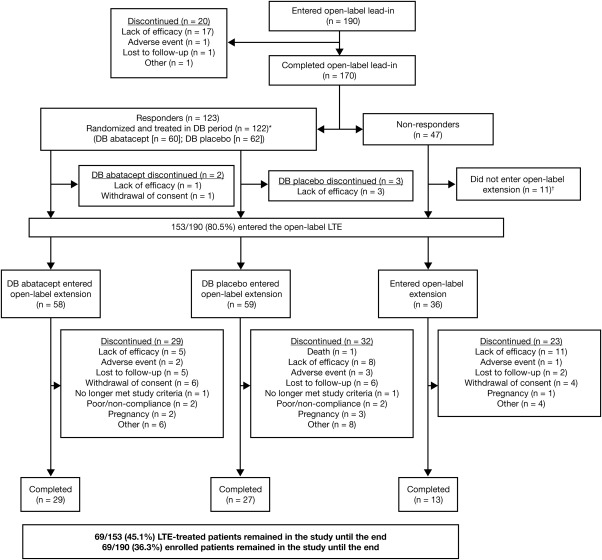
Disposition of the patients with juvenile idiopathic arthritis during the long‐term extension (LTE) phase. ∗ = One patient withdrew consent and left the study during the double‐blind (DB) treatment period. † = The reasons for discontinuation were not reported.
The demographic and clinical characteristics at baseline of the patients who entered the LTE phase are shown in Table 1. The mean disease duration at baseline was 4.1 years. Patients had an average of 16.0 joints with active disease and significant disability (mean C‐HAQ disability index score of 1.2). The most common JIA category was rheumatoid factor–negative polyarthritis (data not shown). The baseline characteristics of the 153 patients who entered the LTE phase and the 190 patients originally enrolled in the phase III trial were comparable.
Table 1.
Demographic and clinical characteristics at baseline, prior to the first abatacept infusion, of the JIA patients who entered the LTE phasea
| Characteristic | Double‐blind abatacept (n = 58) | Double‐blind placebo (n = 59) | Initial nonresponders (n = 36) | Total (n = 153) |
|---|---|---|---|---|
| Age, years | 12.4 ± 2.9 | 12.0 ± 2.9 | 12.7 ± 3.1 | 12.3 ± 2.9 |
| Female sex, no. (%) | 41 (70.7) | 42 (71.2) | 23 (63.9) | 106 (69.3) |
| Duration of JIA, years | 3.8 ± 3.8 | 4.0 ± 3.5 | 4.8 ± 3.9 | 4.1 ± 3.7 |
| No. of joints with active disease | 17.8 ± 11.2 | 14.9 ± 13.0 | 14.9 ± 13.6 | 16.0 ± 12.5 |
| No. of joints with limited range of motion | 16.7 ± 12.2 | 14.6 ± 13.9 | 17.1 ± 18.0 | 16.0 ± 14.3 |
| Physician's global assessment (100‐mm VAS) | 52.9 ± 17.8 | 51.7 ± 20.8 | 51.4 ± 22.4 | 52.1 ± 20.0 |
| Parent's global assessment (100‐mm VAS) | 41.8 ± 22.9 | 40.4 ± 25.1 | 44.2 ± 25.1 | 41.8 ± 24.1 |
| ESR, mm/hour (normal ≤20) | 31.3 ± 27.2 | 31.5 ± 27.7 | 30.6 ± 21.9 | 31.2 ± 26.1 |
| CRP, mg/dl (normal <0.5) | 3.0 ± 4.6 | 2.8 ± 3.5 | 3.8 ± 4.9 | 3.1 ± 4.3 |
| C‐HAQ disability index score, 0–3 scale | 1.3 ± 0.7 | 1.3 ± 0.8 | 1.1 ± 0.9 | 1.2 ± 0.8 |
| CHQ physical summary score | 30.2 ± 13.9 | 31.0 ± 14.9 | 29.5 ± 16.1 | 30.3 ± 14.7 |
| CHQ psychosocial summary score | 43.5 ± 9.4 | 44.2 ± 10.9 | 47.0 ± 9.9 | 44.5 ± 10.2 |
| CSHQ total score | 47.3 ± 8.6 | 45.6 ± 6.4 | 45.6 ± 8.1 | 46.3 ± 7.7 |
| Parent's global assessment of pain (100‐mm VAS) | 43.8 ± 22.8 | 40.0 ± 23.4 | 46.1 ± 23.6 | 42.9 ± 23.2 |
| No. of days of activity missed by parent or caregiver per month | 3.8 ± 10.3 | 3.0 ± 6.3 | 3.0 ± 6.1 | 3.3 ± 8.0 |
| No. of days of school missed per month | 5.6 ± 9.5 | 4.2 ± 8.1 | 2.5 ± 4.2 | 4.3 ± 8.0 |
Except where indicated otherwise, values are the mean ± SD. JIA = juvenile idiopathic arthritis; LTE = long‐term extension; VAS = visual analog scale; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; C‐HAQ = Childhood Health Assessment Questionnaire; CHQ = Child Health Questionnaire; CSHQ = Children's Sleep Habits Questionnaire.
Exposure
Total exposure to abatacept across the entire study for patients treated during the LTE phase was 683.4 patient‐years (300.2, 260.1, and 123.3 patient‐years for the double‐blind abatacept, double‐blind placebo, and initial nonresponder groups, respectively). Forty‐three patients were exposed to abatacept for ≥6 years.
Concomitant medications
At the time of entry into the LTE phase (day 0), 75.2% of patients (47 [81.0%] in the double‐blind abatacept group, 42 [71.2%] in the double‐blind placebo group, and 26 [72.2%] in the initial nonresponder group) were receiving methotrexate (the mean dosage range for the 3 groups was 12.0–13.0 mg/m2 body surface area/week). During the LTE phase, 111 patients (72.5%) were receiving folic acid. A total of 19 patients (12.4%) added a nonbiologic DMARD during the LTE phase, including 10 patients (17.2%), 4 patients (6.8%), and 5 patients (13.9%) from the double‐blind abatacept, double‐blind placebo, and initial nonresponder groups, respectively.
Azathioprine was initiated in 6 patients (3.9%), cyclosporine in 2 patients (1.3%), and leflunomide in 4 patients (2.6%). Forty‐three patients (28.1%) received concomitant oral corticosteroids at baseline (the mean dosage range was 0.13–0.19 mg/kg/day [data for 1 patient were missing]), and 44 patients (28.8%) received corticosteroids at entry into the LTE phase (the mean dosage range was 0.14–0.21 mg/kg/day [data for 1 patient were missing]).
A total of 24 patients had missing information on the route of administration of medication. No patients received intraarticular corticosteroids at baseline, and 2 patients (1.3%; 1 each in the double‐blind abatacept and placebo groups) received 1 intraarticular corticosteroid injection within 6 months prior to the start of the LTE phase. During the LTE period, 63 patients (41.2%) received oral corticosteroids (the mean dosage range for those with available data was 0.16–0.23 mg/kg/day), and 23 patients (15.0%) received intraarticular corticosteroid injections. The mean ± SD numbers of intraarticular injections for the double‐blind abatacept, double‐blind placebo, and initial nonresponder groups, respectively, were 0.05 ± 0.04, 0.14 ± 0.31, and 0.11 ± 0.10 per month, and 0.62 ± 0.41, 0.62 ± 0.63, and 0.90 ± 0.65 per year.
Safety
Safety events reported during the LTE phase, corresponding to a maximum mean ± SD total exposure of 62.1 ± 20.9 months, were comparable in the 3 treatment groups (Table 2). One death occurred during the LTE phase (motorcycle accident) and was considered to be unrelated to the study treatment.
Table 2.
Summary of AEs during the long‐term extension phasea
| Double‐blind abatacept (n = 58) | Double‐blind placebo (n = 59) | Initial nonresponders (n = 36) | Total (n = 153) | |
|---|---|---|---|---|
| Overall AEs | 55 (94.8) | 54 (91.5) | 31 (86.1) | 140 (91.5) |
| Deaths | 0 | 1 (1.7) | 0 | 1 (1.7)b |
| Discontinuations due to AEs | 2 (3.4) | 3 (5.1) | 1 (2.8) | 6 (3.9) |
| Overall SAEs | 9 (15.5) | 12 (20.3) | 9 (25.0) | 30 (19.6) |
| Discontinuations due to SAEs | 1 (1.7) | 2 (3.4) | 0 | 3 (2.0) |
| Most common SAEsc | ||||
| Arthritis disease flare | 3 (5.2) | 0 | 3 (8.3) | 6 (3.9) |
| Arthralgia | 1 (1.7) | 1 (1.7) | 1 (2.8) | 3 (2.0) |
| Rheumatoid arthritisd | 0 | 1 (1.7) | 1 (2.8) | 2 (1.3) |
| Foot deformity | 1 (1.7) | 1 (1.7) | 0 | 2 (1.3) |
| Pyelonephritis | 1 (1.7) | 0 | 1 (2.8) | 2 (1.3) |
| Bacterial arthritis | 0 | 1 (1.7) | 1 (2.8) | 2 (1.3) |
| Appendicitis | 2 (3.4) | 0 | 0 | 2 (1.3) |
| Pyrexia | 1 (1.7) | 1 (1.7) | 0 | 2 (1.3) |
| Vomiting | 1 (1.7) | 1 (1.7) | 0 | 2 (1.3) |
The mean ± SD exposure to abatacept during the long‐term extension phase was 48.2 ± 24.6 months (53.2 ± 21.0 months, 50.0 ± 24.8 months, and 37.4 ± 27.0 months in the double‐blind abatacept, double‐blind placebo, and initial nonresponder groups, respectively). Values are the number (%). AEs = adverse events; SAEs = serious AEs.
Death attributable to motorcycle accident and considered unrelated to the study treatment.
Defined as those occurring in ≥1% of the total group.
As reported using the Medical Dictionary for Regulatory Activities (version 14.1).
The overall IRs of AEs and SAEs reported in the cumulative study period, corresponding to a mean ± SD maximum total exposure of 62.1 ± 20.9 months, were 209.11 (95% CI 179.11–242.70) and 5.62 (95% CI 3.92–7.82) events/100 patient‐years of exposure, respectively. Six patients discontinued participation in the study due to AEs during the LTE phase, including urticaria and bronchospasm (nonserious, considered probably related to the study treatment), worsening vitiligo (nonserious, considered unlikely related), skin lesions (nonserious squamous skin lesions on the head, considered possibly related), temporal lobe epilepsy and multiple sclerosis (serious, considered possibly related [previously reported; see ref. 19]), appendicitis (serious, considered possibly related), and bacterial arthritis (serious, considered probably related). With the exception of worsening arthritis, no individual SAE was reported by >2 patients in any group during the LTE phase. The IR for AEs and SAEs during the LTE period did not increase relative to the short‐term (combined lead‐in and double‐blind) treatment period (Table 3). It is important to note that no patient received abatacept as isolated therapy.
Table 3.
Summary of adverse events during the short‐term and long‐term extension phasesa
| Patients treated during the short‐term phase (n = 190) | Patients who entered the long‐term extension phase (n = 153) | |||
|---|---|---|---|---|
| n | IR (95% CI) | n | IR (95% CI) | |
| AEs | 145 | 433.61 (365.91–510.21) | 140 | 132.39 (111.37–156.22) |
| SAEs | 6 | 6.82 (2.50–14.84) | 30 | 5.60 (3.78–8.00) |
| Infections | 86 | 142.40 (113.90–175.87) | 120 | 64.72 (53.66–77.39) |
| Serious infections | 1 | 1.13 (0.03–6.27) | 10 | 1.72 (0.83–3.16) |
| Malignancies | 1 | 1.12 (0.03–6.27) | 0 | 0 |
| Autoimmune events | 2 | 2.26 (0.27–8.17) | 7 | 1.18 (0.48–2.44) |
The short‐term phase represents the combined lead‐in and double‐blind periods. IR = incidence rate per 100 patient‐years of exposure; 95% CI = 95% confidence interval; AEs = adverse events; SAEs = serious AEs.
The overall IR of infections during the cumulative period was 83.80 (95% CI 70.80–98.49). The IR for most frequent infections reported were nasopharyngitis (8.85 [95% CI 6.52–11.73]), upper respiratory tract infection (6.81 [95% CI 4.84–9.31]), and influenza (5.58 [95% CI 3.84–7.84]). The IR for serious infections during the cumulative abatacept treatment period was 1.65 (95% CI 0.82–2.95). Serious infections that were considered possibly related to the study treatment included appendicitis, limb abscess, impetigo, herpes zoster infection, varicella, and bacterial arthritis, all of which resolved following treatment. The most frequently reported serious infections were pyelonephritis, bacterial arthritis, and appendicitis (2 patients each). The IRs for infections and serious infections during the cumulative period of abatacept treatment did not increase relative to the short‐term treatment period (Table 3).
The overall IR for autoimmune events was 1.18 (95% CI 0.51–2.32). Events during the LTE phase included cutaneous vasculitis, psoriasis, vitiligo, uveitis, type 1 diabetes, multiple sclerosis, and Raynaud's phenomenon (1 event each). The IR for autoimmune events during the cumulative abatacept treatment period did not increase relative to the short‐term treatment period (Table 3); in fact, the incidence decreased, as seen in other LTE studies of biologic agents in JIA 26, 27. One malignancy was previously reported during the short‐term treatment period (leukemia, diagnosed on day 89 of treatment) 12; no malignant neoplasms were reported during the LTE phase (Table 3). Seven patients became pregnant while enrolled in the LTE phase: 1 patient underwent an induced abortion, 4 gave birth to healthy babies, and outcomes for the remaining 2 patients were not reported. Abatacept was discontinued during each of the pregnancies.
Immunogenicity
Two patients developed antibodies to the whole abatacept molecule; in both patients, this occurred during year 2 of the LTE phase (1 patient from the double‐blind abatacept group, and 1 patient was an initial nonresponder). Neither of these 2 patients experienced infusion reactions, and both completed the LTE phase. Thirty‐nine patients exhibited an antibody response to the CTLA‐4 region alone, 31 of whom demonstrated this response during the double‐blind period (22 in the placebo group and 9 in the abatacept group) and 8 during the LTE phase (initial nonresponders). Of the 22 patients from the double‐blind placebo group, only 3 continued to demonstrate an antibody response after being switched back to abatacept therapy during the LTE period. Two of these patients experienced prespecified infusion reactions during the LTE phase (1 patient from the double‐blind abatacept group had mild vomiting [considered by the investigator as being related to treatment], and 1 from the double‐blind placebo group experienced mild dizziness and headache [considered by the investigator as unlikely to be related to treatment]). Nine patients who developed an antibody response to the CTLA‐4 region discontinued treatment during the LTE phase (3 due to lack of efficacy [2 initial nonresponders and 1 from the double‐blind placebo group], 2 who were lost to followup, and 4 due to other reasons).
Clinical efficacy
At the time of entry into the LTE phase, 49 (84.5%), 46 (79.3%), 32 (55.2%), and 24 (41.4%) of the patients who had received abatacept during the double‐blind withdrawal period had achieved ACR Pedi 30, ACR Pedi 50, ACR Pedi 70, and ACR Pedi 90 responses, respectively, whereas 18 patients (31.0%) had achieved clinically inactive disease. Owing to the withdrawal of abatacept for up to 6 months, fewer patients in the double‐blind placebo group (n = 59) had achieved ACR Pedi responses at the time they entered the LTE phase: 40 (67.8%), 31 (52.5%), 18 (30.5%), and 9 (15.3%) had achieved ACR Pedi 30, Pedi 50, Pedi 70, and Pedi 90 responses, respectively, and 6 (10.2%) had clinically inactive disease. The mean ACR Pedi responses for patients in the double‐blind placebo group increased within 6 months of reinstating abatacept therapy during the LTE phase 19, and these responses were therefore combined with those of patients from the double‐blind abatacept group starting from day 1 of the LTE phase (Figure 2A) (additional information is available from the corresponding author). For patients who did not achieve an ACR Pedi 30 response during the 4‐month lead‐in period, and thus entered the LTE phase directly, ACR Pedi responses gradually increased with continued long‐term abatacept therapy (Figure 2B).
Figure 2.
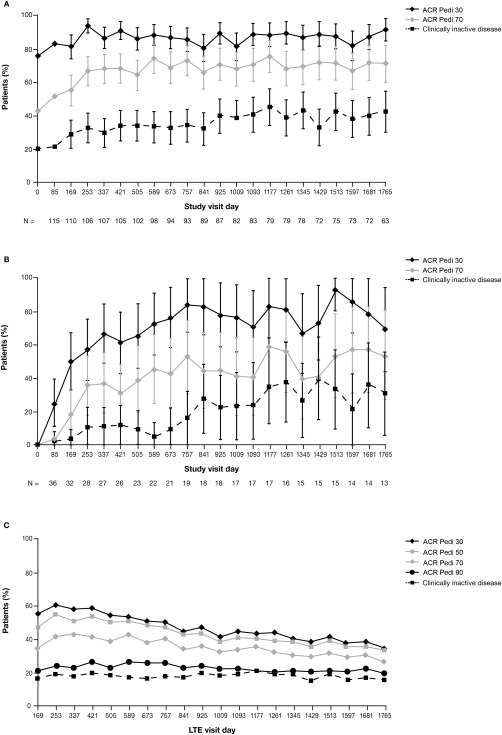
Proportions of juvenile idiopathic arthritis patients meeting the American College of Rheumatology (ACR) Pediatric 30 (Pedi 30), Pedi 50, Pedi 70, and Pedi 90 criteria for improvement and the proportions achieving an inactive disease status over time. A, Combined group of patients who received abatacept during the 6‐month double‐blind withdrawal period and those who received placebo during the 6‐month double‐blind withdrawal period. B, Patients who did not achieve an ACR Pedi 30 response during the initial 4‐month open‐label lead‐in period and who entered the long‐term extension (LTE) phase directly. In A and B, data were derived from as‐observed analyses based on all patients who entered the open‐label LTE. Bars show the 95% confidence intervals. n = number of patients with available data on each visit day. C, Intent‐to‐treat population. ACR Pedi responses throughout the LTE phase were also evaluated in a post hoc intent‐to‐treat analysis based on all 190 patients who had entered the lead‐in phase of the trial, with any patient who discontinued at any point considered a nonresponder. A total of 76 patients remained in the study and had efficacy data available on day 1,765.
ACR Pedi responses throughout the LTE phase were also evaluated in a post hoc intent‐to‐treat (ITT) analysis based on all 190 patients who had entered the lead‐in phase of the trial, with any patient who discontinued at any point or had missing data considered a nonresponder. In this analysis, the proportions of patients achieving an ACR Pedi 30 response, a Pedi 50 response, a Pedi 70 response, a Pedi 90 response, and clinically inactive disease status on day 169 of the LTE phase were as follows: 55.8% (95% CI 48.7–62.9%), 47.4% (95% CI 40.3–54.5%), 35.3% (95% CI 28.5–42.1%), 22.1% (95% CI 16.2–28.0%), and 17.4% (95% CI 12.0–22.8%), respectively. By day 1,765, these responses were achieved by 35.3% (95% CI 28.5–42.1%), 33.7% (95% CI 27.0–40.4%), 27.4% (95% CI 21.0–33.7%), 20.5% (95% CI 14.8–26.3%), and 16.3% (95% CI 11.1–21.6%) of patients, respectively (Figure 2C).
Patient‐reported outcomes
At baseline, the scores of the patients were lower than those of healthy children for all health concepts, as evaluated using the CHQ; following 4 months of abatacept treatment during the open‐label lead‐in period, substantial improvements were seen across all health concepts 18. By the start of the LTE phase, scores for all health concepts in both the double‐blind abatacept and double‐blind placebo groups had reached mean values within 2 SD of the mean for healthy controls. At the end of the LTE phase, mean scores for component items on the CHQ were comparable in all 3 subgroups (double‐blind abatacept, double‐blind placebo, and initial nonresponder groups) (additional information is available from the corresponding author) 22.
At baseline, the mean physical summary scores for the patients were below the range for healthy children (Figure 3A). Patients who were responders during the 4‐month lead‐in phase achieved initial improvements 18 that were sustained with continued long‐term treatment. Initial nonresponders experienced improvements if they continued to receive treatment during the open‐label LTE phase.
Figure 3.
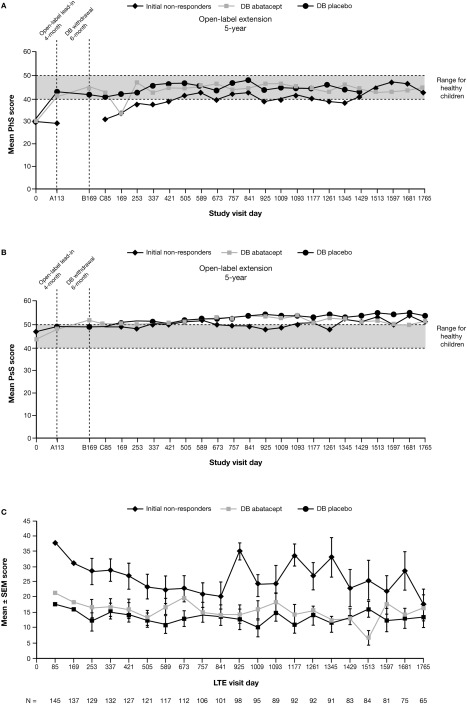
As‐observed and patient‐ and parent‐reported outcomes over time. A and B, Mean Child Health Questionnaire (CHQ) physical summary (PhS) scores (A) and mean CHQ psychosocial summary (PsS) scores (B). On the x‐axis, A, B, and C indicate the 3 study periods. The broken horizontal lines represent a standardized mean score of 50 (and 1 SD below [score of 40]) for the normative population (see ref. 22). C, Parent's global assessment of pain over time. n = the number of patients with available data at each visit day. DB = double‐blind outcomes during the open‐label long‐term extension (LTE) phase. Data were derived from an as‐observed analysis based on all patients who entered the LTE phase.
Physical summary scores in the double‐blind abatacept, double‐blind placebo, and initial nonresponder groups were within the normal range (≥40) in 67.3%, 68.6%, and 43.3% of patients, respectively, on day 169 and in 74.1%, 60.9%, and 66.7%, respectively, on day 1,765. The mean psychosocial summary scores for all 3 treatment groups were within or above the range for healthy children throughout the study (Figure 3B). Psychosocial summary scores were within the normal range (≥40) in 84.6% of patients in the double‐blind abatacept group, 84.3% of those in the double‐blind placebo group, and 80.0% of those in the initial nonresponder group on day 169 and in 85.2%, 100.0%, and 91.7%, respectively, on day 1,765.
During the open‐label lead‐in period, patients in all 3 treatment groups had experienced reductions in pain levels, as assessed using the parent's global assessment of pain, although reductions in the initial nonresponder group were not as great as those in the double‐blind abatacept and placebo groups, in which patients had achieved an ACR Pedi 30 response 18. By the start of the LTE phase, pain scores were further reduced in the double‐blind abatacept group. During the LTE phase, initial improvements in pain scores were maintained over time for patients in all 3 treatment groups who continued to receive treatment (Figure 3C). Freedom from pain (score of 0) was achieved in 20.4% of patients in the double‐blind abatacept group and in 15.7% of those in the placebo group on day 169 and in 17.9% and 16.0% of patients, respectively, on day 1,765. No initial nonresponders were ranked as being pain‐free at either time point.
Mean values for sleep quality had improved in patients during the open‐label lead‐in period and in the double‐blind abatacept group during the 6‐month withdrawal period, as assessed using the CSHQ 18. On day 1,765 of the LTE phase, all groups demonstrated improvement as demonstrated by mean ± SD reductions in the CSHQ total score (−6.36 ± 1.84, −4.63 ± 1.51, and −3.45 ± 1.96 for the double‐blind abatacept [n = 14], double‐blind placebo [n = 16], and initial nonresponder [n = 11] groups, respectively).
Participation in daily activities
Improvements in activity limitations were seen during the initial study periods 18 and were maintained with continued abatacept treatment, as assessed by the 3 questions regarding activity participation. On day 1,765 of the LTE phase, all groups demonstrated mean ± SD reductions in the number of missed parent/caregiver activity days per month (−5.52 ± 3.14, −2.43 ± 0.97, and −3.75 ± 2.27 for the double‐blind abatacept [n = 23], double‐blind placebo [n = 21], and initial nonresponder [n = 12] groups, respectively); reductions in the number of school days missed per month (−6.92 ± 2.45, −4.68 ± 1.88, and −2.08 ± 0.87 [n = 24, n = 22, and n = 12], respectively); and reductions in the number of days of paid care per month (−1.91 ± 1.24, −0.09 ± 0.09, and 0.17 ± 0.17 [n = 23, n = 22, and n = 12], respectively).
DISCUSSION
The LTE phase of this study provided an opportunity to evaluate the safety, clinical efficacy, and patient‐reported QOL in patients with JIA during long‐term treatment with abatacept (up to 7 years). The significantly improved efficacy of abatacept plus methotrexate compared with methotrexate alone in children with JIA who failed to achieve an adequate response to DMARDs, including anti‐TNF therapy, has already been established, with accompanying benefits to HRQOL and consistent safety 12, 18.
Abatacept was generally well tolerated up to a maximum exposure of 7 years, with no new safety signals and comparable safety between the 3 treatment groups (double‐blind abatacept, double‐blind placebo, and initial nonresponders). The IRs for AEs, SAEs, infections, serious infections, malignancies, and autoimmune events did not increase with continued exposure to abatacept during the LTE phase relative to the short‐term phase (combined lead‐in and double‐blind periods). Furthermore, the number of patients with reported immunogenic antibody responses did not increase during the LTE phase, and the presence of anti‐abatacept antibodies was not associated with any significant loss of efficacy or with safety concerns.
Abatacept was associated with clinical efficacy improvements as assessed using ACR Pedi responses during the initial 4‐month lead‐in phase; these improvements were maintained over time with continued treatment. Patients who had been randomized to the double‐blind placebo group during the withdrawal period had lower ACR Pedi responses at the start of the LTE phase compared with patients who had been randomized to the double‐blind abatacept group. By month 6 of the LTE phase, however, the mean ACR Pedi responses increased and were comparable with those observed in the double‐blind abatacept group. Furthermore, continued use of abatacept in patients who did not achieve an ACR Pedi 30 response within the first 4 months of treatment resulted in improved responses over time in some patients.
An objective of this long‐term analysis was to evaluate the maintenance of clinical efficacy with continued treatment. Given that a large proportion of the study population discontinued participation during the course of the LTE phase, 2 analysis methods were used to address this shortcoming. An as‐observed analysis was used to evaluate disease activity in patients who continued to receive abatacept. Of the 153 patients who entered the LTE phase, 69 (45%) remained in the study until the end; of the 84 patients who discontinued, 24 did so due to loss of efficacy of the study drug.
Currently, there is discussion in the rheumatology community regarding the best way to present long‐term followup efficacy data such as these, although no consensus or recommendations have yet been published 28. Therefore, we also performed an analysis of ACR Pedi responses in the ITT population, with nonresponder imputation. In this analysis, the proportions of patients achieving an ACR Pedi 90 response or clinically inactive disease status remained relatively stable during the LTE phase (21–27% and 16–22%, respectively). The proportions of patients determined to be demonstrating ACR Pedi 30 and Pedi 50 responses declined over time, which is likely reflective of patients discontinuing treatment. This represents a conservative assessment, because >50% of discontinuing patients stopped treatment because of reasons other than lack of efficacy or AEs. However, it should be noted that the discontinuation rate for abatacept, with 69 (36.3%) of 190 patients still receiving treatment at the last available followup visit (year 7), was similar to the rates for other biologic agents such as infliximab (36 [29.5%] of 122 patients at year 4) 27 and etanercept (26 [44.8%] of 58 patients at year 8) 26.
Patient‐reported end points allow us to evaluate tangible outcomes that may be more meaningful to patients in terms of their overall physical and psychological health. Such outcomes are recognized as a valuable addition to traditional clinical efficacy outcomes, and together they can provide an overall picture of how a treatment is affecting multiple aspects of a disease and associated QOL. Progressive improvements in the CHQ score were seen throughout the study for patients who continued to receive treatment, with these patients achieving mean scores that were comparable with those for healthy children. Improvements in CHQ summary scores that were gained after the initial short‐term treatment were also maintained with continued long‐term therapy and were generally within the range for healthy children. Patients in all treatment groups experienced reductions in pain and improvement in sleep quality that were maintained with continued treatment. Improvements in activity participation were also maintained with continued treatment and translated into meaningful reductions in the number of days of activity missed by parents and/or caregivers and the number of school days missed per month.
Long‐term followup data for the treatment of JIA with other available biologic therapies are scarce, with the exception of etanercept, for which clinical efficacy and safety have been demonstrated for up to 8 years 26. Sustained clinical efficacy was reported for patients continuing to receive etanercept treatment, with 26 (44.8%) of 58 LTE‐treated patients remaining in the study at final followup, and the safety profile was similar to that observed in patients with rheumatoid arthritis. Indirect comparisons of data from randomized clinical trials in JIA suggest that there are no significant differences in the short‐term efficacy of etanercept, adalimumab, and abatacept 29. No direct comparisons are available for the long‐term efficacy and safety of biologic therapies for the treatment of JIA. However, the maintained improvements in disease activity with long‐term abatacept treatment reported here appear to be consistent with observations for etanercept, suggesting that abatacept is a clinically efficacious alternative option that is also well tolerated and confers high patient acceptance.
These data show that long‐term treatment with abatacept for up to 7 years is associated with consistent safety relative to the short‐term period and, in patients continuing treatment, sustained efficacy and tangible QOL benefits, demonstrating that abatacept is a well‐tolerated and viable treatment for patients with JIA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Lovell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Lovell, Ruperto, Abud‐Mendoza, Burgos‐Vargas, Melo‐Gomes, Block, Giannini, Martini.
Acquisition of data. Lovell, Ruperto, Mouy, Paz, Rubio‐Pérez, Silva, Abud‐Mendoza, Burgos‐Vargas, Gerloni, Melo‐Gomes, Saad‐Magalhaes, Chavez‐Corrales, Huemer, Kivitz, Blanco, Foeldvari, Hofer, Huppertz, Deslandre, Minden, Punaro, Giannini, Martini.
Analysis and interpretation of data. Lovell, Ruperto, Abud‐Mendoza, Burgos‐Vargas, Huemer, Kivitz, Block, Giannini, Martini.
ROLE OF THE STUDY SPONSOR
Bristol‐Myers Squibb funded the study and reviewed and approved the manuscript prior to submission. The authors had ultimate control over the decision to publish and approved the final version of the manuscript submitted for publication. Professional medical writing and editorial assistance were provided by Eve Guichard, BSc (Hons), Caudex Medical, and were funded by Bristol‐Myers Squibb.
ACKNOWLEDGMENTS
We thank the following investigators who participated in the trial: Sheila Oliveira, MD, Flavio Sztajnbok, MD, Claudia Schainberg, MD, Morton Scheinberg, MD (Brazil); Brigitte Bader Meunier, MD, Michael Fischbach, MD, Irene Lemelle, MD, Anne Marie Prieur, MD (France); Javier Orozco, MD (Mexico); Maria Alessio, MD, Angelo Ravelli, MD, Loredana Lepore, MD, Elisabetta Cortis, MD, Fernanda Falcini, MD (Italy); Immaculada Calvo, MD (Spain); Anne B. Eberhard, MD, Phillip Hashkes, MD, Christine Hom, MD, Larry Jung, MD, Nancy Olson, MD, Carol Wallace, MD (US). We also thank Alejandro Flores Nunez, MD, Hospital del Nino Poblano, Puebla, Mexico, for his contributions to the manuscript, as well as Allison Covucci and Marleen Nys, Bristol‐Myers Squibb, for support with statistics.
ClinicalTrials.gov identifier: NCT00095173.
REFERENCES
- 1. Gortmaker SL, Sappenfield W. Chronic childhood disorders: prevalence and impact. Pediatr Clin North Am 1984;31:3–18. [DOI] [PubMed] [Google Scholar]
- 2. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. [DOI] [PubMed] [Google Scholar]
- 3. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 4. Oliveira S, Ravelli A, Pistorio A, Castell E, Malattia C, Prieur AM, et al, for the Pediatric Rheumatology International Trials Organization (PRINTO) . Proxy‐reported health‐related quality of life of patients with juvenile idiopathic arthritis: the Pediatric Rheumatology International Trials Organization multinational quality of life cohort study. Arthritis Rheum 2007;57:35–43. [DOI] [PubMed] [Google Scholar]
- 5. Solari N, Viola S, Pistorio A, Magni‐Manzoni S, Vitale R, Ruperto N, et al. Assessing current outcomes of juvenile idiopathic arthritis: a cross‐sectional study in a tertiary center sample. Arthritis Rheum 2008;59:1571–9. [DOI] [PubMed] [Google Scholar]
- 6. Bruns A, Hilario MO, Jennings F, Silva CA, Natour J. Quality of life and impact of the disease on primary caregivers of juvenile idiopathic arthritis patients. Joint Bone Spine 2008;75:149–54. [DOI] [PubMed] [Google Scholar]
- 7. De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367:2385–95. [DOI] [PubMed] [Google Scholar]
- 8. Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al, for the Pediatric Rheumatology Collaborative Study Group . Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med 2000;342:763–9. [DOI] [PubMed] [Google Scholar]
- 9. Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008;359:810–20. [DOI] [PubMed] [Google Scholar]
- 10. Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double‐blind, placebo‐controlled trial with the interleukin‐1 receptor antagonist anakinra in patients with systemic‐onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis 2011;70:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al, for the Paediatric Rheumatology International Trials Organisation and the Pediatric Rheumatology Collaborative Study Group . A randomized, placebo‐controlled trial of infliximab plus methotrexate for the treatment of polyarticular‐course juvenile rheumatoid arthritis. Arthritis Rheum 2007;56:3096–106. [DOI] [PubMed] [Google Scholar]
- 12. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Perez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 13. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367:2396–406. [DOI] [PubMed] [Google Scholar]
- 14. Ruperto N, Quartier P, Wulffraat N, Woo P, Ravelli A, Mouy R, et al, for the Paediatric Rheumatology International Clinical Trials Organisation . A phase II, multicenter, open‐label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum 2012;64:557–67. [DOI] [PubMed] [Google Scholar]
- 15. Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic‐onset juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled, withdrawal phase III trial. Lancet 2008;371:998–1006. [DOI] [PubMed] [Google Scholar]
- 16. Electronic Medicines Compendium (eMC) . Orencia summary of product characteristics. URL: http://www.medicines.org.uk/emc/medicine/19714/SPC/.
- 17. Yamada A, Salama A, Sayegh M. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol 2002;13:559–75. [DOI] [PubMed] [Google Scholar]
- 18. Ruperto N, Lovell DJ, Li T, Sztajnbok F, Goldenstein‐Schainberg C, Scheinberg M, et al, for the Paediatric Rheumatology International Trials Organisation (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG) . Abatacept improves health‐related quality of life, pain, sleep quality, and daily participation in subjects with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2010;62:1542–51. [DOI] [PubMed] [Google Scholar]
- 19. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Perez N, Silva CA, et al, for the Paediatric Rheumatology International Trials Organization and the Pediatric Rheumatology Collaborative Study Group . Long‐term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum 2010;62:1792–802. [DOI] [PubMed] [Google Scholar]
- 20. Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 21. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 22. Landgraf JM, Abetz L, Ware JE. The CHQ user's manual. 1st ed Boston: The Health Institute, New England Medical Center; 1996. [Google Scholar]
- 23. Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado‐West L, et al. Cross‐cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries: review of the general methodology. Clin Exp Rheumatol 2001;19:S1–9. [PubMed] [Google Scholar]
- 24. Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school‐aged children. Sleep 2000;23:1043–51. [PubMed] [Google Scholar]
- 25. Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school‐aged children. J Dev Behav Pediatr 2000;21:27–36. [DOI] [PubMed] [Google Scholar]
- 26. Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al, for the Pediatric Rheumatology Collaborative Study Group . Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 2008;58:1496–504. [DOI] [PubMed] [Google Scholar]
- 27. Ruperto N, Lovell DJ, Cuttica R, Woo P, Meiorin S, Wouters C, et al. Long‐term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular‐course juvenile rheumatoid arthritis: findings from an open‐label treatment extension. Ann Rheum Dis 2010;69:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buch MH, Aletaha D, Emery P, Smolen J. Reporting of long‐term extension studies: lack of consistency calls for consensus. Ann Rheum Dis 2011;70:886–90. [DOI] [PubMed] [Google Scholar]
- 29. Otten MH, Anink J, Spronk S, van Suijlekom‐Smit LW. Efficacy of biological agents in juvenile idiopathic arthritis: a systematic review using indirect comparisons. Ann Rheum Dis 2013;72:1806–12. [DOI] [PubMed] [Google Scholar]


