Abstract
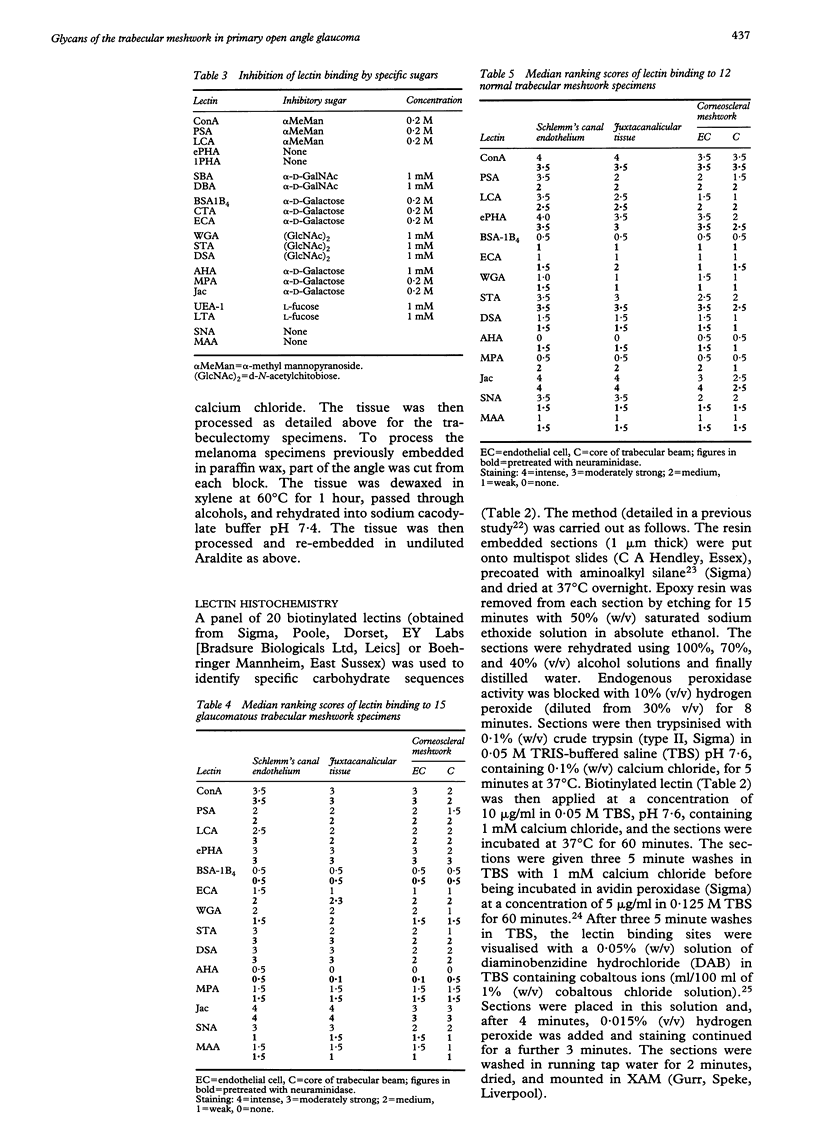
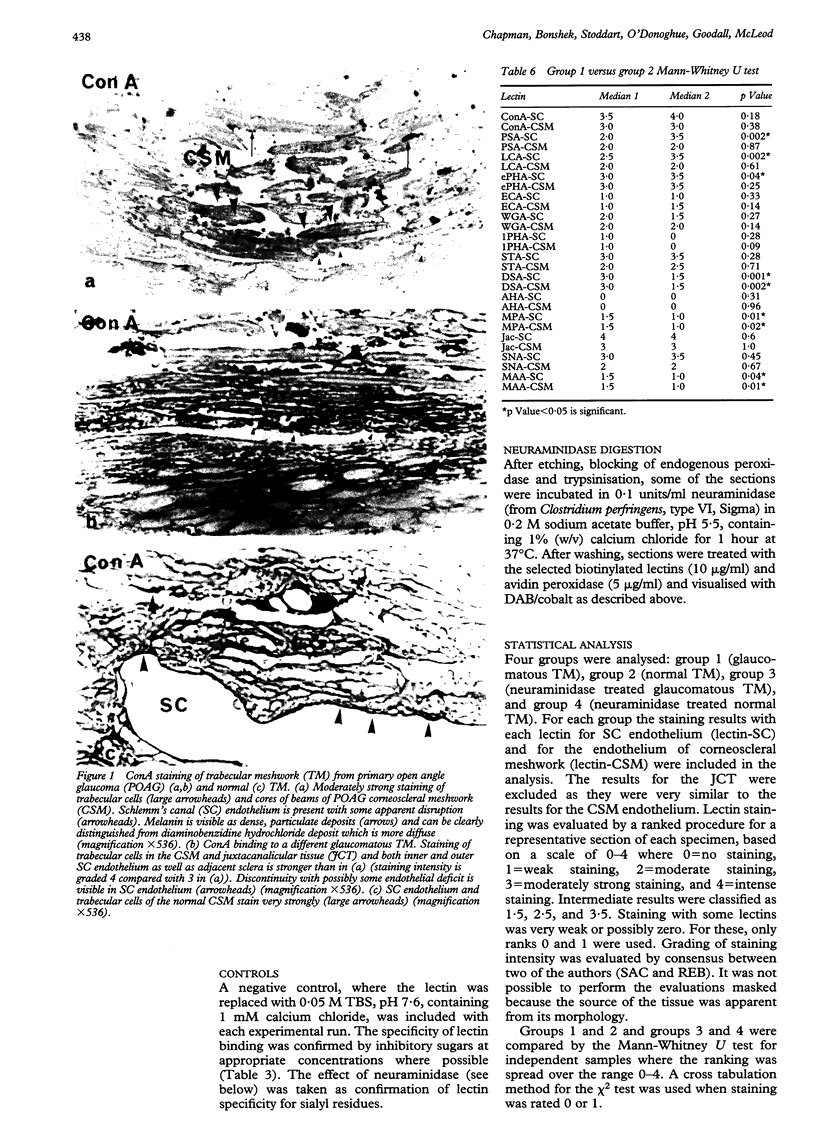
AIMS: Glycan expression was compared in glaucomatous trabecular meshwork (TM) and normal TM in order to determine any differences which may reflect pathological changes underlying primary open angle glaucoma (POAG). METHODS: Resin embedded TM from trabeculectomy specimens from 15 eyes with POAG and from 12 eyes with normal anterior segments were probed with a panel of biotinylated lectins and an avidin-peroxidase revealing system at the light microscope level. Statistical analyses were performed on the comparative staining results. RESULTS: The lectins ConA and ePHA showed strong staining in all areas of both glaucomatous and normal TM; ePHA staining of Schlemm's canal (SC) from POAG TM was significantly less than that from normal TM (ePHA-SC p = 0.04). The lectins PSA, LCA, and SNA bound moderately strongly to SC endothelium and weakly to the endothelium of the corneoscleral meshwork (CSM); glaucomatous SC endothelial binding was significantly less than that of normal SC endothelium for PSA and LCA (PSA-SC p = 0.002, LCA-SC p = 0.002). STA and DSA showed moderately strong binding while WGA, ECA, AHA, and MPA bound weakly throughout the TM; for DSA and MPA this staining was significantly greater in POAG than in normal TM (DSA-SC p = 0.001, DSA-CSM p = 0.002, MPA-SC p = 0.01, MPA-CSM p = 0.02). Jac stained strongly throughout the TM and showed no significant difference in POAG compared with normal TM (Jac-SC p = 0.6, Jac-CSM p = 1). 1PHA, SBA, DBA, CTA, UEA-1 and LTA did not bind to glaucomatous TM or normal TM. There were no age-related changes seen. CONCLUSIONS: The expression of some complex and hybrid, bisected and non-bisected N-linked glycans is significantly diminished in glaucomatous TM compared with normal TM. Some glycans with multiple N-acetylglucosamine residues and O-linked glycans with terminal and subterminal galactosyl groups are significantly increased in POAG TM. Glycan expression does not change significantly with age in POAG or normal TM.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed H., Chatterjee B. P. Further characterization and immunochemical studies on the carbohydrate specificity of jackfruit (Artocarpus integrifolia) lectin. J Biol Chem. 1989 Jun 5;264(16):9365–9372. [PubMed] [Google Scholar]
- Alvarado J. A., Murphy C. G. Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol. 1992 Dec;110(12):1769–1778. doi: 10.1001/archopht.1992.01080240109042. [DOI] [PubMed] [Google Scholar]
- Alvarado J. A., Murphy C. G. Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol. 1992 Dec;110(12):1769–1778. doi: 10.1001/archopht.1992.01080240109042. [DOI] [PubMed] [Google Scholar]
- Alvarado J., Murphy C., Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984 Jun;91(6):564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya L., Haraldsson M., Brewer C. F. Precipitation of galactose-specific lectins by complex-type oligosaccharides and glycopeptides: studies with lectins from Ricinus communis (agglutinin I), Erythrina indica, Erythrina arborescens, Abrus precatorius (agglutinin), and Glycine max (soybean). Biochemistry. 1988 Feb 9;27(3):1034–1041. doi: 10.1021/bi00403a028. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya L., Haraldsson M., Sharon N., Lis H., Brewer F. Binding and precipitating activities of Erythrina lectins with complex type carbohydrates and synthetic cluster glycosides. A comparative study of the lectins from E. corallodendron, E. cristagalli, E. flabelliformis, and E. indica. Glycoconj J. 1989;6(1):141–150. doi: 10.1007/BF01047896. [DOI] [PubMed] [Google Scholar]
- Chapman S. A., Bonshek R. E., Stoddart R. W., Jones C. J., Mackenzie K. R., O'Donoghue E., Mcleod D. Glycoconjugates of the human trabecular meshwork: a lectin histochemical study. Histochem J. 1995 Nov;27(11):869–881. [PubMed] [Google Scholar]
- Chapman S. A., Bonshek R. E., Stoddart R. W., Mackenzie K. R., McLeod D. Localisation of alpha(2,3) and alpha(2,6) linked terminal sialic acid groups in human trabecular meshwork. Br J Ophthalmol. 1994 Aug;78(8):632–637. doi: 10.1136/bjo.78.8.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Damjanov I. Lectin cytochemistry and histochemistry. Lab Invest. 1987 Jul;57(1):5–20. [PubMed] [Google Scholar]
- De Boeck H., Loontiens F. G., Lis H., Sharon N. Binding of simple carbohydrates and some N-acetyllactosamine-containing oligosaccharides to Erythrina cristagalli agglutinin as followed with a fluorescent indicator ligand. Arch Biochem Biophys. 1984 Oct;234(1):297–304. doi: 10.1016/0003-9861(84)90352-7. [DOI] [PubMed] [Google Scholar]
- Debray H., Decout D., Strecker G., Spik G., Montreuil J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem. 1981 Jun;117(1):41–55. doi: 10.1111/j.1432-1033.1981.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Duk M., Sticher U., Brossmer R., Lisowska E. The differences in significance of alpha 2,3Gal-linked and alpha 2,6GalNAc-linked sialic acid residues in blood group M- and N-related epitopes recognized by various monoclonal antibodies. Glycobiology. 1994 Apr;4(2):175–181. doi: 10.1093/glycob/4.2.175. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Kabat E. A. Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry. 1970 Feb 17;9(4):869–877. doi: 10.1021/bi00806a022. [DOI] [PubMed] [Google Scholar]
- Fine B. S., Yanoff M., Stone R. A. A clinicopathologic study of four cases of primary open-angle glaucoma compared to normal eyes. Am J Ophthalmol. 1981 Jan;91(1):88–105. doi: 10.1016/0002-9394(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Finkelstein I., Trope G. E., Basu P. K., Hasany S. M., Hunter W. S. Quantitative analysis of collagen content and amino acids in trabecular meshwork. Br J Ophthalmol. 1990 May;74(5):280–282. doi: 10.1136/bjo.74.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd B. B., Cleveland P. H., Worthen D. M. Fibronectin in human trabecular drainage channels. Invest Ophthalmol Vis Sci. 1985 Jun;26(6):797–804. [PubMed] [Google Scholar]
- Gallagher J. T., Morris A., Dexter T. M. Identification of two binding sites for wheat-germ agglutinin on polylactosamine-type oligosaccharides. Biochem J. 1985 Oct 1;231(1):115–122. doi: 10.1042/bj2310115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Grierson I., Howes R. C. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond) 1987;1(Pt 2):204–210. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- Grierson I. What is open angle glaucoma? Eye (Lond) 1987;1(Pt 1):15–28. doi: 10.1038/eye.1987.3. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Hammarström M. L., Sundblad G., Arnarp J., Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Stoddart R. W. A post-embedding avidin-biotin peroxidase system to demonstrate the light and electron microscopic localization of lectin binding sites in rat kidney tubules. Histochem J. 1986 Jul;18(7):371–379. doi: 10.1007/BF01675218. [DOI] [PubMed] [Google Scholar]
- Kress B. C., Weinreb R. N., Pinney E., Miller A. L. Surface glycoconjugates of cynomolgus monkey trabecular cells. Exp Eye Res. 1991 Dec;53(6):703–707. doi: 10.1016/0014-4835(91)90104-m. [DOI] [PubMed] [Google Scholar]
- Kurosawa A., Elner V. M., Yue B. Y., Elvart J. L., Tso M. O. Cultured trabecular-meshwork cells: immunohistochemical and lectin-binding characteristics. Exp Eye Res. 1987 Aug;45(2):239–251. doi: 10.1016/s0014-4835(87)80147-1. [DOI] [PubMed] [Google Scholar]
- Lee W. R., Grierson I. Relationships between intraocular pressure and the morphology of the outflow apparatus. Trans Ophthalmol Soc U K. 1974 Jul;94(2):430–449. [PubMed] [Google Scholar]
- Lotan R., Sharon N. Peanut (Arachis hypogaea) agglutinin. Methods Enzymol. 1978;50:361–367. doi: 10.1016/0076-6879(78)50043-8. [DOI] [PubMed] [Google Scholar]
- Maddox P. H., Jenkins D. 3-Aminopropyltriethoxysilane (APES): a new advance in section adhesion. J Clin Pathol. 1987 Oct;40(10):1256–1257. doi: 10.1136/jcp.40.10.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov A. P., Batmanov Y. E. Trabecular wall of Schlemm's canal in the early stage of primary open-angle glaucoma. Am J Ophthalmol. 1974 Oct;78(4):639–647. doi: 10.1016/s0002-9394(14)76302-0. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A., Sharon N. Immunochemical studies on the specificity of soybean agglutinin. Carbohydr Res. 1974 Oct;37(1):89–102. doi: 10.1016/s0008-6215(00)87066-4. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Kisailus E. C., Gruezo F., Kabat E. A. Immunochemical studies on the combining site of the blood group H-specific lectin 1 from Ulex europeus seeds. Arch Biochem Biophys. 1978 Jan 15;185(1):108–115. doi: 10.1016/0003-9861(78)90149-2. [DOI] [PubMed] [Google Scholar]
- Petryniak J., Goldstein I. J. Immunochemical studies on the interaction between synthetic glycoconjugates and alpha-L-fucosyl binding lectins. Biochemistry. 1986 May 20;25(10):2829–2838. doi: 10.1021/bi00358a014. [DOI] [PubMed] [Google Scholar]
- Quinton P. M., Philpott C. W. A role for anionic sites in epithelial architecture. Effects of cationic polymers on cell membrane structure. J Cell Biol. 1973 Mar;56(3):787–796. doi: 10.1083/jcb.56.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. M., Katz S. I., Foidart J. M., Spaeth G. L. Collagen, factor VIII antigen, and immunoglobulins in the human aqueous drainage channels. Ophthalmology. 1980 Apr;87(4):337–345. doi: 10.1016/s0161-6420(80)35242-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Spaeth G. L., Sivalingam E., Weinreb S. Histopathology of 150 trabeculectomy specimens in glaucoma. Trans Ophthalmol Soc U K. 1976 Jul;96(2):245–255. [PubMed] [Google Scholar]
- Rohen J. W., Futa R., Lütjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981 Oct;21(4):574–585. [PubMed] [Google Scholar]
- Rohen J. W., Lütjen-Drecoll E., Flügel C., Meyer M., Grierson I. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993 Jun;56(6):683–692. doi: 10.1006/exer.1993.1085. [DOI] [PubMed] [Google Scholar]
- Sarkar M., Wu A. M., Kabat E. A. Immunochemical studies on the carbohydrate specificity of Maclura pomifera lectin. Arch Biochem Biophys. 1981 Jun;209(1):204–218. doi: 10.1016/0003-9861(81)90273-3. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987 Feb 5;262(4):1596–1601. [PubMed] [Google Scholar]
- Shirato S., Murphy C. G., Bloom E., Franse-Carman L., Maglio M. T., Polansky J. R., Alvarado J. A. Kinetics of phagocytosis in trabecular meshwork cells. Flow cytometry and morphometry. Invest Ophthalmol Vis Sci. 1989 Dec;30(12):2499–2511. [PubMed] [Google Scholar]
- Spicer S. S., Schulte B. A. Diversity of cell glycoconjugates shown histochemically: a perspective. J Histochem Cytochem. 1992 Jan;40(1):1–38. doi: 10.1177/40.1.1370305. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Yamashita K., Suzuki K., Kobata A. Structural studies of the sugar chains of cold-insoluble globulin isolated from human plasma. J Biochem. 1980 Dec;88(6):1587–1594. doi: 10.1093/oxfordjournals.jbchem.a133133. [DOI] [PubMed] [Google Scholar]
- Torres B. V., McCrumb D. K., Smith D. F. Glycolipid-lectin interactions: reactivity of lectins from Helix pomatia, Wisteria floribunda, and Dolichos biflorus with glycolipids containing N-acetylgalactosamine. Arch Biochem Biophys. 1988 Apr;262(1):1–11. doi: 10.1016/0003-9861(88)90161-0. [DOI] [PubMed] [Google Scholar]
- Tripathi B. J., Marcus C. H., Tripathi R. C., Millard C. B., Gulcher J., Stefansson K. Monoclonal antibodies and lectins as probes for investigation of the cell biology of human trabecular meshwork: a preliminary report. Ophthalmic Res. 1989;21(1):27–32. doi: 10.1159/000266763. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. Aqueous outflow pathway in normal and glaucomatous eyes. Br J Ophthalmol. 1972 Mar;56(3):157–174. doi: 10.1136/bjo.56.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J., Spaeth G. L. Localization of sialic acid moieties in the endothelial lining of Schlemm's canal in normal and glaucomatous eyes. Exp Eye Res. 1987 Feb;44(2):293–306. doi: 10.1016/s0014-4835(87)80013-1. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Isolation and chemical characterization of a mitogenic lectin from Pisum sativum. J Biol Chem. 1974 Sep 25;249(18):6004–6012. [PubMed] [Google Scholar]
- Wood C., Kabat E. A., Murphy L. A., Goldstein I. J. Immunochemical studies of the combining sites of the two isolectins, A4 and B4, isolated from Bandeiraea simplicifolia. Arch Biochem Biophys. 1979 Nov;198(1):1–11. doi: 10.1016/0003-9861(79)90389-8. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Kobata A. Structural determinants of Phaseolus vulgaris erythroagglutinating lectin for oligosaccharides. J Biol Chem. 1983 Dec 25;258(24):14753–14755. [PubMed] [Google Scholar]
- Yamashita K., Totani K., Ohkura T., Takasaki S., Goldstein I. J., Kobata A. Carbohydrate binding properties of complex-type oligosaccharides on immobilized Datura stramonium lectin. J Biol Chem. 1987 Feb 5;262(4):1602–1607. [PubMed] [Google Scholar]