Abstract
Background:
Diabetes ketoacidosis (DKA) is a life-threatening complication of type 1 diabetes mellitus (T1DM). Reducing DKA admissions in children with T1DM requires a coordinated, comprehensive management plan. We aimed to decrease DKA admissions, 30-day readmissions, and length of stay (LOS) for DKA admissions.
Methods:
A multipronged intervention was designed in 2011 to reach all patients: (1) increase insulin pump use and basal-bolus regimen versus sliding scales, (2) transform educational program, (3) increased access to medical providers, and (4) support for patients and families. A before-after study was conducted comparing performance outcomes in years 2007-2010 (preintervention) to 2012-2014 (postintervention) using administrative data and Wilcoxon rank sum and Fischer exact tests.
Results:
DKA admissions decreased by 44% postintervention (16.7 vs 9.3 per 100 followed patient-years; P = .006), unique patient 30-day readmissions decreased from 20% to 5% postintervention (P = .001), and median LOS significantly decreased postintervention (P < .0001). Although not an original goal of the study, median hemoglobin A1C of a subset of the population transitioned from sliding scale decreased, 10.3% to 8.9% (P < .02).
Conclusions:
When clinical and widespread program interventions were used, significant reductions in DKA hospitalizations, 30-day readmissions, and LOS occurred for pediatric T1DM. Continuous performance improvement efforts are needed for improving DKA outcomes.
Keywords: admissions, diabetes ketoacidosis, length of stay, pediatrics, type 1 diabetes mellitus
Type 1 diabetes mellitus (T1DM) is a disorder in childhood and adolescence, which affects 1.54 per 1000 people younger than 20 years in the United States.1,2 The incidence of T1DM is increasing worldwide3 and has resulted in increased health care expenditures for patients with this disease.1 Moreover, this expense is far greater when associated with complications associated with T1DM. The major acute T1DM complication in childhood is diabetes ketoacidosis (DKA). With poor glycemic control, these DKA episodes can be quite frequent resulting in emergency department visits and admissions to the intensive care unit.4 DKA and other complications, like hypoglycemia, can be reduced by a comprehensive, multidisciplinary approach to disease management. Successful execution, using this approach, results in a significant reduction in patient morbidity and mortality as well as health care costs.4–6 In addition, optimal glycemic control is critical for preventing and delaying long-term complications related to T1DM.7,8
Recently, in the United Kingdom, adults with structured diabetes education and flexible basal-bolus insulin dosing regimens experienced a 61% reduction in risk for DKA and a 64% reduction in cost.9 Finding comparable reductions in pediatric populations is important, as a study done previously by our group, in 2005, showed that 7% of children with T1DM were admitted for DKA and the cost per patient was $4730 for that year of care.4 McEvilly and Kirk6 showed that the use of a multidisciplinary team in the home setting resulted in lower costs when compared with hospital bed use for children with diabetes. A recent report demonstrated a reduction in costs without reducing quality of care in Medicaid pediatric patients with many chronic disorders using the Accountable Care Organization model; however, they failed to improve DKA outcomes in their pediatric patient population.10 We wished to understand the barriers to care and apply the multidisciplinary approach with this understanding to reduce DKA admission using a 3-pronged approach.
We hypothesized that DKA admission rates, 30-day readmission rates (second admission within a 30-day period after the first admission of DKA), and length of stay (LOS) could be reduced in our patients by (1) intensification of insulin management using basal-bolus insulin regimens including increased utilization of insulin pump therapy,11 (2) new diabetes education program,12,13 (3) increased access to clinic appointments,14 and (4) development of patient and family support events and groups.15,16
METHODS
Setting
The hospital where this initiative took place is an urban tertiary care center, which is part of a larger health system where 93 000 patients are admitted each year. In 2014, the hospital saw over 500 unique patients with T1DM and has over 130 diabetes-related admissions per year. Previously, in the Division of Pediatric Endocrinology and Diabetes, there were 6 faculty members who managed diabetes within a faculty practice of 8 faculty members. Since our intervention, there are 7 faculty members who manage patients with diabetes within a faculty practice of 9. Also included in the care team of our patients are 3 nurse practitioners (NPs) who are certified diabetes educators (CDEs), 4 registered nurse (RN) educators, one of which is a CDE and the nurse manager, 2 registered dietitians (RDs) who are CDEs, and 1 licensed social worker (LSW) as well as research staff and administrative staff. In 2010, it was determined by the chief of the department and the nurse manager that the DKA admission rate (16.7 per 100 patient-years followed) was very high for established patients with diabetes. The Pediatric Diabetes Program was evaluated to determine areas that needed improvement.
Planning the intervention
Preintervention assessment involved analysis of identifiable causes and barriers of all diabetes-related admissions, which were divided into 3 categories: (1) clinical, (2) educational, and (3) structural.
Clinical
Assessment showed lack of communication between the multidisciplinary team of physicians, LSW, RDs, RNs, and NPs. Patients were admitted for “DKA” without adequate prerequisite criteria, such as a pH less than 7.30 and a serum bicarbonate less than 15 mmol/L.17 These patients often had high blood glucose levels or social concerns leading to the admission. The majority of patients were managed with sliding scale and neutral protamine Hagedorn (NPH) insulin regimens, which failed to provide required flexibility or intensiveness to manage diabetes well. The percentage of patients on insulin pump therapy lagged behind national rates and even the newer long-acting analogs, such as glargine and detemir, were used sparingly. Patients were frequently unaware of carbohydrate counting and therefore were not using it as a means to determine insulin dosage.
Educational
An initial educational assessment was done for RNs, NPs, and physicians. A lack of knowledge about insulin pump features among staff led to decreased prescribing of pump therapy. Nursing staff did not consistently adhere to national guidelines for diabetes education. This led to a failure to motivate patients to have goals and a failure to empower patients to manage their own diabetes.
A second educational assessment of school RNs suggested that they were unaware of new insulin analogs, insulin pumps, and correct treatment of hypoglycemia. An educational assessment of patients revealed a lack of knowledge about diabetes sick day management in the home setting, ketone testing, and lack of empowerment in making insulin dose changes based on glucose patterns. In addition, a lack of an institutionally approved and standardized diabetes education program and diabetes education manual led to conflicting recommendations given to patients by the staff. Finally, education materials in Spanish were unavailable for Spanish-speaking families who represent 40% of our total diabetes population.
Structural
There was a lack of coordination between providers and ancillary staff. There was also a lack of common goals regarding clinical outcomes for our diabetic patients. These structural deficiencies resulted in poor access to appointments, with increasing wait times. In particular, our diabetes clinic template only allowed for 15-minute visits per patient, which was not long enough to provide adequate education and care.
Intervention design
An intervention was designed to target each of the 3 above-mentioned aspects of our program: clinical, educational, and structural (summary in Table 1).
Table 1. Interventions.
| Intervention | Preassessment | Postassessment |
|---|---|---|
| Clinical |
|
|
| Educational |
|
|
| Structural |
|
|
Abbreviations: ADA, American Diabetes Association; CDE, certified diabetes educator; DKA, diabetes ketoacidosis; DSMES, Diabetes Self-Management Education and Support; NP, nurse practitioner; NPH, neutral protamine Hagedorn; RD, registered dietitian; RN, registered nurse.
First, weekly multidisciplinary preclinic meetings were instituted and all patients scheduled for the following week were discussed. The meeting allowed for an open discussion of patient-related problems and the role of each team member was defined a priori. A concerted effort was made to transition patients who were on sliding scales to basal-bolus regimens, including pumps or long-acting insulin analogs with fast-acting boluses, and the RD taught patients to incorporate carbohydrate counting. Finally, revised criteria for DKA admissions were established to include presence of 1.5 mmol/L of blood ketones, a pH less than 7.30, a serum bicarbonate less than 15 mmol/L, and/or clinical signs of dehydration.17
Second, educational goals were created on the basis of the planning assessments. Our primary objective with the education initiative was to target (a) staff, (b) patients and families, and (c) the community. Staff education for physicians, RNs, RDs, and NPs focused on American Diabetes Association (ADA) guidelines for best diabetes practices. The expectation was for all RNs, RDs, and NPs to become CDEs. Being a CDE requires that the health care provider have standardized knowledge, understanding, and experience in diabetes prevention and diabetes care.18
For patient and family education, our goal was to become an ADA-recognized program. The ADA guidelines support the use of the national standards for Diabetes Self-Management Education and Support (DSMES).19 In addition, we also used Chronicle, a database for diabetes management capture provided by the ADA.20 This program allowed us to track individual patients and their specific clinical goals. Patient educational materials and content were developed both for English and Spanish speakers. All educational programs were based on DSMES guidelines.
For community education, we organized 2 community events for patients annually: the Family Diabetes Day and the Candy Exchange. The Family Diabetes Day program provides information on a variety of topics including global and local diabetes programs, healthy eating and exercise, and insurance and legal rights of patients with diabetes. Guest speakers included prominent public figures living with diabetes as well as other patients with diabetes who have successful lives. The Candy Exchange was developed as an event to promote healthy eating after Halloween. At this event, children exchanged their candy for a toy and are therefore rewarded for choosing to be healthy and not eat all their Halloween candy. The event also gives patients and their families the opportunity to interact with diabetes educators, technology companies, dentists, and ophthalmologists. In addition to these 2 events, we have quarterly educational programs for school RNs and home health RNs in the surrounding area. We have also participated in Shabbatons (educational programs held on Shabbat) for the Orthodox Jewish Community.
Third, structural changes were made when the division hired new physicians and staff who were specifically dedicated to diabetes practice. We increased access for patients by doubling the number of diabetes sessions from 5 to 10 clinic sessions per week. To allow for more comprehensive visits and increased patient interactions, the clinic templates for diabetes were changed from 15- to 30-minute visits. We created a model for physician-NP collaboration, with alternating appointments with an NP and a physician for a total of at least 4 diabetes appointments per year.21 With this approach, our patients now have additional opportunities to see providers at different times of the day and alternate sites closer to home.
Methods of evaluation and analysis
Statistical analysis
The unit of analysis for this study was T1DM patient-years followed. Patients contributed 1 “followed patient-year” if they were seen in the diabetes ambulatory clinic at any point during that calendar year. Admissions were excluded from analysis if the patient was not seen in the diabetes ambulatory clinic in the 12 months prior to that admission or if the admission was not related to diabetes (eg, broken arm). Descriptive statistics (means, medians, standard deviations, and rates) were used to present demographics and outcomes of interest in the pre- (2007-2010) and postintervention (2012-July 2014) periods. Given the nonnormal distribution of the outcomes of interest (admissions, admissions with DKA, and LOS), these outcomes' medians were compared with the Wilcoxon rank sum test, although rates are also presented. All admissions with an LOS greater than 9 days (n = 32) were reviewed and 5 admissions were removed from the LOS calculation (2 preintervention and 3 postintervention). These were removed because the LOS was increased due to non-diabetes-related issues (eg, prolonged hospital stay awaiting placement in foster care). We acknowledge and caution readers that the same patient may have contributed more than one followed patient-year in the pre- and/or postintervention periods, leading to a violation of the independence assumption. In other words, some patients were part of the pre- and postintervention period simply because they were continually followed up in our diabetes center and therefore the comparative populations represent different periods not necessarily different groups of unique patients.
Given the large percentage of patients who had no 30-day readmissions, this outcome was dichotomized into unique patients ever readmitted versus never readmitted in the pre- and postintervention periods. This avoids violating the independence assumption. Once dichotomized, the Fischer exact test was used to examine differences between the pre- and postintervention periods.
In terms of process measures, hemoglobin A1C (HbA1C) data were used to determine the status of glycemic control for the population. Ninety-six subjects were examined between 2009 and 2011 and postintervention. The sample was picked on the basis of the availability of at least 2 appointments per year and those patients that were on sliding scale regimens only. The data were analyzed using the nonparametric Kruskal-Wallis test, and the median HbA1C with 25th and 75th percentiles for this population is reported.
This study was approved by the Ethics Committee of Human Experimentation and in accordance with the Declaration of Helsinki and deemed exempt by the local Institutional review board. There are no conflicts of interest to report.
RESULTS
Demographics of cohort
Table 2 presents patient demographic characteristics. The majority of our patients were of racial minorities: multiracial origin and African American (68% preintervention and 64% postintervention). We have a preponderance of patients who were on Medicaid/Medicare, and over time our insurance distribution among the pre- and postintervention remained constant. Over the course of the intervention, there was an increase in the T1DM population at the hospital.
Table 2. Demographics of Followed Patient-Years Pre- and Postintervention.
| Preintervention (2007-2010) N = 1329a | Postintervention (2012-July 2014) N = 1291a | |
|---|---|---|
| Unique patients followed at any point | 523 | 596 |
| Mean patients followed per year | 332.5 | 516.4 |
| Race, n (%) | ||
| White | 120 (9) | 236 (18) |
| Black or African American | 339 (26) | 305 (24) |
| Asian | 18 (1) | 8 (1) |
| Multiracial | 557 (42) | 521 (40) |
| Declined/unknown | 295 (22) | 218 (17) |
| American Indian or Alaskan Native | – | 1 (0) |
| Native Hawaiian or Pacific Islander | – | 2 (0) |
| Ethnicity | ||
| Hispanic, n (%) | 810 (61) | 687 (53) |
| Insurance type, n (%) | ||
| Commercial | 566 (43) | 523 (41) |
| Medicaid/Medicare | 734 (55) | 738 (57) |
| Self-pay | 29 (2) | 30 (2) |
| Mean age in years, n (SD) | 13.7 (4.6) | 14.2 (4.6) |
aPatients contributed 1 followed patient-year if they were seen in the diabetes ambulatory clinic at any point during that calendar year.
Intervention outcomes
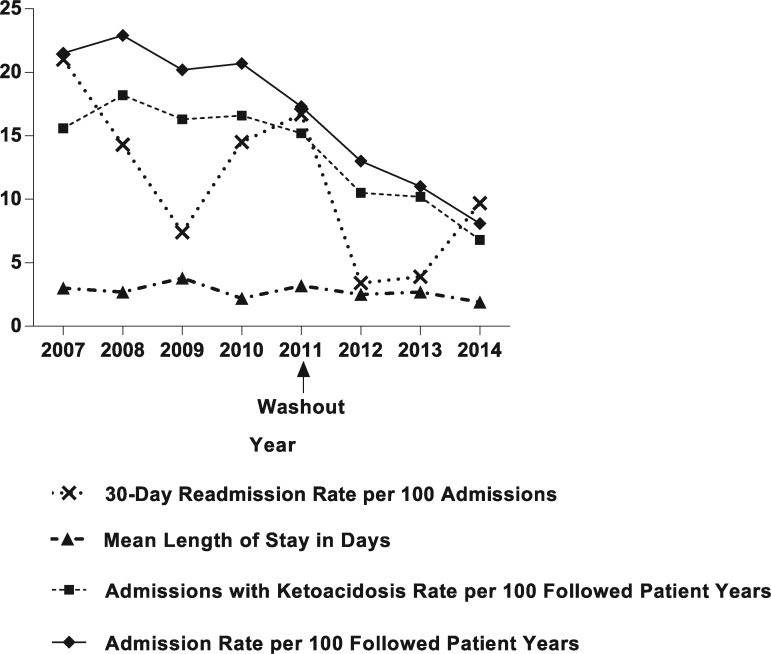
In the intervention period, there were statistically significant reductions in all 3 outcomes of interest: median admissions with ketoacidosis per patient per year (P = .006), median LOS (P < .0001), and unique patient 30-day readmissions (P = .001) (Table 3). These data are also depicted as a run chart from 2007 to 2014 (Figure).
Table 3. Admissions, Lengths of Stay, and Readmissions Pre- and Postintervention.
| Preintervention (2007-2010) | Postintervention (2012-July 2014) | P Value | |
|---|---|---|---|
| Total admissions | 283 | 140 | |
| Admission rate per 100 followed patient-yearsa | 21.3 | 10.8 | |
| Median admissions per patient per year, n (range) | 0 (0-10) | 0 (0-3) | .0005b |
| Admissions with ketoacidosis | 222 | 120 | |
| Admissions with ketoacidosis rate per 100 followed patient-years | 16.7 | 9.3 | |
| Median admissions with ketoacidosis per patient per year, n (range) | 0 (0-8) | 0 (0-3) | .006b |
| Median length of stay in days, n (range)c | 2 (1-47) | 2 (1-38) | <.0001b |
| Total 30-d readmissions | 40 | 7 | |
| 30-d readmission rate per 100 admissions | 14.1 | 5.0 | |
| Unique patient 30-d readmissionsd | |||
| Admitted and readmitted, n (%) | 17 (20) | 6 (5) | .001 |
| Admitted but not readmitted, n (%) | 68 (80) | 114 (95) | |
aPatients contributed 1 followed patient-year if they were seen in the diabetes ambulatory clinic at any point during that calendar year.
bCompares median using Wilcoxon rank sum test.
cFive patients were removed (2 preintervention and 3 postintervention) because length of stay was increased due to non-diabetes-related issues, such as placement in foster care.
dPatients were counted once if they were readmitted at any time in the preperiod and then once again if they were readmitted at any time in the postperiod. Compares pre versus postperiod using the Fischer exact test.
Figure.
Readmission rate, length of stay, and admission rates before and after intervention.
Median and 25th- and 75th-percentile HbA1C for a subset of patients (n = 96) was 10.35 (90 mmol/mol) (9.3% [78 mmol/mol] to 11% [97 mmol/mol]) in 2009 and 8.9% (74 mmol/mol) (8% [64 mmol/mol] to 10.58% [92 mmol/mol]) in 2014 (P < .02). There was a marked improvement in glycemic control in this poorly controlled cohort, previously managed on rapid-acting insulin sliding scales and transitioned to intensive insulin management with insulin pump therapy and/or multidose injections using a basal-bolus regimen. Our interventions had a direct benefit to our patients and there were no risks to our patients. During the intervention, positive effects were seen in our patients; however, the trends we see might be a source of an association rather than interventional effects.
DISCUSSION
This quality improvement initiative provides a model for improving care in an underserved urban population with diabetes. We demonstrate that during our use of a multidisciplinary approach to diabetes management, with the incorporation of more intensive insulin regimens, standardizing diabetes education, and empowering community engagement, we were able to improve DKA admissions and readmissions. Our outcomes improved in the challenging face of increasing total number of patients into our practice and faculty transitions. Although there was faculty turnover during the period being studied, the net total number of faculty caring for patients with diabetes remained approximately the same and in some months, fewer than at onset. There was an increase in NP staff from 1 to 3 and RDs from 1 to 2. A nurse manager was added to coordinate the ADA program and streamline diabetes education. These programmatic changes allowed us to successfully manage our growing patient population without having to increase the number of physician faculty members.
One of our interventions was increased use of insulin pump therapy and multiple daily insulin injection use in our patients. Despite the initial expense and time commitment that is associated with insulin pump therapy, insulin pump therapy is very effective in improving glycemic control. The overall cost-benefit supports its use for diabetic patients.22,23
Improved DSMES in the inpatient setting successfully decreases all-cause diabetes admissions and readmissions.24 Diabetes education handbooks as well as structured diabetes education programs in adults has improved glycemic control as shown in the Dose Adjustments for Normal Eating (DAFNE) trial and post-DAFNE follow-up of patients.25–27 This study also standardized T1DM education for all patients and families. The ADA has guidelines and a recognition program for diabetes education based on the National Standards of Diabetes Self-Management Education and Support (NDSMES).28 The NDSMES aims to support informed decision making, self-care behaviors, problem solving, and active collaboration with the collaborative approach with the health care team to improve health outcomes. In 2013, we became the first ADA-recognized pediatric diabetes program in the area. A similar multidisciplinary team approach decreased LOS for patients with T1DM in the United Kingdom.6 In addition, other studies have shown that expanded T1DM care outside the standard provider offices, such as home health nursing and school-based clinics, improved care and glycemic control.29,30
The setting of this project has a diverse population with a large number of racial minorities. In adult Medicare patients, African Americans and Hispanics had a greater readmission rates for diabetes than other races and ethnicities.31 These disparities in outcomes can be addressed through targeting improvements in access for these patients.32 Increasing access to diabetes appointments, allowing patients to have more frequent visits, and increasing appointments from 15 to 30 minutes allow for greater quality and more time for education during visits. There is paucity of literature on the ideal appointment time that a patient with diabetes requires to have a comprehensive evaluation. Some diabetes centers utilize appreciably longer appointment times to include clinical services to address proper eye, foot, and dental care during each visit.33 Similar to findings from Perros and Frier,34 we decreased wait times for appointments and saw improved T1DM outcomes by increasing the number of sessions and improving the patient-to-provider ratio.
One limitation of our project is being a single-site study, which may not be applicable to all practice settings. The hospital presented here is an Accountable Care Organization35,36 and has a strong culture emphasizing quality of care and efforts to reduce cost and waste. Hence, the hospital was very supportive in providing us the necessary resources to improve care for this challenging population. Other institutions that fail to invest in proper staffing for patient care on the outpatient diabetes management will see increased expenditure on the inpatient hospitalization for DKA, and this should be undertaken despite the costs of additional committed personnel.37 Although we saw an improvement in DKA and glycemic control in our patients, optimal glycemic targets lagged and need improvement. In addition, as this is a before-after study design, unmeasured secular factors may have contributed to our change in outcomes such as individual variation, family support, and socioeconomic factors. Finally, the use of before-after data that may violate the independence assumption of the statistical tests utilized in the research could reduce the significance of findings, although all outcomes were highly significant.
The hospital is always in the process of continuous improvement. We plan to continue to encourage insulin intensification and expand our program through community outreach event offerings, improved educational materials, and increased appointment availability. In 2015, we started a separate diabetes RN educator and RD-run clinic supervised by an NP for our high-risk patients who need to be seen more frequently. It should be noted that this clinic and all these interventions discussed are available for both patients with type 1 and type 2 diabetes. We believe these efforts will continue to show a decrease in DKA admission rates, overall admission rates, and improvement in glycemic control of our patients. We also recently hired a psychologist to address the mental health needs of our patients with diabetes and to offer support to patients as they transition from supervised care to more independent self-management.
There are many further studies that must be done. In the future, we could survey patients and staff before and after initiation of individual interventions, assess long-term success, and include additional interventions and baseline assessments.
CONCLUSIONS
DKA admission rates seem to be reduced by targeting specific aspects of patient care such as diabetes education to patients, families, and health care providers. Successful self-management starts with the provision of proper treatment tools such as insulin pump therapy, easy access to providers, and psychosocial empowerment for patients to take charge of the lives.
Footnotes
Authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Dahlquist G, Mustonen L. Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Swedish Childhood Diabetes Study Group. Acta Paediatr. 2000;89(10):1231–1237. [DOI] [PubMed] [Google Scholar]
- 2.Puett RC, Lamichhane AP, Nichols MD, et al. Neighborhood context and incidence of type 1 diabetes: the SEARCH for diabetes in youth study. Health Place. 2012;18(4):911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannini C, Mohn A, Chiarelli F. Technology and the issue of cost/benefit in diabetes. Diabetes Metab Res Rev. 2009;25(suppl 1)(1):S34–S44. [DOI] [PubMed] [Google Scholar]
- 4.Ying AK, Lairson DR, Giardino AP, et al. Predictors of direct costs of diabetes care in pediatric patients with type 1 diabetes. Pediatr Diabetes. 2011;12(3 Pt 1):177–182. [DOI] [PubMed] [Google Scholar]
- 5.Bratcher CR, Bello E. Traditional or centralized models of diabetes care: the multidisciplinary diabetes team approach. J Fam Pract. 2011;60(11 suppl):S6–S11. [PubMed] [Google Scholar]
- 6.McEvilly A, Kirk J. Twenty years of a multidisciplinary paediatric diabetes home care unit. Arch Dis Child. 2005;90(4):342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Lachin J, Cleary P, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171(5):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott J, Jacques RM, Kruger J, et al. Substantial reductions in the number of diabetic ketoacidosis and severe hypoglycaemia episodes requiring emergency treatment lead to reduced costs after structured education in adults with type 1 diabetes. Diabet Med. 2014;31(7):847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582–e589. [DOI] [PubMed] [Google Scholar]
- 11.Pankowska E, Blazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes. 2009;10(1):52–58. [DOI] [PubMed] [Google Scholar]
- 12.Selea A, Sumarac-Dumanovic M, Pesic M, et al. The effects of education with printed material on glycemic control in patients with diabetes type 2 treated with different therapeutic regimens. Vojnosanit Pregl. 2011;68(8):676–683. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad B, Ramadas A, Kia Fatt Q, Md Zain AZ. A pilot study: the development of a culturally tailored Malaysian Diabetes Education Module (MY-DEMO) based on the Health Belief Model. BMC Endocr Disord. 2014;14(31):31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr CJ, Hopman W, Yen JL, Houlden RL. Long-term efficacy of insulin pump therapy on glycemic control in adults with type 1 diabetes mellitus. Diabetes Technol Ther. 2015;17(1):49–54. [DOI] [PubMed] [Google Scholar]
- 15.Ingerski LM, Anderson BJ, Dolan LM, Hood KK. Blood glucose monitoring and glycemic control in adolescence: contribution of diabetes-specific responsibility and family conflict. J Adolesc Health. 2010;47(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald PE, Wykle ML, Misra R, Suwonnaroop N, Burant CJ. Predictors of social support, acceptance, health-promoting behaviors, and glycemic control in African-Americans with type 2 diabetes. J Natl Black Nurses Assoc. 2002;13(1):23–30. [PubMed] [Google Scholar]
- 17.American Diabetes Association. Hospital admission guidelines for diabetes mellitus. Diabetes Care. 2003;26(suppl 1)(1):S118.. [DOI] [PubMed] [Google Scholar]
- 18.National Certification Board for Diabetes Educators. What is a CDE? http://www.ncbde.org/certification_info/what-is-a-cde/. Published 2015.
- 19.Krebs JD, Parry-Strong A, Gamble E, et al. A structured, group-based diabetes self-management education (DSME) programme for people, families and whanau with type 2 diabetes (T2DM) in New Zealand: an observational study. Prim Care Diabetes. 2013;7(2):151–158. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Siminerio LM. Educators' insights in using chronicle diabetes: a data management system for diabetes education. Diabetes Educ. 2013;39(2):248–254. [DOI] [PubMed] [Google Scholar]
- 21.Heptulla RA, Choi SJ, Belamarich PF. A quality improvement intervention to increase access to pediatric subspecialty practice. Pediatrics. 2013;131(2):e585–e590. [DOI] [PubMed] [Google Scholar]
- 22.Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. [DOI] [PubMed] [Google Scholar]
- 23.Conget Donlo I, Serrano Contreras D, Rodriguez Barrios JM, Levy Mizrahi I, Castell Abat C, Roze S. Cost-utility analysis of insulin pumps compared to multiple daily doses of insulin in patients with type 1 diabetes mellitus in Spain. Rev Esp Salud Publica. 2006;80(6):679–695. [DOI] [PubMed] [Google Scholar]
- 24.Healy SJ, Black D, Harris C, Lorenz A, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care. 2013;36(10):2960–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre HD. DAFNE (Dose Adjustment for Normal Eating): structured education in insulin replacement therapy for type 1 diabetes. Med J Aust. 2006;184(7):317–318. [DOI] [PubMed] [Google Scholar]
- 26.Dinneen SF, O'Hara MC, Byrne M, et al. The Irish DAFNE study protocol: a cluster randomised trial of group versus individual follow-up after structured education for type 1 diabetes. Trials. 2009;10(88):88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snow R, Sandall J, Humphrey C. Use of clinical targets in diabetes patient education: qualitative analysis of the expectations and impact of a structured self-management programme in type 1 diabetes. Diabet Med. 2014;31(6):733–738. [DOI] [PubMed] [Google Scholar]
- 28.Haas L, Maryniuk M, Beck J, et al. National standards for diabetes self-management education and support. Diabetes Educ. 2012;38(5):619–629. [DOI] [PubMed] [Google Scholar]
- 29.Handley MA, Shumway M, Schillinger D. Cost-effectiveness of automated telephone self-management support with nurse care management among patients with diabetes. Ann Fam Med. 2008;6(6):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strawhacker MT. Multidisciplinary teaming to promote effective management of type 1 diabetes for adolescents. J Sch Health. 2001;71(6):213–217. [DOI] [PubMed] [Google Scholar]
- 31.Jiang HJ, Andrews R, Stryer D, Friedman B. Racial/ethnic disparities in potentially preventable readmissions: the case of diabetes. Am J Public Health. 2005;95(9):1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schectman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruger DF, Lorenzi GM, Dokken BB, Sadler CE, Mann K, Valentine V. Managing diabetes with integrated teams: maximizing your efforts with limited time. Postgrad Med. 2012;124(2):64–76. [DOI] [PubMed] [Google Scholar]
- 34.Perros P, Frier BM. An audit of waiting times in the diabetic outpatient clinic: role of patients' punctuality and level of medical staffing. Diabet Med. 1996;13(7)669–673. [DOI] [PubMed] [Google Scholar]
- 35.Lowell KH, Bertko J. The Accountable Care Organization (ACO) model: building blocks for success. J Ambul Care Manage. 2010;33(1):81–88. [DOI] [PubMed] [Google Scholar]
- 36.Farrell P, Barnaby S, Galarza T, et al. Population management of diabetes in a high-need urban community in the Bronx: the experience of Montefiore Medical Center. Diabetes Educ. 2013;39(4):515–522. [DOI] [PubMed] [Google Scholar]
- 37.Martin AL. Changes and consistencies in diabetes education over 5 years: results of the 2010 National Diabetes Education Practice Survey. Diabetes Educ. 2012;38(1):35–46. [DOI] [PubMed] [Google Scholar]