Abstract
AIMS: To evaluate the effects of allopurinol in lens induced uveitis (LIU) by morphological methods and to compare these effects with those of steroids and a combination of both drugs biochemically and morphologically. METHODS: Lipid peroxides (LPO) of the retinal tissue were determined by two different methods (thiobarbituric acid assay (TBA) and high performance liquid chromatography expressed as malondialdehyde-like substances). Myeloperoxidase (MPO) activity in the iris/ciliary body complex was analysed spectrophotometrically. Histological changes on three morphological levels of LIU eyes were evaluated. RESULTS: Both allopurinol and the combination of allopurinol/prednisolone led to a significant reduction in the increaed retinal LPO values. Prednisolone only revealed significant effects on retinal LPO when being measured with the TBA method. MPO activity in iris and ciliary body was significantly reduced in all therapy groups. The morphological evaluation of the sections by two masked investigators revealed a significant reduction (p < 0.05) in the inflammation score in all therapy groups. Morphometric studies using the QUANTIMED system (Leica, Cambridge) showed significantly reduced values (p < 0.05) in the allopurinol group and in the group receiving prednisolone and allopurinol. Prednisolone alone did not lead to a significant reduction in the values. CONCLUSIONS: The findings show that both allopurinol and steroids exert positive effects on the variables determined in LIU. The effects of steroids are believed to be mostly due to their direct action on inflammatory cells. The recently reported scavenging effects of methylprednisolone should play a minor role in this disease model. Allopurinol and oxypurinol act as direct scavengers of free radicals and hypochlorous acid, which is produced via MPO catalysis, thus leading to a reduction in tissue inflammation and tissue damage.
Full text
PDF
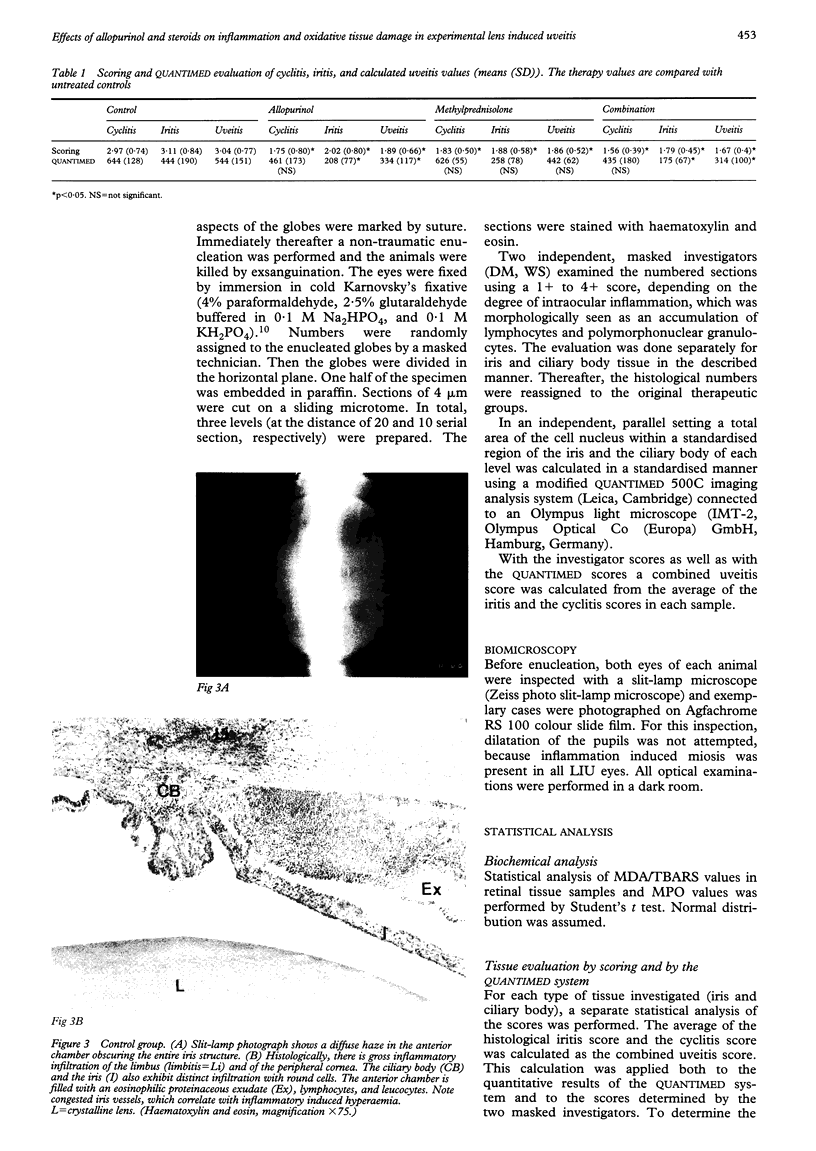
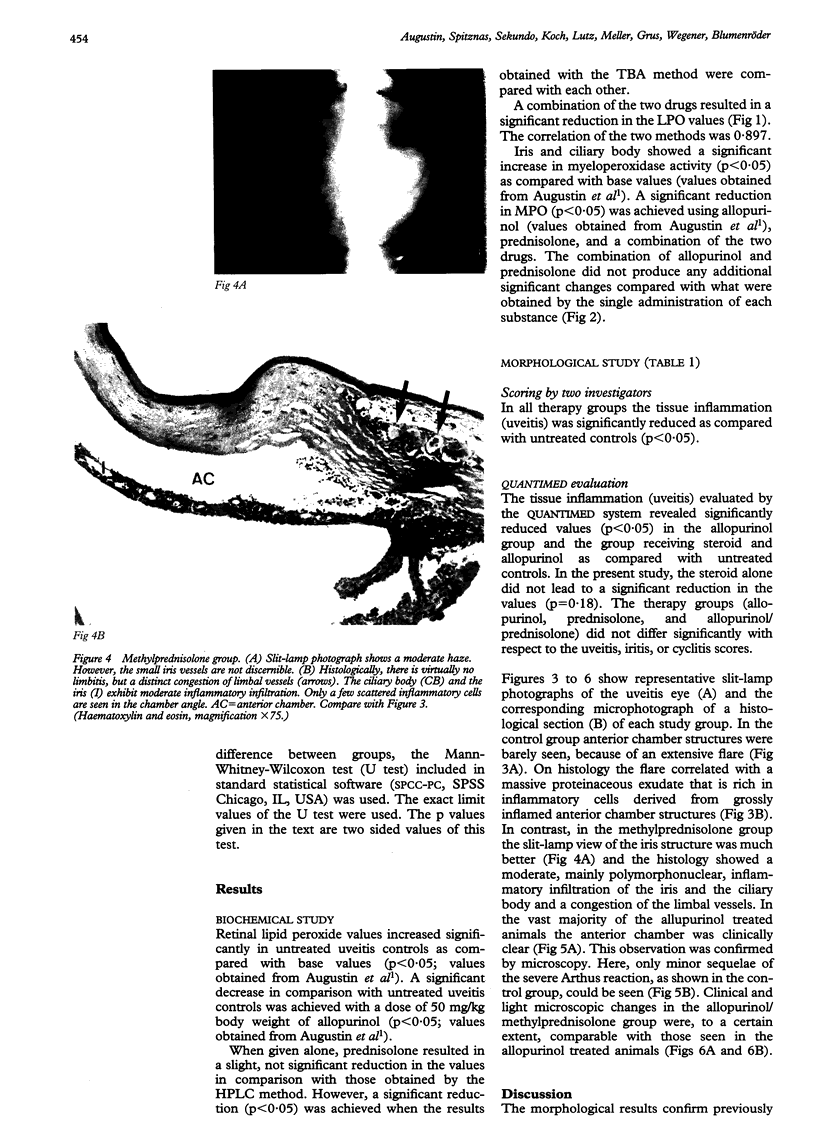

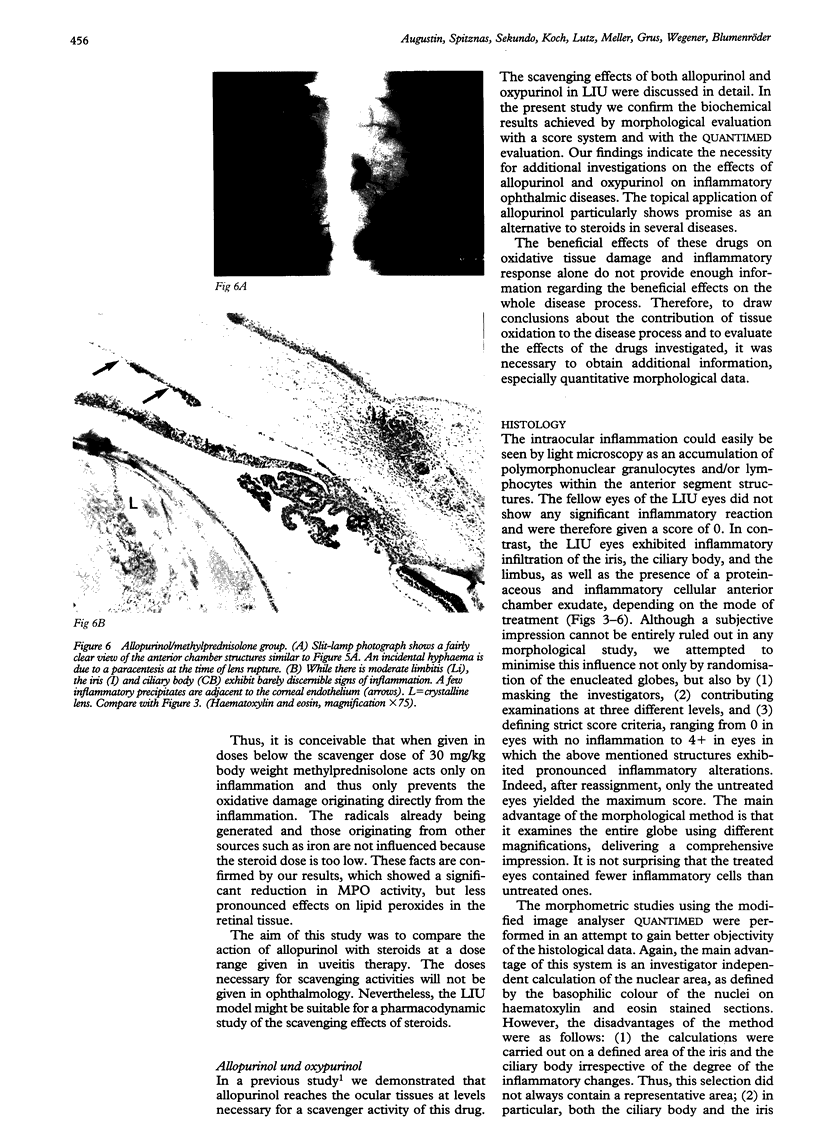

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A. J., Böker T., Blumenröder S. H., Lutz J., Spitznas M. Free radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitis. Invest Ophthalmol Vis Sci. 1994 Oct;35(11):3897–3904. [PubMed] [Google Scholar]
- Augustin A. J., Lutz J. Intestinal, hepatic and renal production of thiobarbituric acid reactive substances and myeloperoxidase activity after temporary aortic occlusion and reperfusion. Life Sci. 1991;49(13):961–968. doi: 10.1016/0024-3205(91)90079-q. [DOI] [PubMed] [Google Scholar]
- Bazan N. G., de Abreu M. T., Bazan H. E., Belfort R Júnior Arachidonic acid cascade and platelet-activating factor in the network of eye inflammatory mediators: therapeutic implications in uveitis. Int Ophthalmol. 1990 Oct;14(5-6):335–344. doi: 10.1007/BF00163553. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Lang J., Zadravec S., Slater T. F. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984;105:319–328. doi: 10.1016/s0076-6879(84)05041-2. [DOI] [PubMed] [Google Scholar]
- Gadaleta D., Verma M., Davis J. M. Inhibition of neutrophil leukotriene generation by the 21-aminosteroid, U-74389F. J Surg Res. 1994 Aug;57(2):233–237. doi: 10.1006/jsre.1994.1137. [DOI] [PubMed] [Google Scholar]
- Goto H., Wu G. S., Gritz D. C., Atalla L. R., Rao N. A. Chemotactic activity of the peroxidized retinal membrane lipids in experimental autoimmune uveitis. Curr Eye Res. 1991 Nov;10(11):1009–1014. doi: 10.3109/02713689109020339. [DOI] [PubMed] [Google Scholar]
- Hall E. D. Lipid antioxidants in acute central nervous system injury. Ann Emerg Med. 1993 Jun;22(6):1022–1027. doi: 10.1016/s0196-0644(05)82745-3. [DOI] [PubMed] [Google Scholar]
- Hall E. D. The effects of glucocorticoid and nonglucocorticoid steroids on acute neuronal degeneration. Adv Neurol. 1993;59:241–248. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Rao N. A., Calandra A. J., Sevanian A., Bowe B., Delmage J. M., Marak G. E., Jr Modulation of lens-induced uveitis by superoxide dismutase. Ophthalmic Res. 1986;18(1):41–46. doi: 10.1159/000265413. [DOI] [PubMed] [Google Scholar]
- Rao N. A. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990;88:797–850. [PMC free article] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. D., Mao G. D., Rabinovitch A., Poznansky M. J. Inhibition of superoxide-generating NADPH oxidase of human neutrophils by lazaroids (21-aminosteroids and 2-methylaminochromans). Biochem Pharmacol. 1993 Jan 7;45(1):241–251. doi: 10.1016/0006-2952(93)90398-g. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Sessa G., Bevans V. Effect of DMSO on the stabilization of lysosomes by cortisone and chloroquine in vitro. Ann N Y Acad Sci. 1967 Mar 15;141(1):326–332. doi: 10.1111/j.1749-6632.1967.tb34897.x. [DOI] [PubMed] [Google Scholar]
- Wu G. S., Sevanian A., Rao N. A. Detection of retinal lipid hydroperoxides in experimental uveitis. Free Radic Biol Med. 1992;12(1):19–27. doi: 10.1016/0891-5849(92)90054-k. [DOI] [PubMed] [Google Scholar]