Background:
The anterolateral thigh (ALT) flap has been widely used for reconstructions. Nevertheless, the atherosclerotic risk factors that affect the lateral circumflex femoral artery (LCFA) are still inconclusive. The aim was to study the effect of atherosclerosis on the LCFA and descending branch (dLCFA) visualized by computer tomographic angiography (CTA) between nonatherosclerosis and atherosclerosis.
Methods:
Retrospective studies of CTA of lower extremity were reviewed. The patients were divided into 2 groups: nonatherosclerotic and atherosclerotic risk factors. The angiographic study of LCFA and dLCFA was analyzed, and atherosclerotic and nonatherosclerotic risk factors were compared.
Results:
Ninety-seven patients with 194 lower extremities were enrolled. Atherosclerotic risks comprised 76 patients. A total of 14, 16, and 46 patients had 1, 2, and 3 risk factors, respectively. Musculocutaneous perforator was 79.38%. The LCFA originated from deep femoral, common femoral, and superficial femoral artery was 97.42%, 2.06%, and 0.52%, respectively. The dLCFA was classified into 5 types depending on its origin. Diameters of LCFA in nonatherosclerotic and atherosclerotic patients were 4.03 ± 0.71 and 4.07 ± 0.97 mm, respectively. No statistical significance was found between both groups in diameters of LCFA. Diameters of dLCFA in nonatherosclerotic patients were 2.28 ± 0.28 mm and in atherosclerotic patients were 2.11 ± 0.28 mm. Statistical significance of diameters of dLCFA was found in patients having 3 risk factors and smoker groups (p < 0.05).
Conclusions:
LCFA is not atherosclerosis resistant. Stenosis of the LCFA and dLCFA occurred in varying degrees in atherosclerosis-risk patients. Preoperative CTA should be considered to evaluate the patency in multiple risk factors patients.
The anterolateral thigh (ALT) flap was first described by Song et al1 in 1984. The advantages of this flap are pliable, long pedicle, good esthetic result, and low donor-site morbidity. The ALT flap has been used widely for both microsurgery and pedicle flap reconstructions. Moreover, ALT can be employed for myocutaneous,2 adipofascial,3,4 and suprafascial flaps.5 The first described vascular supply of the ALT was the descending branch of the lateral circumflex femoral artery (dLCFA), which passed between the vastus lateralis and rectus femoris muscle and was classified as a septocutaneous perforator. Recent studies have reported that the septocutaneous perforator constitutes only 6.97% to 28.6%.6–8 However, most reports focused on the anatomic variation of the vascular pedicle and modification of this flap in both western and Asian populations.6–14
Nevertheless, among elderly patients with multiple underlying diseases, the safety of using this flap for microsurgery reconstruction is unclear and still debated. Only a few studies in the English literature have investigated the patency of the LCFA in atherosclerosis,15–17and the effects of atherosclerotic risk factors on LCFA are inconclusive.
The aim was to study the effect of atherosclerosis on the LCFA and dLCFA visualized by computer tomographic angiography (CTA) between nonatherosclerosis and atherosclerosis.
MATERIALS AND METHODS
Approval of this study was obtained from the ethics committee of Phramongkutklao Hospital and College of Medicine. A 3-year retrospective review of patients who underwent CTA of the lower extremities from January 2013 to December 2015 was conducted. The patients were divided in 2 groups. Group 1 comprised patients with nonatherosclerotic risk factors, and indications for CTA included preoperative vascular assessment for the ALT or fibular free flap for head and neck tumors. The patients in group 2 had atherosclerotic risk factors and indications for CTA, including peripheral vascular disease (PVD) with symptoms or chronic ulcer of the legs or feet. The atherosclerotic risk factors, based on Framingham risk score,11 included total and high-density lipoprotein cholesterol, hypertension, smoking, and diabetes mellitus (DM). Exclusion criteria included previous fracture or vascular injury or surgery of the lower extremities and vessel pathology, such as connective tissue disease (vasculitis).
Data were recorded including age, sex, underlying disease, atherosclerotic risk factor, and indication for CTA. The CT scanner used was a Siemens Somatom Sensation 64 multidetector row CT scanner (Siemens Medical Solutions, Malvern, Pa.). A standard bolus of 100 mL of intravenous ultravist 370 (Berlex Laboratories, Montville, N.J.) or Omnipaque 350 (GE Healthcare, Inc, Princeton, N.J.) was used for contrast. The LCFA and dLCFA were evaluated by a single radiologist. The volumetric data acquired were then used to reconstruct images with a slice width of 1.3 mm and reconstruction interval of 0.6 mm. The angiographic study of the deep femoral artery (DFA), LCFA, and dLCFA were analyzed, including the diameter of vessels, origin of LCFA, type of perforator, length of LCFA, length of anterior superior iliac spine (ASIS) to origin of LCFA, and length of ASIS to the largest perforator of dLCFA. Diameters of each vessel were measured at the origin of its branching off. Atherosclerotic and nonatherosclerotic risk factors were compared. The degree of stenosis was evaluated by 2 radiologists, and scores were defined as 0 for less than 20% greatest stenosis, 1 for 20% to 49% greatest stenosis, 2 for 50% to 99% greatest stenosis, and 3 for totally occluded.18
Statistical Analyses
For comparative study statistics, the chi-square or Mann-Whitney U test for categorical data and Student t test for continuous data were used. A P value <0.05 was considered to indicate statistical significance.
RESULTS
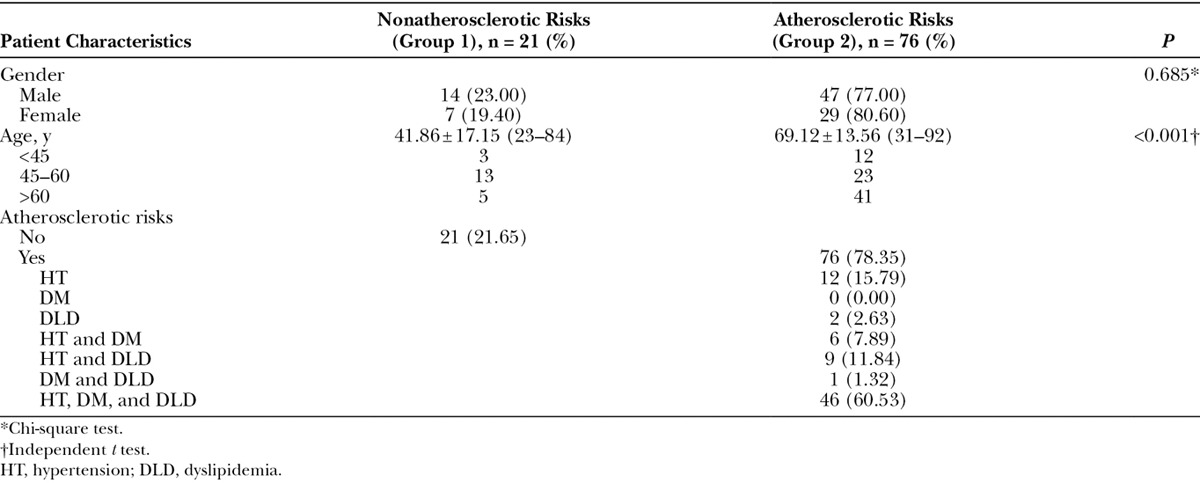
A total of 115 patients who underwent CTA of the lower extremities were recorded. Eighteen patients were excluded because of previous hip or knee arthroplasty and vascular injury at the thigh. The remaining 97 patients with 194 lower extremities were enrolled. All patients were Asian with 61 men (62.89%) and 36 women (37.11%). A total of 76 (78.35%) patients had atherosclerotic risks and 21 (21.65%) patients had nonatherosclerotic risk. The mean ages in nonatherosclerotic and atherosclerotic groups were 41.86 and 69.12 years, respectively. The mean age was statistically significant between groups (Table 1). Among the atherosclerotic risk patients, 14 patients (18.42%) had 1 risk factor (hypertension, DM, or dyslipidemia), 16 patients (21.05%) had 2 risk factors (hypertension and DM, hypertension, and dyslipidemia or DM and dyslipidemia), and 46 patients (60.53%) had 3 risk factors (combined hypertension, DM, and dyslipidemia). Seventy-three patients (96.05 %) had hypertension, 58 patients (76.32%) had dyslipidemia, and 53 patients (69.74 %) had DM.
Table 1.
Patient Characteristics
According to the type of perforator, 154 extremities (79.38%) were musculocutaneous type and 40 extremities (20.62%) were septocutaneous type. No statistical significance was found between nonatherosclerotic and atherosclerotic patients regarding the type of perforator (Table 2).
Table 2.
Characteristics of Lateral Circumflex Femoral Artery and Descending Branch of Lateral Circumflex Femoral Artery
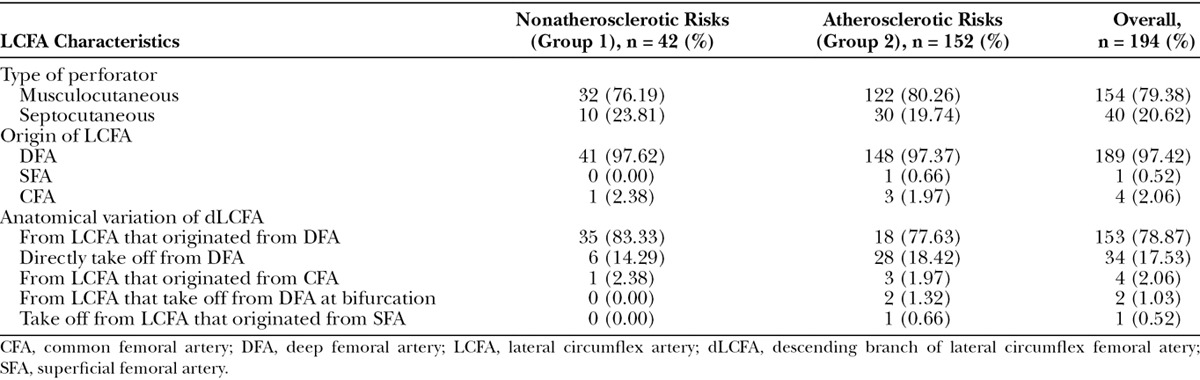
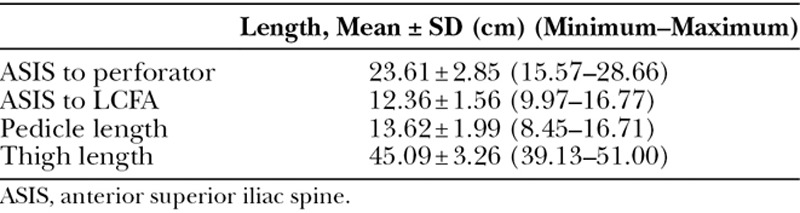
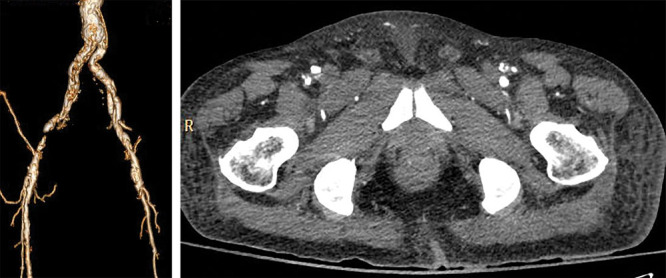
According to origin of LCFA, 189 extremities (97.42%) found that the LCFA originated from the DFA, 4 extremities (2.06 %) originated from the common femoral artery, and 1 extremity (0.52 %) had a takeoff from the superficial femoral artery. No statistical significance was found between nonatherosclerotic and atherosclerotic patients in the origin of LCFA. The dLCFA was classified into 5 types depending on its origin (Fig. 1). A total of 153 extremities (78.87 %) had descending branch takeoff from LCFA that originated from the DFA, 34 extremities (17.53%) had descending branch direct take off from the DFA, 4 extremities (2.06 %) had the LCFA take off from the common femoral artery, 2 extremities (1.03 %) had the LCFA take off from the DFA at bifurcation (Fig. 2), and 1 extremity (0.52 %) had descending branch takeoff from the LCFA that originated from the superficial femoral artery (Fig. 3). No statistical significance was found between nonatherosclerotic and atherosclerotic patients regarding the origin of the dLCFA (Table 2). The average thigh length was 45.09 ± 3.26 cm. The average length from ASIS to perforator was 23.61 ± 2.85 cm. The average length from ASIS to LCFA was 12.36 ± 1.56 cm, and the average pedicle length was 13.62 ± 1.99 cm (Table 3).
Fig. 1.
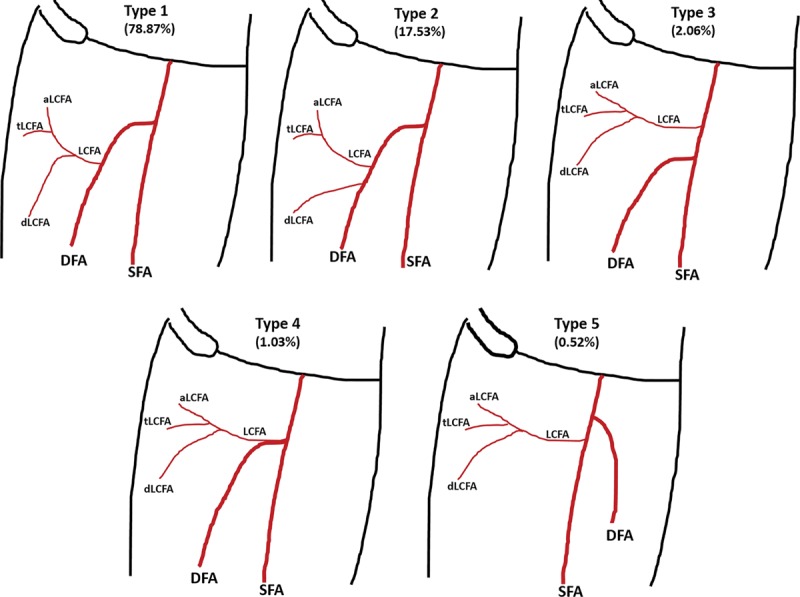
Anatomical variation of LCFA and its branches. aLCFA, ascending branch of LCFA; SFA, superficial femoral artery; tLCFA, transverse branch of LCFA; dLCFA, descending branch of lateral circumglex femoral artery.
Fig. 2.
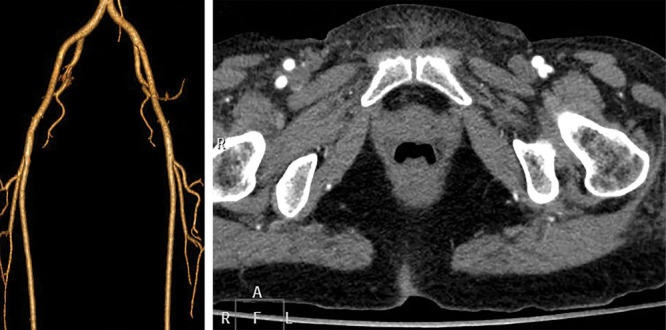
Left LCFA originating at bifurcation of the superficial and deep femoral arteries but right LCFA originating from DFA. CTA 3D reconstruction (A) and axial view (B).
Fig. 3.
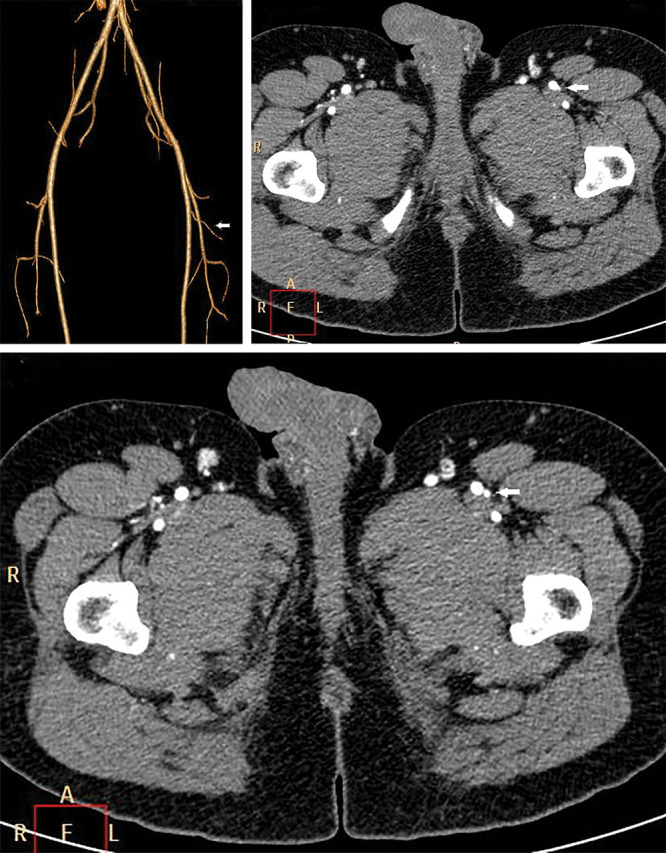
Left LCFA originating from the superficial femoral artery (arrow). CTA 3D reconstruction (A) and axial view (B and C).
Table 3.
Length of LCFA and Perforator
Diameters of the DFA in nonatherosclerotic patients were 5.84 ± 0.99 and 5.93 ± 1.11 mm in atherosclerotic patients. Diameters of DFA in atherosclerotic patients were classified in each risk factor as demonstrated in Table 4. No statistical significance was found between nonatherosclerotic and atherosclerotic patients in diameters of DFA. Diameters of the LCFA in nonatherosclerotic patients were 4.03 ± 0.71 and 4.07 ± 0.97 mm in atherosclerotic patients. Diameters of LCFA in atherosclerotic patients were classified in each risk factor as demonstrated in Table 5. No statistical significance was found between nonatherosclerotic and atherosclerotic patients in diameter of LCFA.
Table 4.
Comparison of Internal Diameter of Deep Femoral Artery, Lateral Circumflex Femoral Artery, and Descending Branch of Lateral Circumflex Femoral Artery Between Nonatherosclerosis and Atherosclerosis-risk Factors
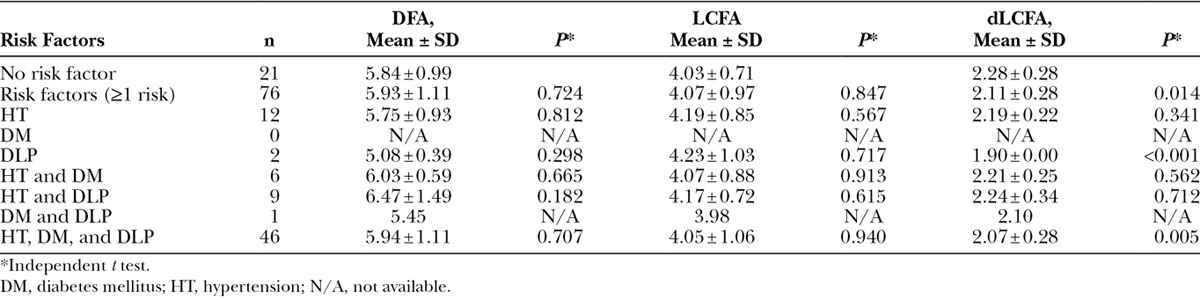
Table 5.
Comparison of Internal Diameter of Deep Femoral Artery, Lateral Circumflex Femoral Artery, and Descending Branch of Lateral Circumflex Femoral Artery in Nonsmoker and Smoker
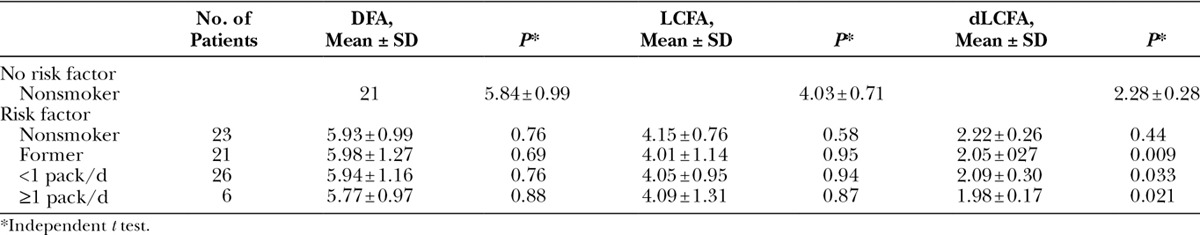
Diameters of dLCFA in nonatherosclerotic patients were 2.28 ± 0.28 and 2.11 ± 0.28 mm in atherosclerotic patients. Statistical significance was found between nonatherosclerotic and atherosclerotic patients in diameters of dLCFA. After subgroup analysis of the diameters of dLCFA in each risk factors, significance was found between nonatherosclerotic and patients having 3 risk factors (P < 0.05). All patients in the nonatherosclerotic risk factor group were nonsmokers. In the atherosclerotic risk factor group, patients were classified as nonsmoking, former smoking, smoking less than 1 pack per day, and smoking equal to or more than 1 pack per day.
The mean diameter of DFA for nonsmokers without risk factor was 5.84 mm, for nonsmokers with risk factor was 5.93 mm, for former smokers with risk factor was 5.98 mm, for smokers of less than 1 pack per day with risk factor was 5.94 mm, and for smokers of more than 1 pack per day with risk factor was 5.77 mm. No statistical significance was found between nonsmoker and smoker groups.
The mean diameter of LCFA in nonsmokers without risk factor was 4.03 mm, in nonsmokers with risk factor was 4.15 mm, in former smokers with risk factor was 4.01 mm, in smokers of less than 1 pack per day with risk factor was 4.05 mm, and in smokers of more than 1 pack per day with risk factor was 4.09 mm. No statistical significance was found between nonsmoker and smoker groups.
The mean diameter of dLCFA in nonsmokers without risk factor was 2.28 mm, in nonsmokers with risk factor was 2.22 mm, in former smokers with risk factor was 2.05 mm, in smokers of less than 1 pack per day with risk factor was 2.09 mm, and in smokers of more than 1 pack per day with risk factor was 1.98 mm. Statistical significance was found between nonsmokers and smokers in the diameters of dLCFA.
According to the sclerosis change of DFA, score 0 was found for 26 extremities (92.86%) in 1 risk factor patient, 28 extremities (87.50%) in 2 risk factors patients, and 76 extremities (82.61%) in 3 risk factors patients (Table 6) (Figs. 4 and 5). According to the sclerosis change of LCFA, score 0 was found for 24 extremities (85.71%) in 1 risk factor patient, 26 extremities (81.25%) in 2 risk factors patients, and 72 extremities (78.26%) in 3 risk factors patients. According to the sclerosis change of dLCFA, score 0 was found for 24 extremities (85.71%) in 1 risk factor patient, 26 extremities (81.25%) in 2 risk factors patients, and 72 extremities (78.26%) in 3 risk factors patients. The occlusion of LCFA more than 50% was found in 4 extremities and only in 2 or more risk factors. No totally occluded (score 3) case was found in all patients.
Table 6.
Comparison of Sclerosis Change of Deep Femoral Artery, Lateral Circumflex Femoral Artery, and Descending Branch of Lateral Circumflex Femoral Artery in Atherosclerosis Risk Factors
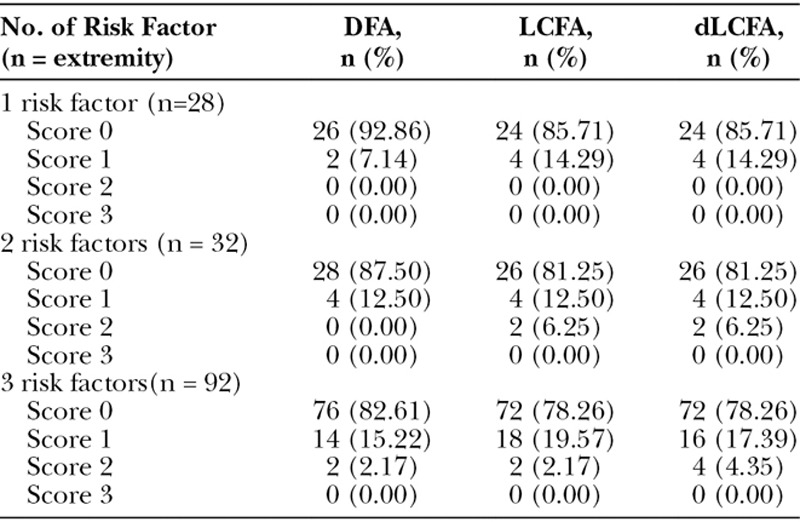
Fig. 4.
Atherosclerosis of the common femoral artery, superficial femoral, deep femoral artery, and lateral circumflex femoral artery. CTA 3D reconstruction demonstrating the irregularity of the wall of vessels (A) and axial view showing the plaque in the right deep femoral artery (B).
Fig. 5.
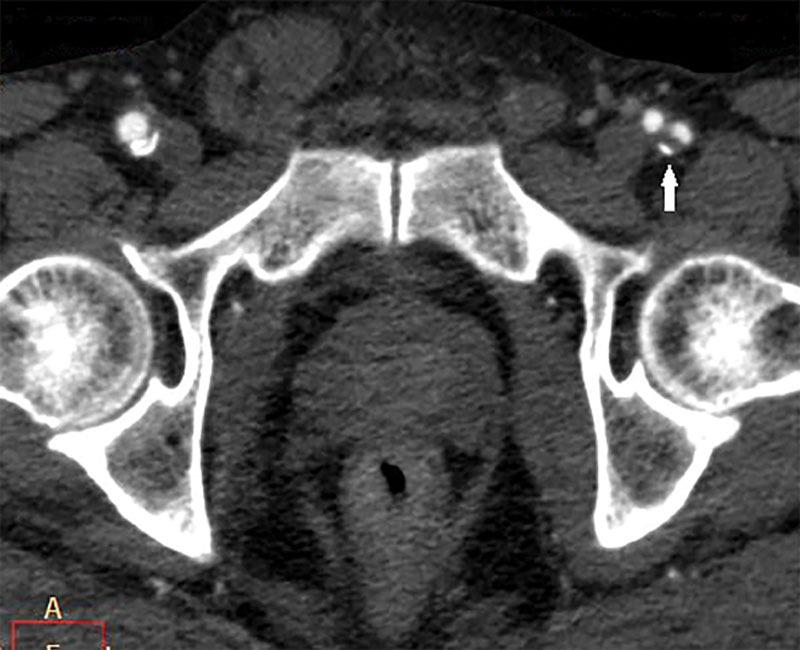
Calcified plaque (white arrow) in the left deep femoral artery (adjusted window width view to clarify the bone and calcified plaque).
DISCUSSION
Although the ALT flap is widely used in reconstructive surgery, the anatomic variation has appeared increasingly. Most of patients with diagnosed head and neck tumors and undergoing ALT microvascular reconstruction are elderly and have multiple underlying diseases. Only a few studies in the English literature have investigated the patency of LCFA in atherosclerosis,15–17 and the effects of these risk factors on LCFA are still inconclusive.
Halvorson et al15 studied patients with vascular disease and concluded that the dLCFA and DFA seem to be relatively spared from atherosclerosis and be relatively atherosclerosis resistant. Ahn et al17 showed that the dLCFA is not affected by patient comorbidity, including PVD when compared with medically fit controls. Even in individuals with severe disease of the superficial vascular system, sparing of the deep system was observed often.17
Kamdar et al19 argued that LCFA is not always atherosclerosis resistant and reported the reconstructed anterior scalp defect in a 75-year-old man with hypertension and coronary artery disease, undergoing a renal transplant 12 years ago. Preoperatively, no evidence was found for significant lower extremity vascular disease. Two lateral thigh perforating vessels were identified using Doppler ultrasonography, but the entirety of the LCFA was extensively and completely calcified, making microanastomosis of these vessels extremely difficult.19 Although the stenosis of the superficial femoral artery was 67.2%, the stenosis of the DFA was 12.5 %, the LCFA was 10.9%, and the dLCFA was 10% to 12.5 %, in this, 7% were severe atherosclerotic changes.15–17
Interestingly, Ahn et al17 reported that 24.0% of patients without PVD showed some degree of stenotic changes in the dLCFA. This unexpected finding could be a result of those patients in the no-symptom group having undiagnosed PVD.17 These data demonstrated that the dLCFA, LCFA, and DFA were not atherosclerosis resistant and experienced atherosclerotic change. The effect of atherosclerosis risk factor was supported in the study by Choi et al.16 They analyzed the degree of stenosis in the dLCFA in regard to each risk factor having shown that hypertension, impaired pulmonary function, history of lower limb amputation, and total score of 11 risk factors were statistically significant. In the same direction with our study, the size of internal diameters of DFA and LCFA was not significant between nonatherosclerosis and atherosclerosis groups. However, the size of the internal diameters of the dLCFA was statistically significant in the combined 3 risk factors and smoking group.
According to the anatomic variation of the LCFA, the dLCFA that originated from the common femoral artery or superficial femoral artery may have higher incidence of atherosclerosis. The subgroup analysis of the dLCFA and LCFA from the origin will be beneficial. When the dLCFA arises from the superficial femoral artery, it may in theory be more prone to atherosclerosis.15 However, some studies have revealed that the proximal tract of the superficial femoral artery is not narrowed by atherosclerosis to a significantly higher degree than that portion of the DFA situated 1 cm distal to the origin of the LCFA.20
Although, the free ALT in severe atherosclerosis is not contraindicated and the successful microsurgical ALT for limb salvage in diabetic foot ulcer was demonstrated,21–23 the failure rate increased in the smoking and multiple atherosclerotic risk factor groups.24 The clinical significance of the differences in arterial diameter between atherosclerosis and nonatherosclerosis is still inconclusive, but the effects of atherosclerotic risk factors caused stenosis of the dLCFA, LCFA, and DFA occurred in varying degrees. So, in the patients with multiple atherosclerotic risk factors, preoperative CTA should be considered to evaluate the patency of the dLCFA before reconstruction.
The pathologic variation may be occurred from atherosclerotic change. Some reports have shown the aneurysm of the LCFA in multiple atherosclerosis.25,26 Although, the ALT flap is commonly used and the perforator from the dLCFA is the vascular supply, the anatomic variation still occurred and appeared increasingly. Furthermore, the absent perforator was found overall at 1.8% to 4.8% (0.85% in Asian and 3.08% in western countries),8,27 and the knowledge of anatomical variation will help surgeons to plan their operations. Our study found the anatomic variation of the dLCFA and LCFA in 5 types. The LCFA takeoff from the DFA and dLCFA takeoff from the LCFA are classic in anatomy and found at 78.87%, and that correlated with another study that reported 75%.28
The dLCFA direct takeoff from the DFA was found at 17.53% in our study and 6% to 13% in other studies.12,29,30 The LCFA takeoff from common femoral artery was 2.06%, whereas other studies found 10% to 25%.7,28,31 Our study showed 0.52% LCFA takeoff from the superficial femoral artery that has never been reported and 1.03% LCFA takeoff from the DFA at bifurcation of the deep and superficial femoral arteries. Some anatomic variations were not found in our study, such as the dLCFA from the DFA at bifurcation of the LCFA 17.1%,12 the dLCFA from the common femoral artery 1% to 6%,12,30,32 and the LCFA from the external iliac artery 6%.13 Moreover, the perforators arising from the transverse branch of the LCFA were found in 4% to 32.4%14,33,34 and from the oblique branch were found at 14%, which was first described by Wong et al.10
Preoperative planning and investigation are important. In nonatherosclerosis, Doppler ultrasonography is commonly used for preoperative planning, and the accuracy depends on the experience of the surgeon and type of Doppler ultrasonography. Color Doppler ultrasonography is significantly more accurate than acoustic Doppler ultrasonography.35 Furthermore, the accuracy of Doppler ultrasonography decreased when body mass index increased.12 One study using color Doppler ultrasonography showed 19.04% and did not find any perforator. Moreover, 11.76% that detected the perforator by Doppler ultrasonography did not find the perforator intraoperative.36
Preoperative investigations are controversial regarding atherosclerosis. Ahn et al17 reported that surgical reconstruction using the ALT flap was a safe procedure for patients even with multiple comorbidities, including significant PVD.17 Hage and Woerdeman37 reported a partial necrosis of the foot and calf caused by the interruption of the dLCFA, which acted as critical collateral for the obstructed superficial femoral artery. They recommended preoperative angiography of the donor leg in patients in whom palpable popliteal pulsations are lacking. In our study, we found the calcified plaque in LCFA and dLCFA and we suggest preoperative angiography in the multiple atherosclerosis and smoking group even Doppler ultrasonography is detectable.
CONCLUSIONS
In summary, LCFA and its descending branch are not atherosclerosis resistant. Stenosis of the dLCFA, LCFA, and DFA occurred in varying degrees in atherosclerosis-risk patients. Statistical significance in the diameters of the dLCFA was found among multiple atherosclerotic patients. For patients with multiple atherosclerotic risk factors, preoperative CTA should be considered to evaluate the patency of the dLCFA before reconstruction.
ACKNOWLEDGMENT
We thank Mrs. Supak Cae-ngow, statistician and the research assistant of the Office of Research Development, Phramongkutklao College of Medicine for her kind help in the statistical analysis of this article.
Footnotes
Trial database registered: Thai Clinical Trials Registry (member of WHO registry network): registration number: TCTR20150728001
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984;37:149–159. doi: 10.1016/0007-1226(84)90002-x. [DOI] [PubMed] [Google Scholar]
- 2.Wong CH, Ong YS, Wei FC. The anterolateral thigh - Vastus lateralis conjoint flap for complex defects of the lower limb. J Plast Reconstr Aesthet Surg. 2012;65:235–239. doi: 10.1016/j.bjps.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Weichman K, Allen RJ, Jr, Thanik V, et al. Adipofascial Anterolateral Thigh Free Flaps for Oncologic Hand and Foot Reconstruction. J Reconstr Microsurg. 2015;31:684–687. doi: 10.1055/s-0035-1558431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanasono MM, Skoracki RJ, Silva AK, et al. Adipofascial perforator flaps for “aesthetic” head and neck reconstruction. Head Neck. 2011;33:1513–1519. doi: 10.1002/hed.21637. [DOI] [PubMed] [Google Scholar]
- 5.Hong JP, Chung IW. The superficial fascia as a new plane of elevation for anterolateral thigh flaps. Ann Plast Surg. 2013;70:192–195. doi: 10.1097/SAP.0b013e3182367c2f. [DOI] [PubMed] [Google Scholar]
- 6.Ribuffo D, Atzeni M, Saba L, et al. Angio computed tomography preoperative evaluation for anterolateral thigh flap harvesting. Ann Plast Surg. 2009;62:368–371. doi: 10.1097/SAP.0b013e31817fe4c5. [DOI] [PubMed] [Google Scholar]
- 7.Alkureishi LW, Shaw-Dunn J, Ross GL. Effects of thinning the anterolateral thigh flap on the blood supply to the skin. Br J Plast Surg. 2003;56:401–408. doi: 10.1016/s0007-1226(03)00125-5. [DOI] [PubMed] [Google Scholar]
- 8.Lakhiani C, Lee MR, Saint-Cyr M. Vascular anatomy of the anterolateral thigh flap: a systematic review. Plast Reconstr Surg. 2012;130:1254–1268. doi: 10.1097/PRS.0b013e31826d1662. [DOI] [PubMed] [Google Scholar]
- 9.Wei FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109:2219–26; discussion 2227. doi: 10.1097/00006534-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wong CH, Wei FC, Fu B, et al. Alternative vascular pedicle of the anterolateral thigh flap: the oblique branch of the lateral circumflex femoral artery. Plast Reconstr Surg. 2009;123:571–577. doi: 10.1097/PRS.0b013e318195658f. [DOI] [PubMed] [Google Scholar]
- 11.Rozen WM, Ashton MW, Pan WR, et al. Anatomical variations in the harvest of anterolateral thigh flap perforators: a cadaveric and clinical study. Microsurgery. 2009;29:16–23. doi: 10.1002/micr.20550. [DOI] [PubMed] [Google Scholar]
- 12.Kimata Y, Uchiyama K, Ebihara S, et al. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconstr Surg. 1998;102:1517–1523. doi: 10.1097/00006534-199810000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Valdatta L, Tuinder S, Buoro M, et al. Lateral circumflex femoral arterial system and perforators of the anterolateral thigh flap: an anatomic study. Ann Plast Surg. 2002;49:145–150. doi: 10.1097/00000637-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Yu P. Characteristics of the anterolateral thigh flap in a Western population and its application in head and neck reconstruction. Head Neck. 2004;26:759–769. doi: 10.1002/hed.20050. [DOI] [PubMed] [Google Scholar]
- 15.Halvorson EG, Taylor HO, Orgill DP. Patency of the descending branch of the lateral circumflex femoral artery in patients with vascular disease. Plast Reconstr Surg. 2008;121:121–129. doi: 10.1097/01.prs.0000293862.68476.97. [DOI] [PubMed] [Google Scholar]
- 16.Choi HJ, Jung KH, Wee SY. Clinical analysis of risk factors of the patency of the descending branch of the lateral circumflex femoral artery. J Plast Surg Hand Surg. 2014;48:396–401. doi: 10.3109/2000656X.2014.899243. [DOI] [PubMed] [Google Scholar]
- 17.Ahn JH, Mirza T, Thomas D, et al. Effect of peripheral vascular disease on the blood supply of the anterolateral thigh free flap: a radiographic study. J Reconstr Microsurg. 2014;30:509–514. doi: 10.1055/s-0034-1378133. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 19.Kamdar MR, Rohde C, Spector JA. Lateral circumflex femoral artery: not always atherosclerosis-resistant. Plast Reconstr Surg. 2008;122:1597–8; author reply 1598. doi: 10.1097/PRS.0b013e318186cb9e. [DOI] [PubMed] [Google Scholar]
- 20.Vaas F. Atherosclerotic lesions at the bifurcation of the common femoral artery. Neth J Surg. 1982;34:168–173. [PubMed] [Google Scholar]
- 21.Kim JY, Lee YJ. A study of the survival factors of free flap in older diabetic patients. J Reconstr Microsurg. 2007;23:373–380. doi: 10.1055/s-2007-992345. [DOI] [PubMed] [Google Scholar]
- 22.Hong JP. Reconstruction of the diabetic foot using the anterolateral thigh perforator flap. Plast Reconstr Surg. 2006;117:1599–1608. doi: 10.1097/01.prs.0000207057.16292.8f. [DOI] [PubMed] [Google Scholar]
- 23.Kolbenschlag J, Hellmich S, Germann G, et al. Free tissue transfer in patients with severe peripheral arterial disease: functional outcome in reconstruction of chronic lower extremity defects. J Reconstr Microsurg. 2013;29:607–614. doi: 10.1055/s-0033-1354739. [DOI] [PubMed] [Google Scholar]
- 24.Christy MR, Lipschitz A, Rodriguez E, et al. Early postoperative outcomes associated with the anterolateral thigh flap in Gustilo IIIB fractures of the lower extremity. Ann Plast Surg. 2014;72:80–83. doi: 10.1097/SAP.0b013e31825737b9. [DOI] [PubMed] [Google Scholar]
- 25.Lancashire MJ, Galland RB. Aneurysm of lateral circumflex femoral artery in association with multiple atherosclerotic aneurysms. Ann Vasc Surg. 1992;6:289–291. doi: 10.1007/BF02000276. [DOI] [PubMed] [Google Scholar]
- 26.Feldman AJ, Berguer R. Rupture of isolated atherosclerotic aneurysm of lateral femoral circumflex artery. Surgery. 1981;90:914–916. [PubMed] [Google Scholar]
- 27.Lu JC, Zelken J, Hsu CC, et al. Algorithmic approach to anterolateral thigh flaps lacking suitable perforators in lower extremity reconstruction. Plast Reconstr Surg. 2015;135:1476–1485. doi: 10.1097/PRS.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 28.Cipriani R, Contedini F, Caliceti U, et al. Three-dimensional reconstruction of the oral cavity using the free anterolateral thigh flap. Plast Reconstr Surg. 2002;109:53–57. doi: 10.1097/00006534-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Garvey PB, Selber JC, Madewell JE, et al. A prospective study of preoperative computed tomographic angiography for head and neck reconstruction with anterolateral thigh flaps. Plast Reconstr Surg. 2011;127:1505–1514. doi: 10.1097/PRS.0b013e318208d23e. [DOI] [PubMed] [Google Scholar]
- 30.Sananpanich K, Tu YK, Kraisarin J, et al. Flow-through anterolateral thigh flap for simultaneous soft tissue and long vascular gap reconstruction in extremity injuries: anatomical study and case report. Injury. 2008;39(Suppl 4):47–54. doi: 10.1016/j.injury.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Choi SW, Park JY, Hur MS, et al. An anatomic assessment on perforators of the lateral circumflex femoral artery for anterolateral thigh flap. J Craniofac Surg. 2007;18:866–871. doi: 10.1097/scs.0b013e3180a03304. [DOI] [PubMed] [Google Scholar]
- 32.Wolff KD, Kesting M, Thurmüller P, et al. The anterolateral thigh as a universal donor site for soft tissue reconstruction in maxillofacial surgery. J Craniomaxillofac Surg. 2006;34:323–331. doi: 10.1016/j.jcms.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Qiao Q, Chen GY, et al. Clinical experience and surgical anatomy of 32 free anterolateral thigh flap transplantations. Br J Plast Surg. 1991;44:91–96. doi: 10.1016/0007-1226(91)90038-l. [DOI] [PubMed] [Google Scholar]
- 34.Shieh SJ, Chiu HY, Yu JC, et al. Free anterolateral thigh flap for reconstruction of head and neck defects following cancer ablation. Plast Reconstr Surg. 2000;105:2349–2357; discussion 2358. doi: 10.1097/00006534-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Kimata Y, Uchiyama K, Ebihara S, et al. Anterolateral thigh flap donor-site complications and morbidity. Plast Reconstr Surg. 2000;106:584–589. doi: 10.1097/00006534-200009030-00009. [DOI] [PubMed] [Google Scholar]
- 36.Kimata Y, Uchiyama K, Ebihara S, et al. Versatility of the free anterolateral thigh flap for reconstruction of head and neck defects. Arch Otolaryngol Head Neck Surg. 1997;123:1325–1331. doi: 10.1001/archotol.1997.01900120075012. [DOI] [PubMed] [Google Scholar]
- 37.Hage JJ, Woerdeman LA. Lower limb necrosis after use of the anterolateral thigh free flap: is preoperative angiography indicated? Ann Plast Surg. 2004;52:315–318. doi: 10.1097/01.sap.0000100422.66597.13. [DOI] [PubMed] [Google Scholar]


