Abstract
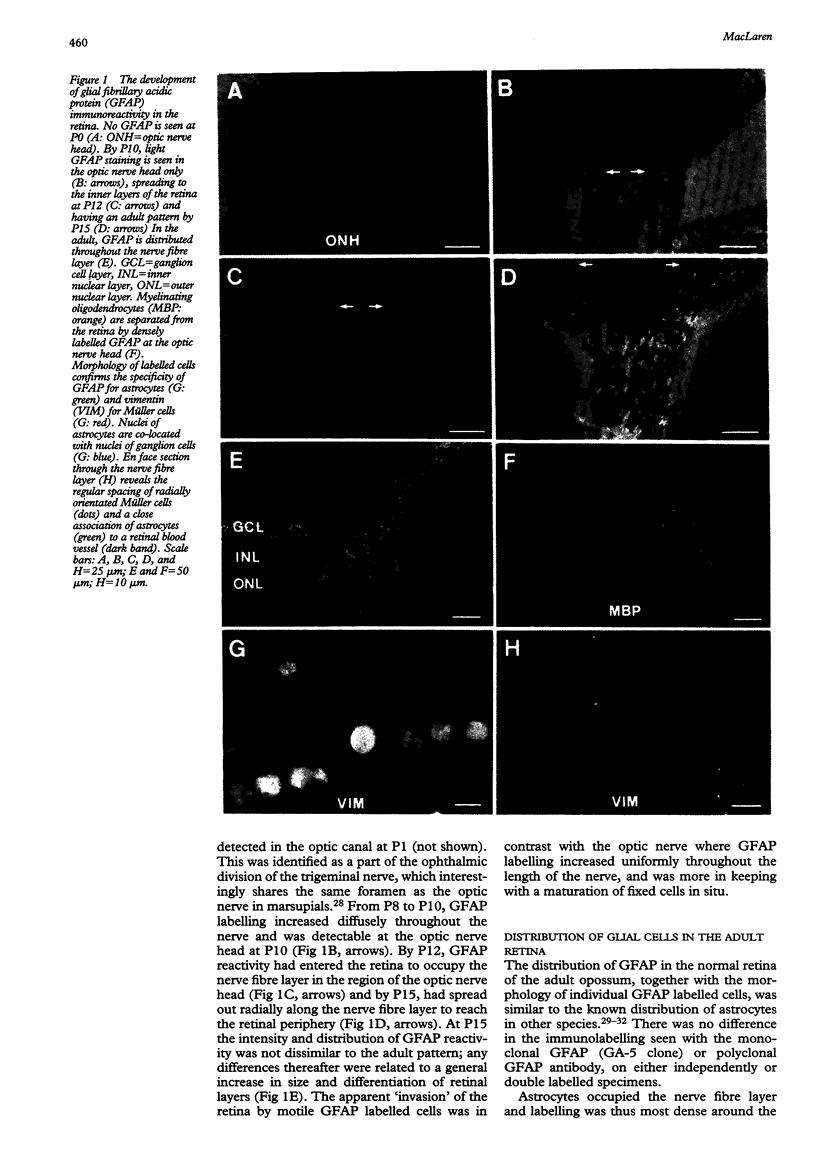
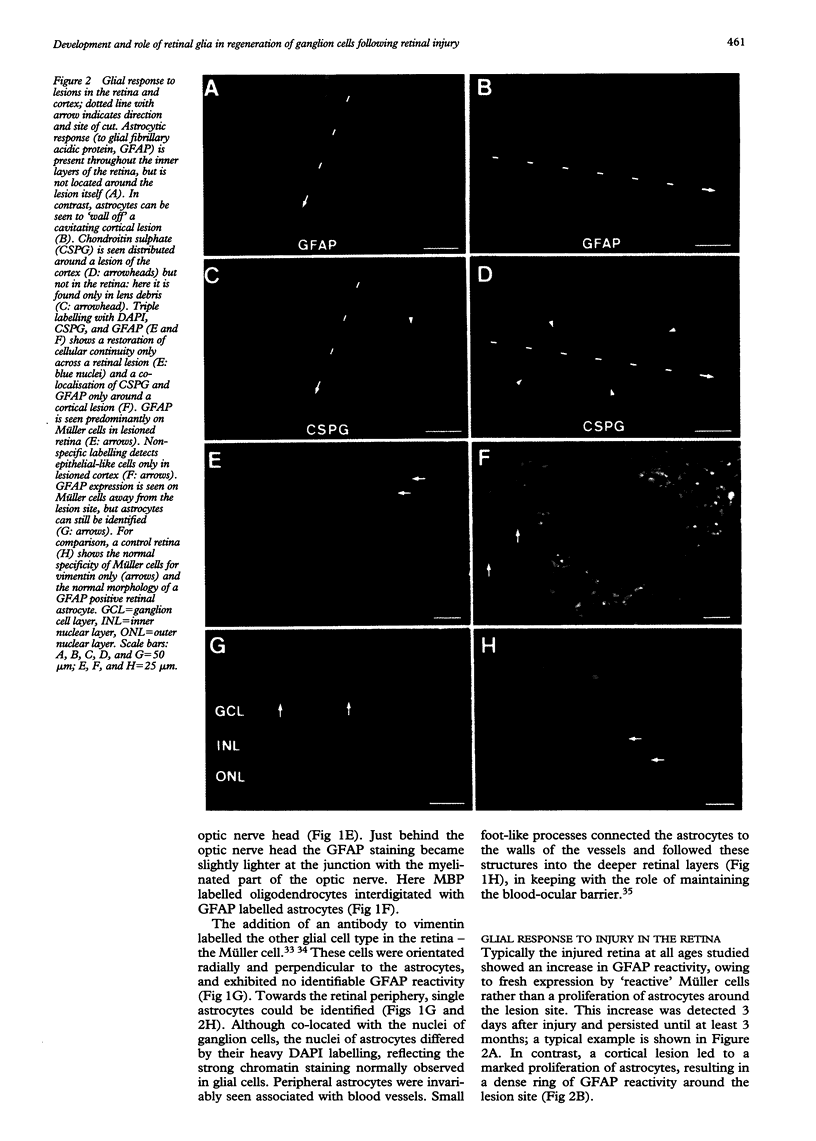
AIMS/BACKGROUND: Recent observations have shown that the glial scar resulting from a surgical lesion of the immature retina differs from elsewhere in the central nervous system, in that it permits the through growth and reconnection of regenerating axons. This study in the opossum examines in detail the development and reaction to injury of retinal glia at different developmental stages, and specifically examines the distribution of the gliosis related inhibitory molecule, chondroitin sulphate proteoglycan (CSPG), making comparisons with a control site of gliosis in the cerebral cortex. METHODS: A linear slit was cut into the retina or cortex with a fine tungsten probe. After a variable time delay, immunocytochemistry of the resulting gliosis was employed to detect astrocytes with glial fibrillary acidic protein (GFAP), Müller cells with vimentin, and CSPG with CS-56 antibodies. GFAP was also used at different ages to examine the normal development of astrocytes in the retina of this species. RESULTS: Astrocytes entered the retina 12 days after birth (P12), closely associated with blood vessels in the nerve fibre layer. In experiments at all ages studied, cellular continuity was re-established across the lesioned retina, which did not result in a significant astrocyte proliferation or CSPG expression. In contrast, cortical injury led to the development of a cystic cavity surrounded by astrocytes and CSPG. Müller cells expressed GFAP but not CSPG in the lesioned retina. CONCLUSION: Successful regrowth of ganglion cells through a retinal lesion may be partly the result of the scarcity of astrocytes in the retina, which results in minimal gliosis, or of their apparent inability to express inhibitory molecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allodi S., Cavalcante L. A., Hokoç J. N., Bernardes R. F. Genesis of neurons of the retinal ganglion cell layer in the opossum. Anat Embryol (Berl) 1992;185(5):489–499. doi: 10.1007/BF00174086. [DOI] [PubMed] [Google Scholar]
- Berry M., Hall S., Rees L., Carlile J., Wyse J. P. Regeneration of axons in the optic nerve of the adult Browman-Wyse (BW) mutant rat. J Neurocytol. 1992 Jun;21(6):426–448. doi: 10.1007/BF01191507. [DOI] [PubMed] [Google Scholar]
- Bregman B. S. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 1987 Aug;431(2):265–279. doi: 10.1016/0165-3806(87)90214-8. [DOI] [PubMed] [Google Scholar]
- Caroni P., Schwab M. E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988 Apr;106(4):1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E. Y., So K. F. Characterization of the sprouting response of axon-like processes from retinal ganglion cells after axotomy in adult hamsters: a model using intravitreal implantation of a peripheral nerve. J Neurocytol. 1992 Aug;21(8):589–603. doi: 10.1007/BF01187119. [DOI] [PubMed] [Google Scholar]
- Distler C., Weigel H., Hoffmann K. P. Glia cells of the monkey retina. I. Astrocytes. J Comp Neurol. 1993 Jul 1;333(1):134–147. doi: 10.1002/cne.903330111. [DOI] [PubMed] [Google Scholar]
- Dreher Z., Wegner M., Stone J. Müller cell endfeet at the inner surface of the retina: light microscopy. Vis Neurosci. 1988;1(2):169–180. doi: 10.1017/s0952523800001449. [DOI] [PubMed] [Google Scholar]
- Dusart I., Schwab M. E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994 May 1;6(5):712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Erickson P. A., Fisher S. K., Guérin C. J., Anderson D. H., Kaska D. D. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res. 1987 Jan;44(1):37–48. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., Housden E., Smith-Thomas L., Meyer R. L. The growth of axons in three-dimensional astrocyte cultures. Dev Biol. 1989 Oct;135(2):449–458. doi: 10.1016/0012-1606(89)90193-0. [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., Rokos J., Bakst I. Oligodendrocytes repel axons and cause axonal growth cone collapse. J Cell Sci. 1989 Jan;92(Pt 1):93–100. doi: 10.1242/jcs.92.1.93. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Miller R. H., Burne J. F., Raff M. C. Evidence that migratory oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells are kept out of the rat retina by a barrier at the eye-end of the optic nerve. J Neurocytol. 1988 Feb;17(1):13–25. doi: 10.1007/BF01735374. [DOI] [PubMed] [Google Scholar]
- Ghooray G. T., Martin G. F. Development of an astrocytic response to lesions of the spinal cord in the North American opossum: an immunohistochemical study using anti-glial fibrillary acidic protein. Glia. 1993 Sep;9(1):10–17. doi: 10.1002/glia.440090103. [DOI] [PubMed] [Google Scholar]
- Godement P., Salaün J., Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984 Dec 20;230(4):552–575. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Holash J. A., Stewart P. A. The relationship of astrocyte-like cells to the vessels that contribute to the blood-ocular barriers. Brain Res. 1993 Dec 3;629(2):218–224. doi: 10.1016/0006-8993(93)91323-k. [DOI] [PubMed] [Google Scholar]
- Holder N., Clarke J. D. Is there a correlation between continuous neurogenesis and directed axon regeneration in the vertebrate nervous system? Trends Neurosci. 1988 Mar;11(3):94–99. doi: 10.1016/0166-2236(88)90151-8. [DOI] [PubMed] [Google Scholar]
- Iwashita Y., Kawaguchi S., Murata M. Restoration of function by replacement of spinal cord segments in the rat. Nature. 1994 Jan 13;367(6459):167–170. doi: 10.1038/367167a0. [DOI] [PubMed] [Google Scholar]
- Kalil K., Reh T. Regrowth of severed axons in the neonatal central nervous system: establishment of normal connections. Science. 1979 Sep 14;205(4411):1158–1161. doi: 10.1126/science.472734. [DOI] [PubMed] [Google Scholar]
- Ling T. L., Mitrofanis J., Stone J. Origin of retinal astrocytes in the rat: evidence of migration from the optic nerve. J Comp Neurol. 1989 Aug 15;286(3):345–352. doi: 10.1002/cne.902860305. [DOI] [PubMed] [Google Scholar]
- Ling T. L., Stone J. The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Brain Res Dev Brain Res. 1988 Nov 1;44(1):73–85. doi: 10.1016/0165-3806(88)90119-8. [DOI] [PubMed] [Google Scholar]
- MacLaren R. E., Taylor J. S. A critical period for axon regrowth through a lesion in the developing mammalian retina. Eur J Neurosci. 1995 Oct 1;7(10):2111–2118. doi: 10.1111/j.1460-9568.1995.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Maxwell W. L., Follows R., Ashhurst D. E., Berry M. The response of the cerebral hemisphere of the rat to injury. I. The mature rat. Philos Trans R Soc Lond B Biol Sci. 1990 Jun 26;328(1250):479–500. doi: 10.1098/rstb.1990.0121. [DOI] [PubMed] [Google Scholar]
- Maxwell W. L., Follows R., Ashhurst D. E., Berry M. The response of the cerebral hemisphere of the rat to injury. II. The neonatal rat. Philos Trans R Soc Lond B Biol Sci. 1990 Jun 26;328(1250):501–513. doi: 10.1098/rstb.1990.0122. [DOI] [PubMed] [Google Scholar]
- McKeon R. J., Schreiber R. C., Rudge J. S., Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991 Nov;11(11):3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G., Doherty P., Walsh F. S., Crocker P. R., Filbin M. T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994 Sep;13(3):757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reese B. E., Colello R. J. Neurogenesis in the retinal ganglion cell layer of the rat. Neuroscience. 1992;46(2):419–429. doi: 10.1016/0306-4522(92)90062-7. [DOI] [PubMed] [Google Scholar]
- Reier P. J., Bregman B. S., Wujek J. R. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986 May 15;247(3):275–296. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- Robinson S. R., Dreher Z. Müller cells in adult rabbit retinae: morphology, distribution and implications for function and development. J Comp Neurol. 1990 Feb 8;292(2):178–192. doi: 10.1002/cne.902920203. [DOI] [PubMed] [Google Scholar]
- Rudge J. S., Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990 Nov;10(11):3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy P. V., Fu M. Transcriptional activation of an intermediate filament protein gene in mice with retinal dystrophy. DNA. 1989 Jul-Aug;8(6):437–446. doi: 10.1089/dna.1.1989.8.437. [DOI] [PubMed] [Google Scholar]
- Saunders N. R., Adam E., Reader M., Møllgård K. Monodelphis domestica (grey short-tailed opossum): an accessible model for studies of early neocortical development. Anat Embryol (Berl) 1989;180(3):227–236. doi: 10.1007/BF00315881. [DOI] [PubMed] [Google Scholar]
- Sauvé Y., Sawai H., Rasminsky M. Functional synaptic connections made by regenerated retinal ganglion cell axons in the superior colliculus of adult hamsters. J Neurosci. 1995 Jan;15(1 Pt 2):665–675. doi: 10.1523/JNEUROSCI.15-01-00665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer J., Schnitzer J. Intraorbital transection of the rabbit optic nerve: consequences for ganglion cells and neuroglia in the retina. J Comp Neurol. 1991 Oct 8;312(2):175–192. doi: 10.1002/cne.903120202. [DOI] [PubMed] [Google Scholar]
- Schnell L., Schwab M. E. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci. 1993 Sep 1;5(9):1156–1171. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Distribution and immunoreactivity of glia in the retina of the rabbit. J Comp Neurol. 1985 Oct 8;240(2):128–142. doi: 10.1002/cne.902400203. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Immunocytochemical studies on the development of astrocytes, Müller (glial) cells, and oligodendrocytes in the rabbit retina. Brain Res Dev Brain Res. 1988 Nov 1;44(1):59–72. doi: 10.1016/0165-3806(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Schreyer D. J., Jones E. G. Growing corticospinal axons by-pass lesions of neonatal rat spinal cord. Neuroscience. 1983 May;9(1):31–40. doi: 10.1016/0306-4522(83)90044-1. [DOI] [PubMed] [Google Scholar]
- Small R. K., Riddle P., Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Rutishauser U., Silver J., Miller R. H. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990 Apr;138(2):377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Snow D. M., Lemmon V., Carrino D. A., Caplan A. I., Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990 Jul;109(1):111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Snow D. M., Watanabe M., Letourneau P. C., Silver J. A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development. 1991 Dec;113(4):1473–1485. doi: 10.1242/dev.113.4.1473. [DOI] [PubMed] [Google Scholar]
- So K. F., Aguayo A. J. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985 Mar 4;328(2):349–354. doi: 10.1016/0006-8993(85)91047-9. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987 Jan 1;255(1):35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Guillery R. W. Early development of the optic chiasm in the gray short-tailed opossum, Monodelphis domestica. J Comp Neurol. 1994 Dec 1;350(1):109–121. doi: 10.1002/cne.903500108. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Jack J. L., Easter S. S. Is the capacity for optic nerve regeneration related to continued retinal ganglion cell production in the frog? Eur J Neurosci. 1989;1(6):626–638. doi: 10.1111/j.1460-9568.1989.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Thanos S., Mey J. Type-specific stabilization and target-dependent survival of regenerating ganglion cells in the retina of adult rats. J Neurosci. 1995 Feb;15(2):1057–1079. doi: 10.1523/JNEUROSCI.15-02-01057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S., Mey J., Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993 Feb;13(2):455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos Solon. Adult Retinofugal Axons Regenerating Through Peripheral Nerve Grafts Can Restore the Light-induced Pupilloconstriction Reflex. Eur J Neurosci. 1992;4(8):691–699. doi: 10.1111/j.1460-9568.1992.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Trimmer P. A., Wunderlich R. E. Changes in astroglial scar formation in rat optic nerve as a function of development. J Comp Neurol. 1990 Jun 15;296(3):359–378. doi: 10.1002/cne.902960303. [DOI] [PubMed] [Google Scholar]
- WINDLE W. F., CLEMENTE C. D., CHAMBERS W. W. Inhibition of formation of a glial barrier as a means of permitting a peripheral nerve to grow into the brain. J Comp Neurol. 1952 Apr;96(2):359–369. doi: 10.1002/cne.900960207. [DOI] [PubMed] [Google Scholar]
- Weibel D., Cadelli D., Schwab M. E. Regeneration of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitors. Brain Res. 1994 Apr 11;642(1-2):259–266. doi: 10.1016/0006-8993(94)90930-x. [DOI] [PubMed] [Google Scholar]
- Weibel D., Kreutzberg G. W., Schwab M. E. Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve. Brain Res. 1995 May 15;679(2):249–254. doi: 10.1016/0006-8993(95)00238-l. [DOI] [PubMed] [Google Scholar]
- Xu X. M., Martin G. F. Developmental plasticity of the rubrospinal tract in the North American opossum. J Comp Neurol. 1989 Jan 15;279(3):368–381. doi: 10.1002/cne.902790304. [DOI] [PubMed] [Google Scholar]
- Yan Q., Elliott J., Snider W. D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992 Dec 24;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]