Sir:
In view of recent United States Food and Drug Administration (USFDA) approval of deoxycholate (DC) as a constituent for lipolysis,1 we would like to share our experience of using it in combination with phosphatidylcholine (PC) for injection lipolysis. PC and DC have independently been used in lipolysis and are both USFDA approved; however, a combination of both has not been approved by the USFDA. DC is a bile salt and fundamentally increases the solubility of PC, which is structurally a phospholipid.2 The first use of this class of drugs was done to dissolve the intramural accumulations of atherosclerotic plaques in systemic arteries and was later followed by usage to treat the pulmonary fat embolism. We have been using the combination since 2004 and have had good results in 956 patients so far. We have used the PC-DC mixture in varied age groups, both sexes, and over different target areas. The amount injected per site was about 50% less on the face when compared to any other body part, and the amount injected at each prick was not more than 0.3 cc. The maximum dose used in 1 sitting is 5 g and the minimum interval between 2 subsequent sittings we followed has been 4 weeks. The maximal appreciable effect is achieved in 80% of patients by day 10; however, the drug continues to act for 6–8 weeks. The effects are more easily appreciated over the face (malar and submental areas) and arms and show average effects in the back and around the knees. We have also used lipolysis as primary modality and a touch-up modality following liposuction. The results are better appreciated in primary lipolysis. The need for follow-up sessions (1–2 sessions) of lipolysis and the quantification of results in subsequent sessions reveal that maximal improvement is achieved in the first session. We have also successfully used this technique of lipolysis in post free fat grafting cases, where patient desired minimal undercorrection subsequent to fat hypertrophy.
The injection sites are guided by a custom-made predesigned grid, which ensures that the injections are adequately spaced, and the projected cone of lipolysis has a minimal overlap ensuring a smoother transition into zone of neighboring injection (Fig. 1). The most common complaint after the procedure is a stinging type of a pain. Other complaints after the procedure were erythema, edema, induration, and formation of subcutaneous nodules, which resolved over a period of time. Accidental injection into the muscle causes excruciating pain due to myonecrosis and hence we recommend pinch technique for injections.
Fig. 1.
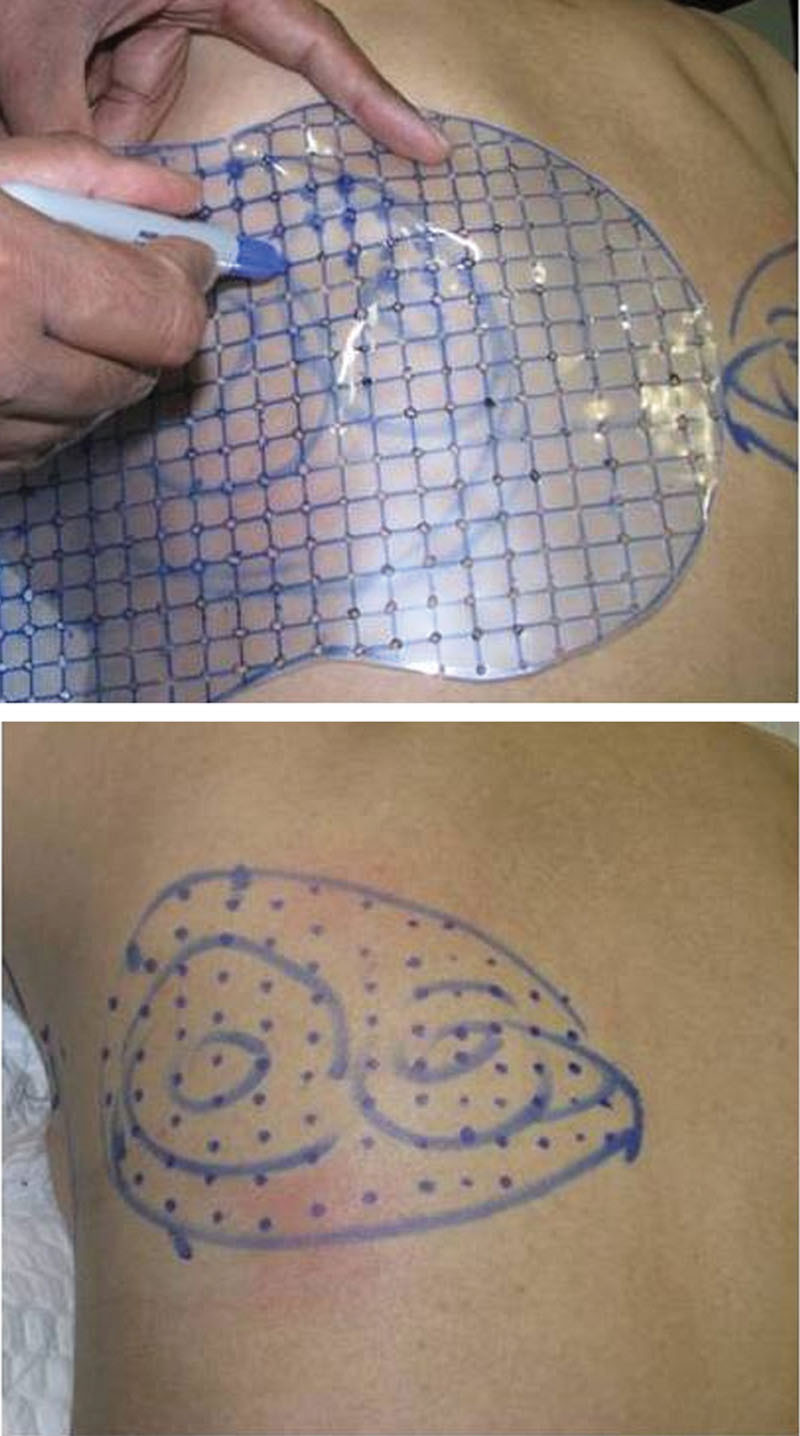
Custom-made marking grid for injection lipolysis.
Although liposuction is and is destined to remain the gold standard for getting rid of body fat, injection lipolysis has advantages of minimal downtime, less invasiveness, and most important to a patient’s psychology is that no surgery is involved in the process. The drugs, dosages, and techniques are not standardized and with acceptance by the USFDA we look forward to a systematic study and code for injection lipolysis in near future.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Fischer A. FDA News Release - FDA approves treatment for fat below the chin. 2015. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm444978.htm. Accessed on April 30,2015.
- 2.Klein SM, Schreml S, Nerlich M, et al. In vitro studies investigating the effect of subcutaneous phosphatidylcholine injections in the 3T3-L1 adipocyte model: lipolysis or lipid dissolution? Plast Reconstr Surg. 2009;124:419–427. doi: 10.1097/PRS.0b013e3181adce61. [DOI] [PubMed] [Google Scholar]


