Background:
The use of cultured epithelial autografts for the treatment of extensive burn wounds has become popular in recent years. We examined extensive burn wounds in 14 patients by using a combination of autograft and cultured epithelial autografts developed in Japan (JACE).
Methods:
We undertook a skin biopsy at 2, 4, and 6 weeks after transplantation with JACE. By using electron microscopy we observed the engraftment process.
Results:
In transmission electron microscope findings, we recognized the engraftment process of JACE. Keratinocytes matured gradually. Collagen fibers formed thick bundles in the dermis layer. In scanning electron microscope findings, we observed papillary dermis development on the artificial dermis.
Conclusions:
After managing wound bed preparation by using artificial dermis, we were able to recognize the good result of grafting JACE on meshed 6:1 split thickness autografts. This is because the auto dermis from autograft extended under the JACE, binding between JACE, and the dermis became strong.
Patients with massive burns need many clinical treatments including burn resuscitation, cardiovascular, respiratory, renal support, nutritional support, infection control, pain management, and surgery. The advancement of medical treatments contributes to improve survival rates.1,2 Methods for handling burn wounds have changed in recent decades. Cultured epithelial autografts were used clinically for the first time in the early 1980s. Since a report in 1984 described that extensively burned children recovered with the use of cultured epithelial autografts, the usefulness of Green-type cultured epithelial autografts has become widely known.3–5 The use of cultured epithelial autografts for the treatment of extensive burn wounds has now become popular.6–10
In Japan, the cultured epithelial autograft developed in Japan (JACE) was accepted as a health insurance adaptation starting on January 1, 2009. JACE was the first tissue-engineered skin substitute using human cells and human cell organization used in Japan.11 On the other hand, the cultured epithelial autograft developed in the United States (EPICEL) has been used in the United States since 1988. It is a Green-type autologous cultured epidermis and is recognized as the first tissue-engineered medical product in the world.12 After 20 years, JACE was developed and is also a Green-type autologous cultured epidermis. However, this was not a late-coming version of EPICEL because the process of manufacturing it is different.
We examined the medical treatment of severely burned patients.13 They were implanted with artificial dermis so as to reconstruct the dermis and grafted with JACE on meshed 6:1 split thickness autografts. Surgical results were very good; the skin was soft and did not peel off. After JACE transplantation, we performed a skin biopsy. With the use of electron microscopy, we observed the engraftment process. The purpose of this article is to report the observation of structural changes in the dermis components during the cultured epithelial autograft engraftment process.
PATIENTS AND METHODS
Between May 2010 and January 2015, 14 burn patients were enrolled in this study. All necessary medical equipment was readily available for burn treatment in this hospital. Physicians specialized in critical care, anesthesiologists, and nurses were in the intensive care unit on a 24-hour basis, and plastic surgeons were taking charge of the operation.
Data were reviewed for the following details: gender, age, cause, inhalation injury, percent of total body surface area, result, operation times, percent of cultured epithelial autograft graft take, and length of stay (Table 1).
Table 1.
Patient Information and Results
All 14 patients, 9 men and 5 women, were aged from 20 to 85 years with a mean age of 57.2 years. The mechanism of burn injuries was 12 flame burns, 1 chemical burn, and 1 scald. All patients underwent primary and general care in the intensive care unit. Respiration unit management was launched for 9 inhalation injury patients.
Adaptation of cultured epithelial autografts was discussed with all patients within 1 day of admission. JACE was ordered from the J-TEC company (Aichi, Japan) after consent was received from the patients’ families. We harvested full thickness normal skin from unburned areas within 48 hours. A 10-cm2 specimen of unburned tissue was obtained. We were able to use JACE 3 weeks after harvesting this.
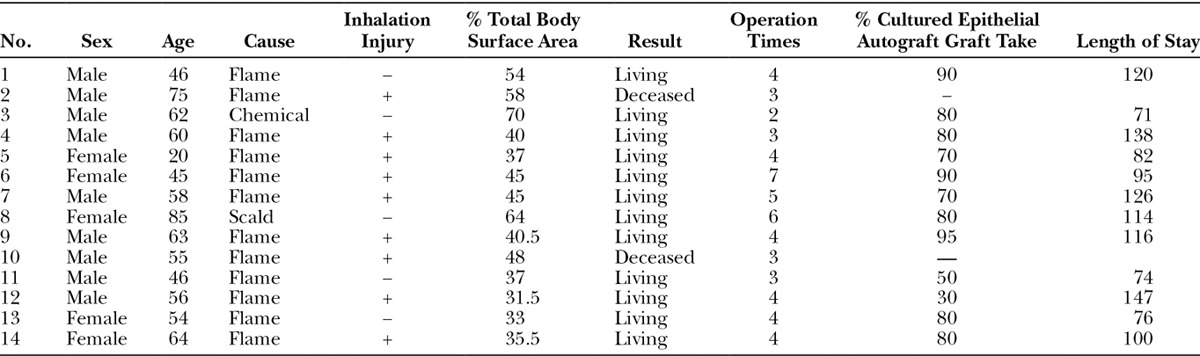
All patients received surgical treatment with tangential excision or avulsion within three days from admission, and all necrotic tissue was removed within 1 week. We implanted artificial dermis immediately after we finished debridement. After artificial dermis was implanted, dressings were applied with Vaseline petroleum jelly and Trafermin (Fibrast, Kaken Pharmaceutical Co., Ltd., Tokyo, Japan) every day to construct a wound bed (Fig. 1A, B).14,15 These were applied with meshed 6:1 split thickness autografts onto the recipient wound bed and covered with JACE (Fig. 1C). After implanting JACE, we followed the protocol described by Sood et al16 of cultured epithelial autograft dressings. In the immediate postoperative period, the grafted areas were left open to air 4 hours each day to allow the JACE grafts to dry. Takedown, removal of the silicone gauze from the JACE graft, generally occurred 1 week after placement of the grafts (Fig. 1D). Dressings were applied with Vaseline petroleum jelly after takedown, and the grafted areas were exposed to air daily for 1 more week. Trafermin was used on the other areas while raw surfaces rested. Almost all wounds were closed within 3 to 4 weeks.
Fig. 1.
A, Flame burn patient, percent of total body surface area of 45, first day. B, Two weeks after debridement and artificial dermis graft. C, Meshed 6:1 split thickness autografts were applied on the recipient wound bed and covered with JACE. D, A week after JACE graft. E, Two years after operation. F, We showed persistent skin covering. The skin was soft enough to pinch.
RESULTS
The percent of total body surface area burns in this study group ranged from 31.5% to 70%, with a mean of 45.6%. The overall survival rate in this study group was 85.7% (12 of 14 patients). Patients underwent operations 2 to 7 times, with a mean of 4.0 times. The take rate of the JACE sheets was 30% to 95%, with a mean of 74.6% 4 weeks post operation. The length of stay in hospital was 71 to 147 days, with a mean of 104.9 days, excluding 2 mortalities.
Eight patients were reviewed more than 2 years after being grafted with JACE. Almost all of these patients were on a good course. We did not recognize any complications of scar contracture and peeling skin. The transplanted skin had become soft enough to be able to pick (Fig. 1E, F).
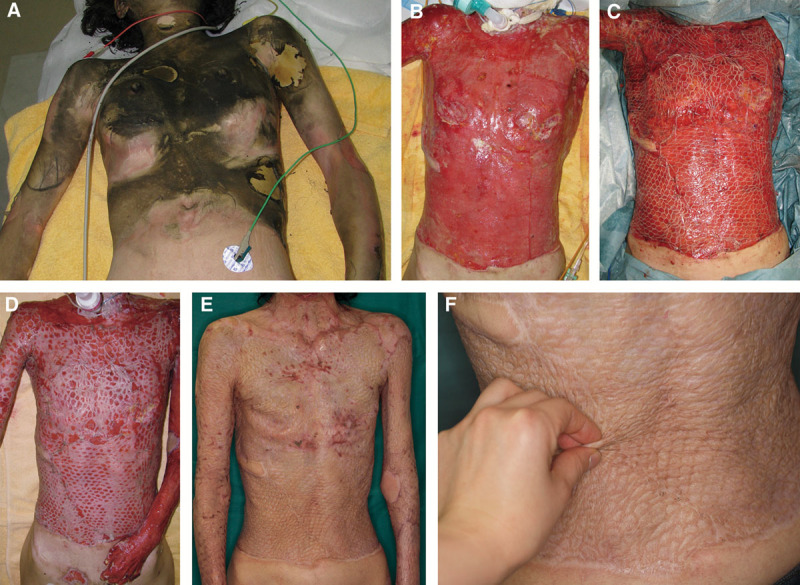
We undertook a skin biopsy at 2, 4, and 6 weeks after transplantation of JACE for patient number 6 and patient number 13 (Fig. 2). In the area of biopsy, there was a dermis-like tissue with artificial dermis on the bottom. We recognized the autograft on both ends. We used a transmission electron microscope (TEM) to observe patient number 6. The samples of tissues for TEM were fixed in 0.1 mol/L phosphate buffered 2% glutaraldehyde and subsequently postfixed in 2% osmium tetroxide for 3 hours in the ice bath. Then, the specimens were dehydrated in graded ethanol and embedded in the epoxy resin. Ultrathin sections were obtained using an ultramicrotomy technique. Ultrathin sections stained with uranyl acetate for 10 minutes and modified Sato’s lead solution17 for 5 minutes were submitted to TEM observation (JEM-1200EX, JEOL, Tokyo, Japan). We then observed patient number 13 by scanning electron microscope (SEM). We macerated epithelium by the alkali water cell-maceration method. The details were as follows: these samples of tissues for SEM were fixed in 0.1 mol/L phosphate-buffered 2% glutaraldehyde and subsequently postfixed in 2% osmium tetraoxide for 3 hours in the ice bath after alkali hydromaceration by 2 N NaOH for 7 days. Then, the specimens were dehydrated in graded ethanol and CO2 critical point dried. Dried specimens were coated by osmium plasma ion coater and were submitted to SEM observation (JEM-6320F, JEOL).
Fig. 2.
We undertook a skin biopsy at 2, 4, and 6 wk after transplantation of JACE. In the area of biopsy, there was a dermis-like tissue with artificial dermis on the bottom. We recognized the autologous skin graft on both ends.
TEM Findings
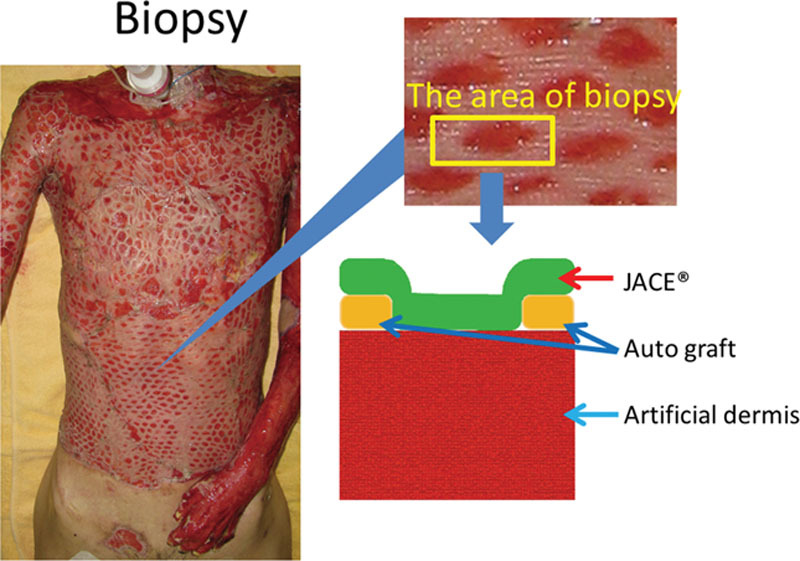
Two weeks after grafting, we biopsied the skin of patient number 6. At first, we observed the upper surface at each end of the specimen. This was an autograft and control finding (Fig. 3). In the upper half of the figure, there was abundant keratinocyte. We also recognized thick desmosomes between the keratinocytes. Basement membrane was observed in the lowest layer of keratinocytes. Under the basement membrane, various thicknesses of collagen fibers were oriented in various directions. These collagen fibers formed the dermal layer.
Fig. 3.
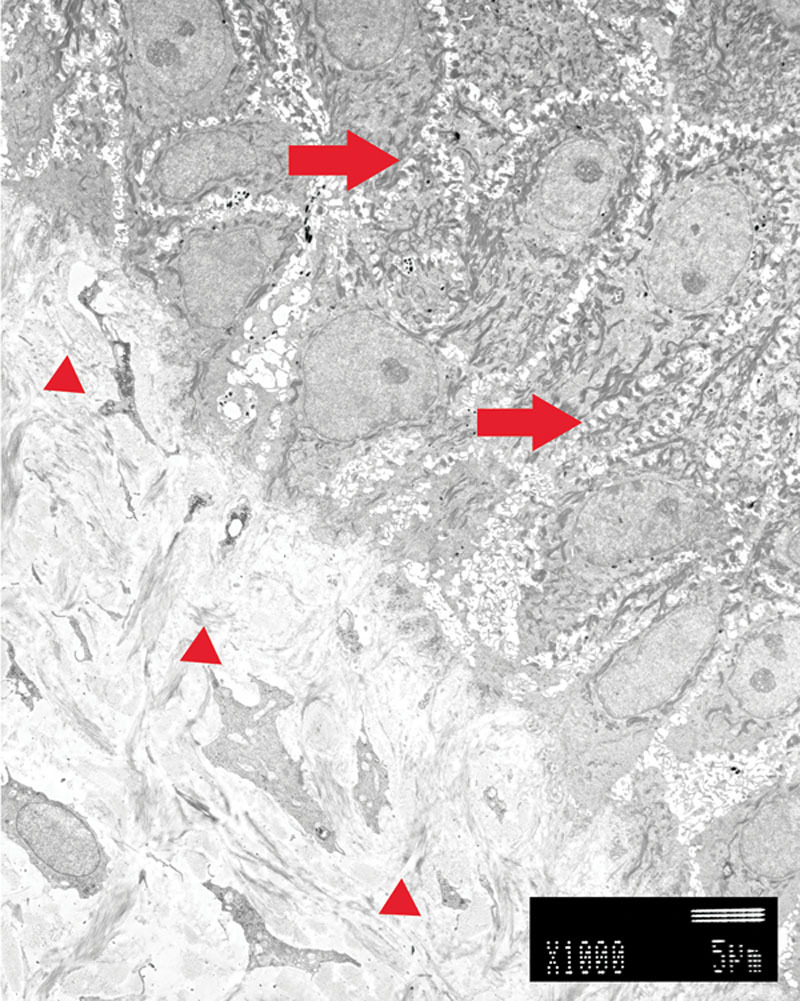
Transmission electron microscope findings of autograft. Keratinocytes were located in the upper part. Dermal layer was located in the lower part. We recognized a lot of thick desmosomes between the keratinocytes (right arrows). Basement membrane was observed in the lowest layer of keratinocytes. In the dermal layer, various thickness collagen fibers were oriented in various directions (small arrowheads).
In the center of the same specimen, there were keratinocytes made of JACE above and there was a dermis-like tissue made of artificial dermis below (Fig. 4A). Compared with the control, the keratinocytes were thin and flat with desmosomes being fewer and narrow. The density of the dermal layer was low. At high magnification, the basement membrane was incomplete (Fig. 5A). Collagen of the dermis layer was thin and short.
Fig 4.
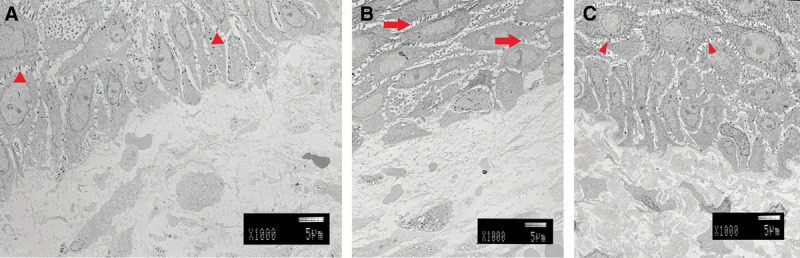
A, Two weeks after grafting. There were keratinocytes made of JACE above, and a dermis-like tissue made of artificial dermis below. Keratinocytes were thin and flat. Desmosomes were fewer and narrow (small arrowhead). The density of the dermal layer was low. B, Four weeks after grafting. There were various forms of keratinocytes. Desmosomes became thicker, and the number has increased (right arrows). C, Six weeks after grafting. Both keratinocytes and dermal layer were almost the same as autografts. There were a lot of desmosomes (small arrowheads). The density of the dermal layer was very high.
Fig. 5.
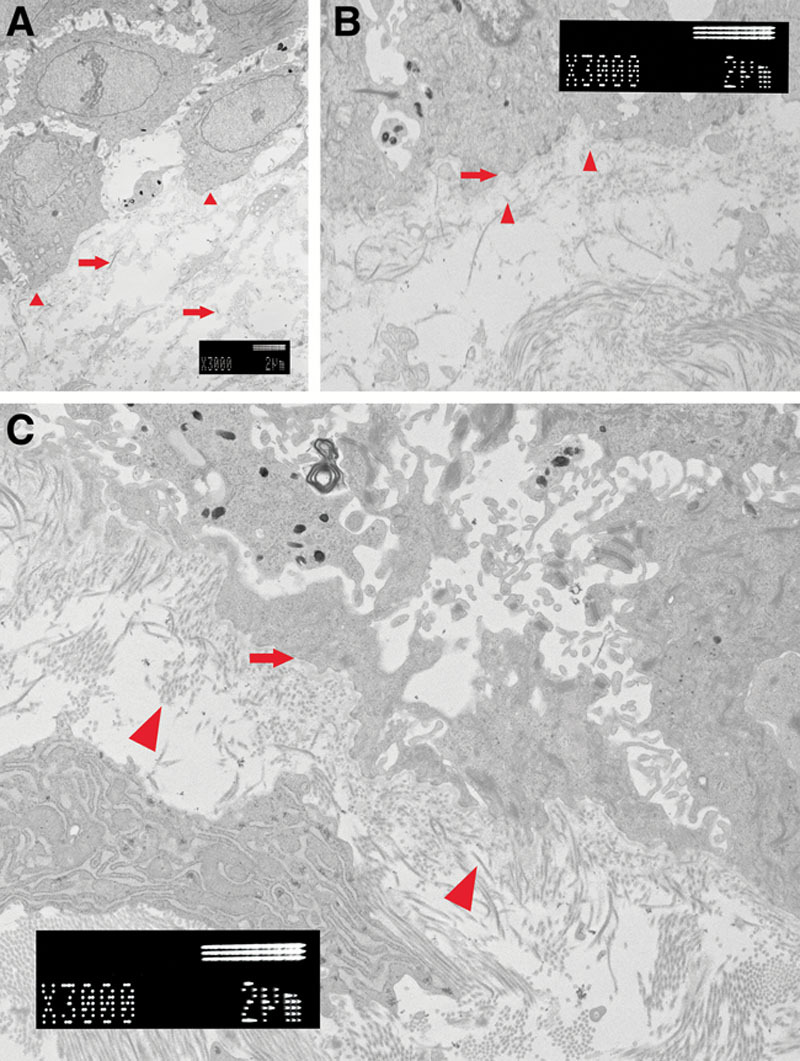
A, Two weeks after grafting. The basement membrane did not connect (small arrowheads). Collagens of the dermis layer were thin and short (right arrows). B, Four weeks after grafting. The basement membrane is recognized under all keratinocytes (right arrow). Collagen fibers became thick and were longer. Type VII collagen was observed between the basement membrane and the collagen of the dermis layer (small arrows). Type VII collagen played a role of the anchor. C, Six weeks after grafting. Basement membrane was recognized clearly (right arrow). A lot of collagen fibers were thick and facing various directions (small arrowheads).
Four weeks after grafting, we biopsied from the same patient. We observed the center of the specimen (Fig. 4B). There were various forms of keratinocytes. Desmosomes had become thicker and the number had increased. At high magnification, the basement membrane was complete (Fig. 5B). Collagen fibers had become thick and were longer. Type VII collagen anchoring to the basement membrane was also observed.
Six weeks after grafting, we undertook a third skin biopsy. We observed the center of the specimen (Fig. 4C). Both keratinocytes and the dermal layer were almost the same as the control. At high magnification, the basement membrane was also completely connected (Fig. 5C). The basement membrane was recognized clearly. Collagen fibers were thick and facing various directions.
SEM Findings
Two weeks after grafting, we biopsied the skin of patient number 13. After epithelium was macerated by the alkali water cell-maceration method, we were able to confirm the dermal-like tissue made of artificial dermis at the bottom and confirmed autodermis at both ends (Fig. 6A). On the upper surface of both sides, there were a lot of concaves and convexes and these were papillary dermis. At high magnification, both ends of the papillary dermis extended toward the center of the top of the dermal-like tissue (Fig. 6C).
Fig. 6.
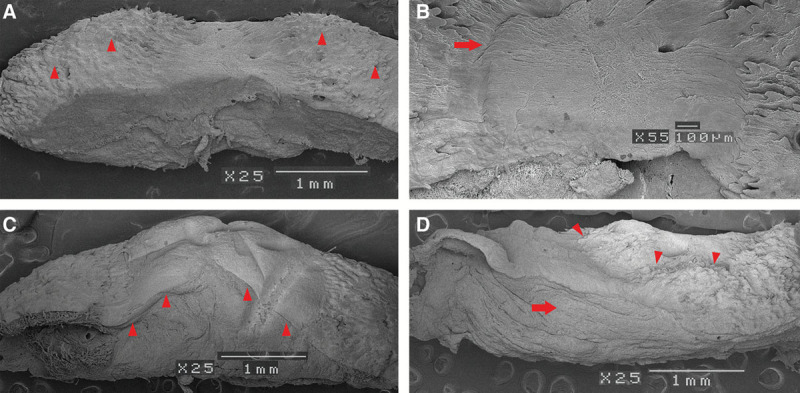
A, Scanning electron microscope findings. Two weeks after grafting. Because the epithelium disappeared, we were able to observe the structure of the dermis layer. At both ends, we recognized papillary dermis (small arrowheads). The uneven surface formed a rete ridge. B, Two weeks after grafting. Both ends of the papillary dermis were extended toward the center of the top of the dermal-like tissue (right arrow). C, Four weeks after grafting. New network of collagen fibers continuing from the papillary dermis were cross-linked from both ends and covered all dermal-like tissue (small arrowheads). D, Six weeks after grafting. On the upper surface of the specimen, irregularities similar to papillary dermis were observed (small arrowheads). The structure of the dermis-like tissue was almost the same as autodermis (right arrow).
Four weeks after grafting, we performed a second biopsy on this patient. New networks of collagen fibers continuing from the papillary dermis were cross-linked from both ends and covered all dermal-like tissue (Fig. 6A). When observed at high magnification, collagen fiber bundles appeared narrow, coarse, and had a low density in the dermis-like tissue. However, collagen fiber bundles that connected to the papillary dermis were thick, fine meshed, high density, and appeared the same as the autodermis. So JACE had adhered to the collagen fiber bundles that extended from the autodermis.
Six weeks after grafting, we took a third skin biopsy. On the upper surface of the specimen, irregularities similar to papillary dermis were observed (Fig. 6C). The structure of the dermis-like tissue appeared almost the same as autodermis.
In each of the specimens observed by TEM and SEM, the degree of maturation was almost the same.
DISCUSSION
In 1963, Todaro and Green18 found 3T3 cells from mouse embryo cells. In 1975, Rheinwald and Green19 described the technique of cultivating autologous keratinocytes for autografting. In 1986, Cuono20 described that the dermal layer of allograft could be used as a wound bed preparation for cultured epithelial autografts. Sood et al16 suggested the use of cryopreserved cadaver allograft to prepare the wound bed. They needed operations every 3 to 5 days to change an insufficient allograft into a new allograft.16 The average final take rate was 72.7%.
In Japan, there were no sufficient cryopreserved cadaver allografts; therefore, we managed the wound bed using artificial dermis instead of allografts. There are three types of artificial dermis available for use: Terudermis (Olympus Terumo Biomaterials Corp., Tokyo, Japan), Pelnac (Gunze Corp., Tokyo, Japan),21–23 and Integra (Integra Life Sciences Corporation, Ibafaki, Japan). Almost all of the wound bed was covered with a fine tissue-like dermis in 2 weeks using Trafermin every day after we implanted the artificial dermis. Meshed 6:1 split thickness autografts were placed onto the recipient wound bed under the JACE. The epidermalization was almost complete within 3 to 4 weeks. Braye et al24 described that the combination of autograft and cultured epithelial autografts appeared effective in a retrospective study. The average final take rate was 84% (±12), which was similar to our result. The postoperative care followed the protocol described by Sood et al.16 We used Trafermin until almost all wounds were closed. Trafermin has also proved effective as an early cure.14,15
However, disappointing results with cultured epithelial autograft grafts were also reported.25 Manufacturing the cultured epithelial autograft sheets was costly and time-consuming. Cultured epithelial autografts were also poorly resistant to infection.26 Even if the cultured epithelial autografts were engrafted, long-term durability was not provided.27 When we used the cultured epithelial autografts for the first time, we had a similar experience.13 However, after meshed 6:1 split thickness autografts were placed onto the recipient wound bed under the JACE, the majority of the skin did not peel off. Initially, it seemed that only mesh was engrafted. We did not know how to find out whether cultured epithelial autografts were engrafted. Because we could not distinguish between autograft and the cultured epithelial autografts histopathologically, we chose to observe the tissue with an electron microscope.
We undertook skin biopsies at 2, 4, and 6 weeks after transplantation of JACE. We observed the engraftment process of JACE. Around the keratinocytes, desmosomes gradually increased and became thicker (Fig. 3). We confirmed the process of maturation of JACE. Desmosomes reached almost the same form as the autograft after 6 weeks (Fig. 3). After 2 weeks, the basement membrane was observed as still sparse (Fig. 5A). However, after 4 weeks, it was completely continuous under all of the keratinocytes (Fig. 5B). Compton et al28 reported that the dermal–epidermal junction was complete within 3 to 4 weeks. Anchoring fibrils, type VII collagen, tethered the basement membrane and the dermis. After 2 weeks, very thin anchoring fibrils were tethered to the basement membrane from the dermis (Fig. 5A). Anchoring fibrils became gradually thicker. After 6 weeks, they became similar in form to the autograft (Fig. 5B). Mommaas et al29 reported that type VII collagen was first observed on the lower part of lamina densa at day 11 and steadily increased, reaching normal values 5 weeks after grafting. Density of the collagen amount gradually increased in the dermis layer (Fig. 5). Collagen fibers became wider and longer. After 6 weeks, they became almost the same density as the autograft (Fig. 3).
We focused on the boundary of the autograft and JACE (Fig. 7). In the image after 4 weeks, we could distinguish the difference between autodermis and the artificial dermis in the dermis layer (Fig. 3). Density of the collagen fibers in the autodermis was higher. We found a clear boundary of many layers and fewer layers in the epidermis. Fewer layers were keratinocytes from JACE. However, autologous dermis was under the JACE; we therefore assumed autodermis was extended. Yamamoto et al30 proposed the epithelialization unit. They experimented with ulceration of the rat. Skin ulcer was healed immediately. They observed epidermis, basement membrane, and papillary dermis development on the granulation surface accompanying epidermal migration. Their healing process was similar to our cases. Autograft was engrafted on the burn wound and we recognized epidermal migration by JACE.
Fig. 7.
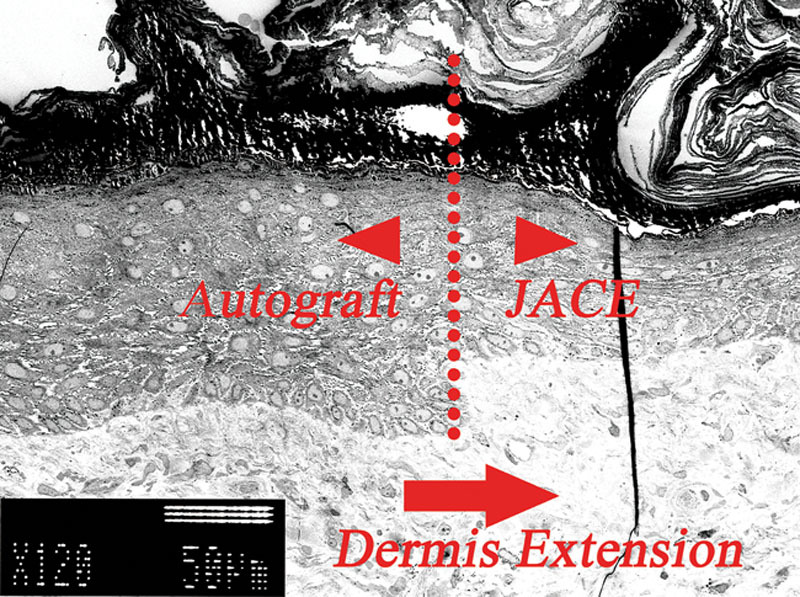
Four weeks after grafting. This was the boundary of the autograft and JACE. We found a clear boundary of many layers and fewer layers in the epidermis (dotted line). The density of the dermal layer was the same under both keratinocytes. We hypothesized dermis extension (right arrow).
In the SEM, it was observed that papillary dermis migrated toward the central portion after 2 weeks (Fig. 6C). After 4 weeks, papillary dermis led and made 1 layer (Fig. 6A). After 6 weeks, artificial dermis was mature, and the boundaries of autoreticular dermis and the artificial dermis were lost (Fig. 6C). In our cases, mature papillary dermis existed under JACE, and anchoring fibrils were also matured. JACE was able to cover burn wounds permanently. Because not only autodermis was extended but also the artificial dermis was mature, the skin became soft enough to pinch.
The advantages of combining JACE and meshed autograft included a high engraftment ratio and softness of the skin. If only the autodermis was extended, we could use the combination of JACE and autodermis without autoepidermis. We could harvest the skin twice in 1 operation at a thick dermis part such as the back. In the future, we are going to continue to perform the above method and observe the engraftment process.
CONCLUSIONS
JACE is one of the cultured epithelial autografts. Although we managed wound bed preparation by using artificial dermis instead of cryopreserved cadaver allograft, we were able to recognize the good result of grafting JACE on meshed 6:1 split thickness autografts. We observed the engraftment process by TEM and SEM. This showed us that the autodermis from autograft extended under the JACE, and binding between JACE and the dermis became strong. JACE was able to cover burn wounds permanently and the skin became soft enough to pinch. If we could succeed using the combination of JACE and meshed autodermis, the strategy of burn surgery should be expanded.
PATIENT CONSENT
All content and procedures described within conform to the principles outlined in the Declaration of Helsinki. All patients or patient’s families included provided written informed consent to participate; the institutional ethics committee approved the study protocol.
ACKNOWLEDGMENTS
We thank Dr. Hideyuki Muramatsu, previous manager of the Department of Plastic and Reconstructive Surgery; Dr. Minoru Nakano, manager of the Advanced Medical Emergency and Critical Care Center; and all the medical staff for their work and enthusiasm.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Herndon DN, Barrow RE, Rutan RL, et al. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg. 1989;209:547–552. doi: 10.1097/00000658-198905000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira C, Murphy K, Herndon D. Outcome measures in burn care. Is mortality dead? Burns. 2004;30:761–771. doi: 10.1016/j.burns.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor NE, Mulliken JB, Banks-Schlegel S, et al. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1:75–78. [PubMed] [Google Scholar]
- 4.Gallico GG, 3rd, O’Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 5.Gallico GG, 3rd, O’Connor NE. Cultured epithelium as a skin substitute. Clin Plast Surg. 1985;12:149–157. [PubMed] [Google Scholar]
- 6.Munster AM, Weiner SH, Spence RJ. Cultured epidermis for the coverage of massive burn wounds. A single center experience. Ann Surg. 1990;211:676–679. doi: 10.1097/00000658-199006000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sood R, Balledux J, Koumanis DJ, et al. Coverage of large pediatric wounds with cultured epithelial autografts in congenital nevi and burns: results and technique. J Burn Care Res. 2009;30:576–586. doi: 10.1097/BCR.0b013e3181ac02de. [DOI] [PubMed] [Google Scholar]
- 8.Cirodde A, Leclerc T, Jault P, et al. Cultured epithelial autografts in massive burns: a single-center retrospective study with 63 patients. Burns. 2011;37:964–972. doi: 10.1016/j.burns.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Yim H, Yang HT, Cho YS, et al. Clinical study of cultured epithelial autografts in liquid suspension in severe burn patients. Burns. 2011;37:1067–1071. doi: 10.1016/j.burns.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Lee H. Outcomes of sprayed cultured epithelial autografts for full-thickness wounds: a single-centre experience. Burns. 2012;38:931–936. doi: 10.1016/j.burns.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Sai K, Ohjimi H, Takagi S, et al. Clinical experience with commercially prepared autologous cultured epidermis with artificial dermis for extensive burns. J Wound Tech. 2010;10:28–32. [Google Scholar]
- 12.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi M, Muramatsu H, Nakano M, et al. Experience of using cultured epithelial autografts for the extensive burn wounds in eight patients. Ann Plast Surg. 2014;73:25–29. doi: 10.1097/SAP.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 14.Akita S, Akino K, Imaizumi T, et al. The quality of pediatric burn scars is improved by early administration of basic fibroblast growth factor. J Burn Care Res. 2006;27:333–338. doi: 10.1097/01.BCR.0000216742.23127.7A. [DOI] [PubMed] [Google Scholar]
- 15.Akita S, Akino K, Imaizumi T, et al. A basic fibroblast growth factor improved the quality of skin grafting in burn patients. Burns. 2005;31:855–858. doi: 10.1016/j.burns.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31:559–568. doi: 10.1097/BCR.0b013e3181e4ca29. [DOI] [PubMed] [Google Scholar]
- 17.Hanaichi T, Sato T, Iwamoto T, et al. A stable lead by modification of Sato’s method. J Electron Microsc (Tokyo) 1986;35:304–306. [PubMed] [Google Scholar]
- 18.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 20.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet 1986;1:1123–1124. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda K, Suzuki S, Isshiki N, et al. Re-freeze dried bilayer artificial skin. Biomaterials. 1993;14:1030–1035. doi: 10.1016/0142-9612(93)90197-a. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Matsuda K, Maruguchi T, et al. Further applications of “bilayer artificial skin”. Br J Plast Surg. 1995;48:222–229. doi: 10.1016/0007-1226(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 23.Kawai K, Suzuki S, Tabata Y, et al. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 24.Braye F, Oddou L, Bertin-Maghit M, et al. Widely meshed autograft associated with cultured autologous epithelium for the treatment of major burns in children: report of 12 cases. Eur J Pediatr Surg. 2000;10:35–40. doi: 10.1055/s-2008-1072320. [DOI] [PubMed] [Google Scholar]
- 25.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32:395–401. doi: 10.1016/j.burns.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Sheridan RL, Morgan JR, Cusick JL, et al. Initial experience with a composite autologous skin substitute. Burns. 2001;27:421–424. doi: 10.1016/s0305-4179(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 27.Desai MH, Mlakar JM, McCauley RL, et al. Lack of long-term durability of cultured keratinocyte burn-wound coverage: a case report. J Burn Care Rehabil. 1991;12:540–545. doi: 10.1097/00004630-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Compton CC, Gill JM, Bradford DA, et al. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989;60:600–612. [PubMed] [Google Scholar]
- 29.Mommaas AM, Teepe RG, Leigh IM, et al. Ontogenesis of the basement membrane zone after grafting cultured human epithelium: a morphologic and immunoelectron microscopic study. J Invest Dermatol. 1992;99:71–77. doi: 10.1111/1523-1747.ep12611862. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N, Kiyosawa T, Arai K, et al. Dermal neoformation during skin wound healing as demonstrated using scanning electron microscopy. Ann Plast Surg. 2004;52:398–406. doi: 10.1097/01.sap.0000106982.98568.92. [DOI] [PubMed] [Google Scholar]


