Background:
Standard isolation of adipose stromal vascular fraction (SVF) requires the use of collagenase and is considered more than “minimally manipulated” by current good manufacturing practice requirements. Alternatively, nonenzymatic isolation methods have surfaced using physical forces to separate cells from the adipose matrix. The purpose of this study was to review the literature on the use of mechanical isolation protocols and compare the results. The implication for use as a standard procedure in practice is discussed.
Methods:
A systematic review of the literature was performed on mechanical isolation of SVF with a search of six terms on PubMed and Medline databases. One thousand sixty-six articles were subject to evaluation by predetermined inclusion and exclusion criteria.
Results:
Two level 2 evidence articles and 7 in vitro studies were selected. SVF was isolated using automated closed systems or by subjecting the lipoaspirate to centrifugation only or by shaking or vortexing followed by centrifugation. Six articles reported isolation in laboratory settings and three inside the operating room. Stromal vascular cells expressed CD34, and CD44, CD73, CD90, and CD105, and differentiated along adipogenic and osteogenic lineages. When compared with enzymatic methods, mechanical isolation required less time but yielded fewer cells. Both case–control studies reported improved volume retention with cell-supplemented fat grafts for breast reconstruction.
Conclusions:
Mechanical isolation methods are alternatives to circumvent safety issues posed by enzymatic protocols. However, randomized comparative studies with long-term clinical outcomes using mechanically isolated stromal vascular cells are needed to identify their ideal clinical applications.
Adipose stromal vascular fraction (SVF) has moved further into the focus of stem cell research, regenerative medicine, and fat grafting with the development of new industries worldwide. Tissue engineering involving adipose stromal vascular cells (SVCs) represents an interesting research field for different diseases, including degenerative, congenital, or traumatic conditions, and bone, articular, and soft-tissue defects. In plastic surgery, these cells have been used mostly to supplement fat grafts, improving graft retention and long-term outcomes.1–3
Adipose SVF consists of a heterogeneous, mesenchymal population of cells that includes not only adipose stromal, hematopoietic stem, and progenitor cells but also endothelial cells, erythrocytes, fibroblasts, lymphocytes, monocyte/macrophages, and pericytes, among others.4 SVF can be isolated by enzymatic nonenzymatic dissociation, manually or in an automated closed system. The most widely used isolation protocol consists of washing the lipoaspirate, enzymatic digestion with collagenase, centrifugation, and red blood cell lysis.5 Although efficient, this enzymatic isolation protocol involves the use of xenogenic components that may pose certain risks and safety issues, such as exposure to infectious agents and immune reactions.6 Thus, xeno-free enzymatic products have been used and shown that they can replace the current research grade products effectively without any negative effect in the yield or function of human adipose stem cells (ASCs).7 To circumvent the need for manual and external manipulation, single devices have been used to separate and concentrate SVCs from the adipose matrix,8 which may be mixed with fat9 to improve results in fat-grafting procedures. Such systems may decrease the risk of infections and operator dependency. Still, the complexity of current good manufacturing practice requirements has created many obstacles to the translation of enzymatic SVF isolation protocols, whether manual or automated, to clinical scenarios.
Nonenzymatic protocols have been attempted consisting of mechanically dissociating SVF using different devices or an automated closed system, resulting in ready-to-use SVF or SVF-supplemented fat. The cellular composition of SVF can differ according to the isolation protocols used and may have an effect on its capabilities of differentiation, angiogenesis, and regeneration.
This review article summarizes the published literature on nonenzymatic isolation of adipose SVF and compares both the techniques and the results. The purpose of this systematic review of the literature is to improve our understanding of the current, available mechanical protocols and to potentially provide guidance for improvements of the methods going forward.
METHODS
A comprehensive search of the Pubmed and MEDLINE databases was conducted in January 2016 using the following search terms: “isolation,” “dissociation,” “adipose,” “fat,” “stromal vascular fraction,” and “stem cells.” The inclusion criteria were studies in the English literature, documenting the use of mechanical methods for isolating SVF of human adipose tissue. Articles that described enzymatic methods or mechanical dissociation combined with enzymatic digestion to obtain SVF were excluded. Not only articles that described fat-processing methods lacking steps to specifically separate SVF but also those that used explant culture to extract only mesenchymal stem cells (MSCs) or isolated cells from the lipoaspirate fluid (infranatant or bottom layer) were excluded.
Data collected included the following: donor information (age, sex, and body mass index), fat-harvesting technique, processing techniques, characterization studies, such as multilineage properties of the isolated cells, phenotyping of markers associated with SVF, specific gene expression, and in vivo outcomes.
Statistical Analysis
A formal statistical analysis of the eligible studies was not performed because of the methodological heterogeneity and novel nature of these methods. A detailed systematic review and comparison of the diverse findings was undertaken instead.
RESULTS
The primary search yielded 1,066 articles; of which, 754 titles passed initial screening. After duplicates were removed, 450 articles remained, and their abstracts were reviewed. The method sections of 278 articles were read in their entirety. Nine articles met our predetermined inclusion and exclusion criteria and were selected (Fig. 1). The journal types in which these articles were published were diverse. Four articles were published in plastic surgery journals, three articles in cellular therapy journals, and two articles in basic biology journals. Countries that contributed articles were Brazil (3), Italy (3), United States (2), and Russia (1). There were 2 prospective comparative studies of level 2 evidence and 7 basic science studies (Table 1). Summaries of the findings in the discussed articles are presented in Tables 2 and 3.
Fig. 1.
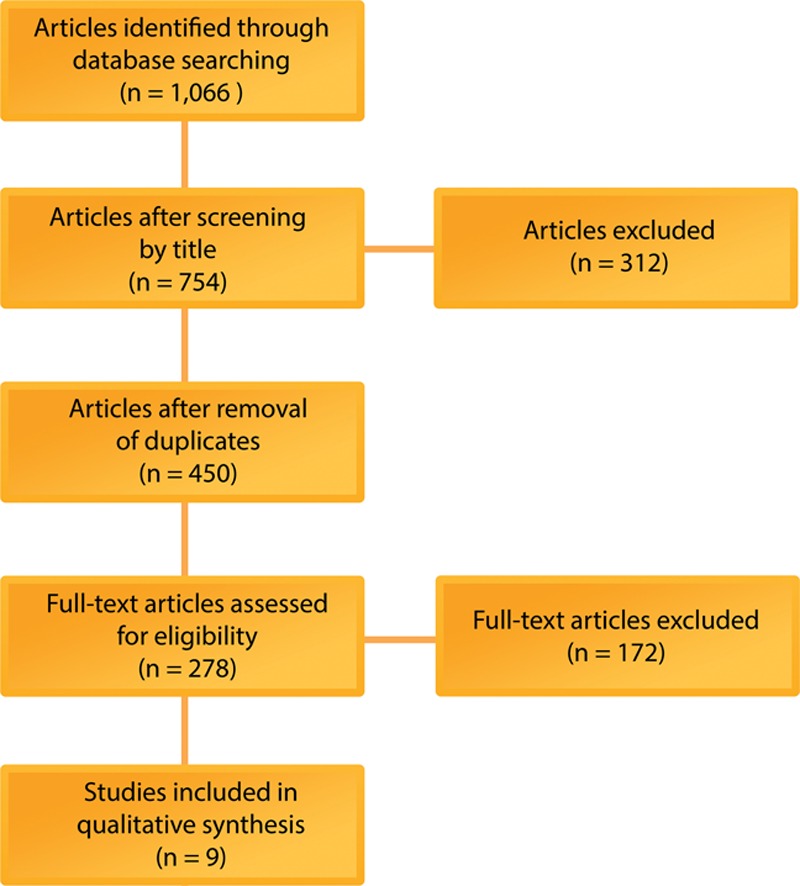
Flow diagram of the search and selection strategy of included articles for mechanical dissociation of adipose-derived stromal vascular fraction.
Table 1.
List of Retained Publications on Mechanical Isolation of Adipose-derived Stromal Vascular Fraction with Their Study Population and Journal Distribution
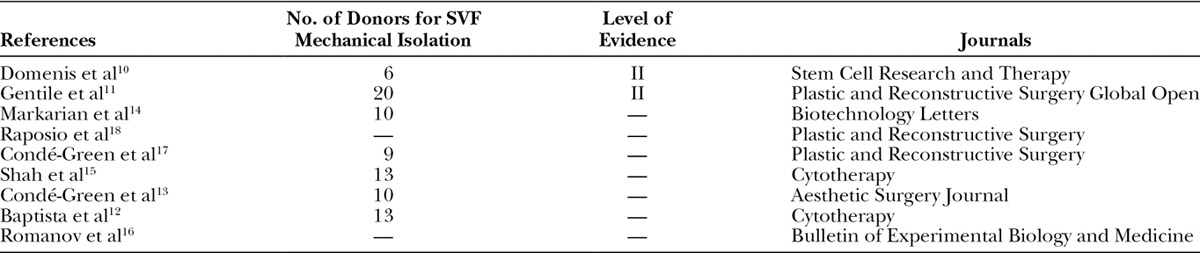
Table 2.
Stromal Vascular Cell and Adipose-derived Stem Cell Yield Obtained from the Mechanical Isolation Protocols
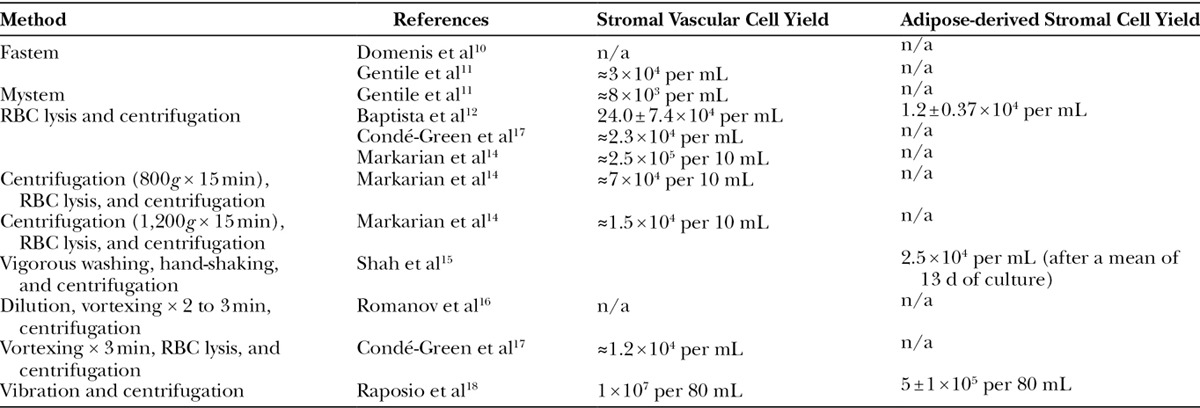
Table 3.
Summary of Articles on Mechanical Isolation of Adipose-derived Stromal Vascular Fraction Including the Mechanical Methods Used and the Relevant Findings

Prospective Comparative Studies
The effects of commercially available SVF-isolation methods on in vitro activity and in vivo outcomes were studied in 2 prospective comparative studies. Eighty-six patients underwent fat grafting for breast reconstruction with or without SVF enrichment, 26 of whom received injection of fat enriched with mechanically isolated SVF.
Domenis et al10 used an automatic system using filtration and centrifugation, the Fastem (CORIOS Soc. Coop, San Giuliano Milanese MI, Italy), to isolate SVF. Characterization of the mechanically isolated cells determined the presence of MSCs. Immunophenotyping studies indicated positivity for CD44, CD73, CD90, and CD105 and negativity for CD45. The cells expressed the pluripotent-linked genes, NANOG, Oct-4, Sox-2, and c-kit. These cells were used for fat-graft enrichment in patients undergoing breast reconstruction. The in vitro and in vivo results were compared with those of fat grafting enriched with enzymatically isolated SVF (Cytori Celution System; Cytori Ltd, Deeside, United Kingdom, and Lipokit Medikhan System; Medikan International Inc, Pusan, Korea) and nonenriched fat grafts. No significant difference in the frequency of CD31+, CD73+, and CD90+ was observed among the 3 isolation methods. The proportion of CD45−/CD31−/CD34+ cells was lower in Fastem-enriched samples than in enzymatically enriched samples. In Fastem-isolated ASCs, the CD45−/CD31−/CD34+ fractions demonstrated reduced ability to differentiate along adipogenic, myogenic, and vasculogenic lineages compared with enzymatically isolated ASCs. The gain in breast subcutaneous thickness observed with Fastem cell–enriched fat was not significantly different from fat enriched with enzymatically isolated cells. After 12 months, significant improvement in volume maintenance was observed with enriched fat from both enzymatic and mechanical methods. This study was limited by the small sample sizes of each group (Fastem, n = 6; Cytori, n = 9; Lipokit, n = 5; nonenriched, n = 16).
Gentile et al11 also used an automatic system with washing and filtration cycles, the Mystem (Mystem LLC, Wilmington, Del.) and Fastem, to isolate SVF. Cell yields from these 2 mechanical methods and 3 enzymatic methods (Cytori, Medikhan, and manual collagenase digestion) were compared. The overall cell yield from Mystem was significantly lower than that from Fastem, which was significantly lower than that from Cytori and manual enzymatic digestion. There was no further analysis of the isolated cell population throughout the methods. The focus of this study was on clinical outcomes in breast reconstruction with fat enriched by each method (Mystem, n = 10; Fastem, n = 10; Cytori, n = 10; Medikhan, n = 10; and nonenriched, n = 10). Enrichment with Fastem and Cytori provided significantly greater contour and volume maintenance compared with nonenriched controls. Comparison of outcomes between the different isolation methods was not reported. The authors observed the presence of oil cysts and cytosteatonecrotic areas at 12 months without specifying which method contributed the most to these complications.
In Vitro Studies
Seven articles reported the in vitro properties of mechanically isolated SVF. The protocols involved manual separation of SVC population as opposed to separation in automated closed systems. Three articles described subjecting lipoaspirate to centrifugation, and four articles described primarily using shaking by hand or electronically or vortexing plus centrifugation to obtain SVCs.
Centrifugation
Baptista et al12 isolated SVF by performing red blood cell (RBC) lysis of the lipoaspirate followed by centrifugation at 900g for 15 minutes and resuspension of the SVF-containing pellet. A mean of 24.0 ± 7.4 × 104 mechanically processed lipoaspirate cells per millimeter were obtained, 1.2 ± 0.37 × 104 cells per millimeter of which showed plastic adherence. The latter cells were CD45−, CD73−, CD31+, CD44+, CD90+, CD105+, and CD34+. This method was compared with manual enzymatic isolation, which showed a greater yield of total and plastic-adherent cells (58.4 ± 17.8 × 104 cells per millimeter and 8.5 ± 6.7 × 104 cells per millimeter, respectively) but required a greater amount of time. They also performed cryopreservation at –196°C, which was associated with a decreased cell yield.
Condé-Green et al13 isolated SVF after the previously described method by Baptista et al12 and compared the population of mechanically processed lipoaspirate cells in centrifuged and decanted lipoaspirates. They looked at the population of cells isolated mechanically from common fat-processing methods to be used in fat grafting. Samples of each processed lipoaspirate and the pellet issued from centrifugation were analyzed. The pellet showed a significantly higher quantity of MSCs and endothelial cells than the other processed samples. However, there was no comparison with enzymatic isolation and limited characterization and differentiation of cells.
Markarian et al14 reported 3 modified versions of the mechanical isolation method described by Baptista et al.12 The first one subjected lipoaspirates to RBC lysis, centrifugation at 600g for 10 minutes. The second and third methods added a centrifugation step at 800g and 1,280g, respectively, for 15 minutes followed by RBC lysis, centrifugation at 600g for 10 minutes. Viable cells were isolated from all 3 methods; however, those from the second and third protocols did not proliferate after 14 days. These 3 mechanical methods were compared with collagenase and trypsin isolation methods. Collagenase isolation provided a significantly greater cell yield than trypsin digestion or the 3 mechanical isolation methods. However, no significant difference in cell yield was observed between the first mechanical isolation and trypsin digestion. No further analysis was performed on mechanically isolated SVCs because of limited growth in cell culture.
Shaking or Vortexing and Centrifugation
Shah et al15 performed repeated cycles of washing with PBS, vigorous hand shaking, and centrifugation at 1,200 rpm for 5 minutes to isolate SVF. This method was compared with collagenase-based isolation. In nonenzymatically isolated samples, there was an increase in CD45+ cells with a decrease in expression of markers for both MSCs and ASCs. However, the changes in the phenotype were not clear since no significant difference was noted for CD29 between enzymatically and nonenzymatically isolated SVF. A significant increase in CD44+ was observed in nonenzymatically isolated SVF. At passage 0, there were significantly greater numbers of MSC markers, based on phenotype, for nonenzymatically isolated cells. The increase in MSC markers correlated with a decrease in contaminating hematopoietic cells, as evidenced by decreases in cells positive for CD34 and CD45. These cells demonstrated comparable adipogenic and osteogenic differentiation to enzymatically isolated cells. Mechanical isolation required, at most, a third of the time required for collagenase isolation yielding 19-fold fewer cells. This study only reported flow cytometry but no additional studies on freshly isolated SVF.
Romanov et al16 isolated SVF by diluting lipoaspirate, vortexing for 2 to 3 minutes, centrifuging at 600g for 10 minutes. Cultured cells homogenously expressed anti–α smooth actin and produced type 1 collagen and fibronectin. Endothelial markers, high positivity for human leukocyte antigen-1, and vimentin were noted. Cells differentiated toward adipogenic, osteogenic, and neurogenic lineages. Analysis of freshly isolated SVF and comparison with enzymatic isolation were not performed.
Condé-Green et al17 isolated SVF using 2 different mechanical methods, subjecting lipoaspirate to centrifugation or vortexing for 3 minutes followed by centrifugation. Overall cell yield from centrifugation was double that of vortexing, but high cell viability was observed with both methods. Comparison was performed with collagenase digestion. SVC yield from collagenase isolation was 10-fold greater than that from centrifugation. In addition, mechanically isolated SVF contained a greater proportion of hematopoietic cells and monocytes/macrophages and fewer ASCs and endothelial cells. There was a lack of quantitative reporting of flow cytometry findings and further in vitro studies as the authors published this study as an abstract with few details on the entire protocol. The findings are nonetheless still relevant and were referenced in related studies.
Raposio et al18 reported isolation of SVF in the operating room, submitting lipoaspirate to vibration in a shaker at 6,000 vibrations per minute for 6 minutes followed by centrifugation at 1,600 rpm for 6 minutes. A mean of 1 × 107 SVCs for 80 mL lipoaspirate was obtained, 5% of which were ASCs. There was no comparison with other isolation methods, only limited in vitro characterization and no in vivo outcome measurements, thus limiting conclusions of this method’s clinical benefits.
DISCUSSION
Enzymatic SVF isolation is standard but limits the volume of lipoaspirate to be processed as it is a lengthy process of in vitro manipulation with costly enzymes. Adipose tissue exposed to collagenase has also been considered more than “minimally manipulated,” which has been defined by U.S. Food and Drug Administration guidance documents as “processing that does not alter the original relevant biological characteristics of cells.”19 Furthermore, when considering the clinical use of SVCs, one must be cognizant of the fact that the use of tissue processing and cell-based product use pose risks for contamination and damage to cells.20 Numerous mechanical cell isolation systems have been developed and commercialized21,22 (Fig. 2). However, the small number of published studies limits their use and credibility. Our analysis of the 9 articles describing mechanical SVF isolation methods has shown promising results. These methods require substantially less time to perform than enzymatic methods.12,15 Although enzymatic digestion yields significantly more SVCs, mechanical protocols isolate a similar population of cells with great cell viability and pluripotency.15,16 Centrifugation achieved the highest cell yield12 followed by vortexing and centrifugation,17 followed by manual shaking.15 Some protocols were performed inside the operating room,10,11,18 suggesting potential easy application to clinical practice. Automated mechanical devices, such as Fastem and Mystem, as opposed to manual manipulation, isolate cells entirely within a single device and were used to enrich fat in patients undergoing breast reconstruction, resulting in significant volume maintenance improvement.10,11
Fig. 2.
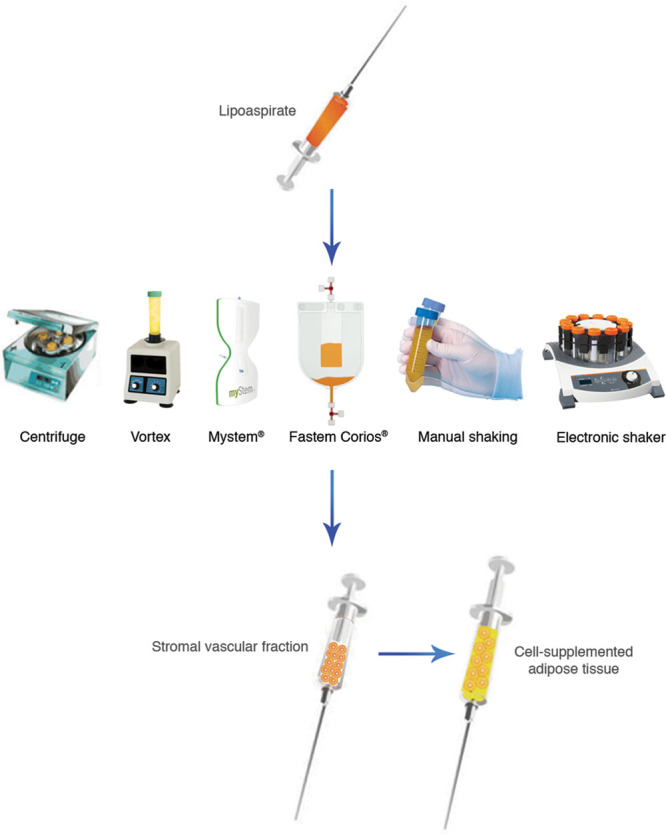
The equipment used for mechanical dissociation of adipose-derived stromal vascular fraction in the discussed articles.
Despite these promising results, determining the most efficient mechanical isolation method among these studies is challenging, as a diverse range of mechanical forces was featured throughout the methods and only three articles directly compared different mechanical isolation methods. Repeated mechanical manipulation of lipoaspirate may negatively impact cell yield17 and growth.14 Mechanically isolated SVF seems to contain a larger proportion of CD45+ cells, representing an increase in potentially contaminating cells, and a lower number of CD34+ cells representing ASCs, which could negatively impact fat-grafting outcomes.10,15,17 Some studies have suggested that CD34+ cells play an important role in promoting fat-graft retention, highlighted by their high degree of proliferation in the first 2 to 4 weeks after fat grafting.23,24 In addition, only 3 studies reported the ASC yield as opposed to the total SVC yield.12,15,18 ASCs have been proposed to play important roles in fat-graft retention because of their immunomodulatory, angiogenic, and multipotent characteristics.25,26 However, it is important to note that other cell types in the heterogeneous SVC population contribute important interactions, which contribute to favorable outcomes.
There is a lack of comparison between the automated, mechanical isolation methods, Fastem and Mystem, and with enzymatic isolation in terms of clinical outcomes and complications. These comparisons are needed to demonstrate if the improved cell yield and cell population composition observed with enzymatic methods translate to improved outcomes and justify the increased time and cost of these equipment (Table 4).
Table 4.
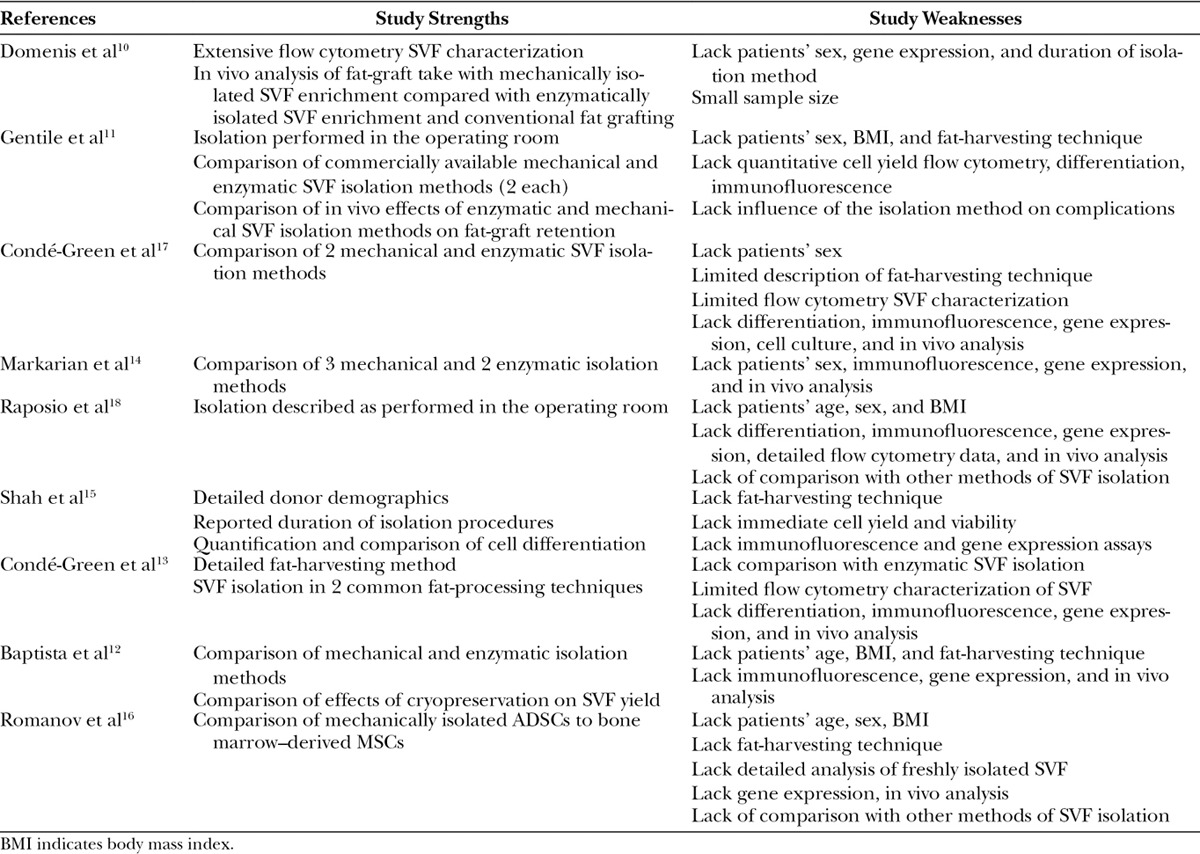
Included Articles Describing Mechanical Isolation Of Adipose-derived Stromal Vascular Fraction with Their Strengths and Weaknesses
Adipose SVF offers tremendous potential for aesthetic and reconstructive applications. In specific circumstances, patients having autologous fat transfer may benefit greatly from enrichment of fat with SVCs to accelerate the regeneration process, further improving outcomes of the procedure.27,28 Mechanical processing of lipoaspirate with steps other than standard electronic centrifugation29 has been described to further fragment adipose tissue particles, aiming to improve fat-graft take. Tonnard et al30 used repeated shear forces, passing lipoaspirate between 2 syringes 30 times followed by filtration, to obtain a product with altered adipocytes and intact viable MSCs. Bianchi et al31 used a closed, handheld system using filtration with simultaneous emulsification and washing of the lipoaspirate. Although these methods have been demonstrated to maintain or increase the proportion of ASCs in the resulting adipose tissue product to be grafted, there are no reports of isolating SVF as a final product through these methods but rather a ready-to-use fat-grafting material instead.
Concerns regarding the safety of collagenase, centered around potential residual enzyme activity after injection, have not been supported by the published literature. Therefore, mechanical isolation methods most likely do not provide additional safety benefits through the avoidance of xenogenic enzyme. To ensure the highest possible safety for patients, a precisely defined procedure with high-quality control is required instead. Automation of the process within a closed system separates the cells from the external environment and reduces opportunity for error and contamination, further improving the safety of the isolation procedure. Moving forward, we must incorporate principles of evidence-based medicine into all aspects of our studies, with adequate control groups, and insist on reporting our fat grafting or cell grafting procedures on the GRAFT registry,32 a U.S.-based nation-wide registry of fat grafting, so that these procedures can become more versatile and reliable in the hands of all plastic surgeons.
CONCLUSIONS
The standard protocol for SVF isolation, although effective, suffers from high costs, long protocol durations, and regulatory scrutiny. Nonenzymatic alternatives address these concerns and isolate populations containing adipose-derived regenerative cells. Mechanically isolated SVCs have demonstrated clinical benefit through contribution to improved volume retention of fat grafts. However, there is a lack of literature comparing different mechanical isolation methods, and published methods yield fewer SVCs than enzymatic isolation. Larger proportions of hematopoietic cells and fewer regenerative cells are isolated when using mechanical protocols. Future studies should compare and refine these protocols to improve cell yield and quality and reduce the proportion of contaminating cell populations. In addition, development of closed, automated systems may improve standardization and reproducibility of results, reducing operator-dependent variations, and simplify the techniques, making them more approachable in a clinical practice. Randomized control studies are needed to analyze long-term outcomes in volume retention and tissue quality after fat grafting supplemented with mechanically isolated cells. Further analysis of the duration and cost of these methods would be of great benefit. These techniques for nonenzymatic SVF isolation are still in infancy with much to be learned about their potential efficacy for research and clinical applications. They may carry significant implications for advancing and making SVC-based therapies more accessible.
Footnotes
Supported by the New Jersey Cancer Commission for publication.
Presented at the American Society of Plastic Surgeons Aesthetica Meeting, June 2016, Wash. This study will also be presented at Plastic Surgery The Meeting 2016, the annual meeting of the American Society of Plastic Surgeons, September 2016, Los Angeles, Calif.
This work is in partial fulfillment for a PhD degree to Alexandra Condé-Green.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the FM Kirby Foundation.
REFERENCES
- 1.Condé-Green A, Wu I, Graham I, et al. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: a pilot study. Aesthet Surg J. 2013;33:713–721. doi: 10.1177/1090820X13487371. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55; discussion 56. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marra KG, Rubin JP. The potential of adipose-derived stem cells in craniofacial repair and regeneration. Birth Defects Res C Embryo Today. 2012;96:95–97. doi: 10.1002/bdrc.21001. [DOI] [PubMed] [Google Scholar]
- 4.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 6.Chang H, Do BR, Che JH, et al. Safety of adipose-derived stem cells and collagenase in fat tissue preparation. Aesthetic Plast Surg. 2013;37:802–808. doi: 10.1007/s00266-013-0156-7. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho PP, Gimble JM, Dias IR, et al. Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng Part C Methods. 2013;19:473–478. doi: 10.1089/ten.TEC.2012.0465. [DOI] [PubMed] [Google Scholar]
- 8.Doi K, Tanaka S, Iida H, et al. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med. 2013;7:864–870. doi: 10.1002/term.1478. [DOI] [PubMed] [Google Scholar]
- 9.Kakudo N, Tanaka Y, Morimoto N, et al. Adipose-derived regenerative cell (ADRC)-enriched fat grafting: optimal cell concentration and effects on grafted fat characteristics. J Transl Med. 2013;11:254. doi: 10.1186/1479-5876-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenis R, Lazzaro L, Calabrese S, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther. 2015;6:2. doi: 10.1186/scrt536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentile P, Scioli MG, Orlandi A, et al. Breast reconstruction with enhanced stromal vascular fraction fat grafting: what is the best method? Plast Reconstr Surg Glob Open. 2015;3:e406. doi: 10.1097/GOX.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baptista LS, do Amaral RJ, Carias RB, et al. An alternative method for the isolation of mesenchymal stromal cells derived from lipoaspirate samples. Cytotherapy. 2009;11:706–715. doi: 10.3109/14653240902981144. [DOI] [PubMed] [Google Scholar]
- 13.Condé-Green A, Baptista LS, de Amorin NF, et al. Effects of centrifugation on cell composition and viability of aspirated adipose tissue processed for transplantation. Aesthet Surg J. 2010;30:249–255. doi: 10.1177/1090820X10369512. [DOI] [PubMed] [Google Scholar]
- 14.Markarian CF, Frey GZ, Silveira MD, et al. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 2014;36:693–702. doi: 10.1007/s10529-013-1425-x. [DOI] [PubMed] [Google Scholar]
- 15.Shah FS, Wu X, Dietrich M, et al. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15:979–985. doi: 10.1016/j.jcyt.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Romanov YA, Darevskaya AN, Merzlikina NV, et al. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 17.Condé-Green A, Rodriguez RL, Slezak S, et al. Comparison between stromal vascular cells’ isolation with enzymatic digestion and mechanical processing of aspirated adipose tissue. Plast Reconstr Surg. 2014;134:54. [Google Scholar]
- 18.Raposio E, Caruana G, Bonomini S, et al. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133:1406–1409. doi: 10.1097/PRS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 19.Code of Federal Regulations. Human Cells, Tissues, and Cellular and Tissue-based Products. 2011. 21CFR1271.3(f)
- 20.McArdle A, Senarath-Yapa K, Walmsley GG, et al. The role of stem cells in aesthetic surgery: fact or fiction? Plast Reconstr Surg. 2014;134:193–200. doi: 10.1097/PRS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberbauer E, Steffenhagen C, Wurzer C, et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen (Lond) 2015;4:7. doi: 10.1186/s13619-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. doi: 10.1186/s40064-015-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philips BJ, Grahovac TL, Valentin JE, et al. Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast Reconstr Surg. 2013;132:845–858. doi: 10.1097/PRS.0b013e31829fe5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H, Mineda K, Eto H, et al. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133:303e–313e. doi: 10.1097/PRS.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 25.Aronowitz JA, Lockhart RA, Hakakian CS, et al. Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg. 2015;75:666–671. doi: 10.1097/SAP.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 26.Navarro A, Marín S, Riol N, et al. Human adipose tissue-resident monocytes exhibit an endothelial-like phenotype and display angiogenic properties. Stem Cell Res Ther. 2014;5:50. doi: 10.1186/scrt438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condé-Green A, Marano AA, Lee ES, et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg. 2016;137:302–312. doi: 10.1097/PRS.0000000000001918. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 29.Coleman SR, Katzel EB. Fat grafting for facial filling and regeneration. Clin Plast Surg. 2015;42:289–300, vii. doi: 10.1016/j.cps.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–1026. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22:2063–2077. doi: 10.3727/096368912X657855. [DOI] [PubMed] [Google Scholar]
- 32.The Plastic Surgery Foundation. General Registry of Autologous Fat Transfer (GRAFT). Available at: http://www.thepsf.org/research/clinical-impact/general-registryautologous-fat-transfer.htm. Accessed April 4, 2016.


