Objective:
To explore the potential viability and limitations of 3D printed models of children with cleft palate deformity.
Background:
The advantages of 3D printed replicas of normal anatomical specimens have previously been described. The creation of 3D prints displaying patient-specific anatomical pathology for surgical planning and interventions is an emerging field. Here we explored the possibility of taking rare pediatric radiographic data sets to create 3D prints for surgical education.
Methods:
Magnetic resonance imaging data of 2 children (8 and 14 months) were segmented, colored, and anonymized, and stereolothographic files were prepared for 3D printing on either multicolor plastic or powder 3D printers and multimaterial 3D printers.
Results:
Two models were deemed of sufficient quality and anatomical accuracy to print unamended. One data set was further manipulated digitally to artificially extend the length of the cleft. Thus, 3 models were printed: 1 incomplete soft-palate deformity, 1 incomplete anterior palate deformity, and 1 complete cleft palate. All had cleft lip deformity. The single-material 3D prints are of sufficient quality to accurately identify the nature and extent of the deformities. Multimaterial prints were subsequently created, which could be valuable in surgical training.
Conclusion:
Improvements in the quality and resolution of radiographic imaging combined with the advent of multicolor multiproperty printer technology will make it feasible in the near future to print 3D replicas in materials that mimic the mechanical properties and color of live human tissue making them potentially suitable for surgical training.
Cleft palate is an infrequent condition worldwide, with a prevalence as an isolated finding of approximately 4.50 per 10,000 live births1 and 6.64 per 10,000 births in conjunction with cleft lip deformity,2 giving a combined total of approximately 10.14 per 10,000 births. The prime method of treatment involves definitive surgical palatoplasty with long-term follow-up for evaluation of growth and to determine the necessity of a return to theater in the event of further deformity.3 As there are few clinics available for medical students and surgical trainees to attend and participate in, and cleft palate surgery does not necessitate a long inpatient stay,4 it is rare that a medical student or surgical trainee will have the opportunity to appreciate the 3D nature of cleft palate pathology and its management.
In the realms of surgical education, it is commonly accepted that the length and cost of a surgical case increase when a junior surgeon (or resident) participates.5,6 The more cases a surgical trainee has the opportunity to participate in, the lesser effects they have on surgical times. This is especially the case when the junior doctor is made the primary surgeon for any case they are unfamiliar with.7 To create an adjuvant to intraoperative experience, and to allow a greater access of teaching materials for the training surgeon, we have created a small series of 3D printed models of children at the age of 8 and 14 months with various forms of cleft palate using a combination of magnetic resonance imaging (MRI) and additive manufacturing or 3D printing technology. Recently, we have outlined methods for creating 3D printed models of normal anatomical cadaveric dissections8 and have recently shown their effectiveness in education of medical students in a double-blind randomized trial.9 A logical extension of these studies is to create 3D prints of patient bespoke pathology for education at the surgical candidate level.
Although antenatal evaluation of craniofacial abnormalities has been performed using MRI,10 to our knowledge no one has created a haptic 3D printed model of a craniofacial abnormality at the postnatal stage, which would be suitable for surgical trainee education. Imaging postbirth also allows for greater accuracy compared with current in utero imaging because of higher resolution scanning, and size of the subject, which aids in distinguishing pathology.
The aim of this study was therefore to create 3D printed models of children with cleft palate deformities using MRI scans, with a view to visualizing an incomplete cleft palate deformity, a complete cleft palate deformity, and a submucosal cleft palate deformity. Secondly, using emerging technology of multimaterial printing, we aimed to produce a prototype 3D replica of a cleft palate with a soft, deformational material, which could be used as a training model.
METHODS
The initial step toward making these 3D cleft palate models involved the acquisition of appropriate imaging data. Ideally a combination of CT scan and MRI would allow an accurate representation of hard and soft tissues, respectively, making the process of thresholding, segmentation, and modeling of the tissues and anatomical structures of interest easier. It is only by being able to segment the data of interest from radiographic data that 3D modeling and printing can occur.
A search of Monash Health’s imaging database from 2003 to 2014 was conducted with the keyword search “cleft palate” required in the reported results of the scans. The availability of CT or MRI head scans in children under the age of 12 months, however, was limited. The search was subsequently expanded to include patients of less than 24 months of age, whereupon 6 MRI scans were found. Each of these scans was performed for reasons other than the patient’s cleft palate—the cleft itself was an incidental finding on the MRI reports. These scans were deidentified by Monash Radiology and were transferred in DICOM (Digital Imaging and Communications in Medicine) format, where the MRI scans could be analyzed further. The scans were evaluated individually to determine whether they were of satisfactory quality to create a 3D model. This was determined by a trial upload to a computer program with virtual modeling capabilities. From this trial, 2 scans were identified as having satisfactory quality and resolution that would allow the creation of a 3D print model. The first scan (Case 1) showed an incomplete soft-cleft palate deformity. The patient was 14 months of age, and the scan was taken for multiple congenital abnormalities—including Joubert syndrome and cleft palate. The second scan (Case 2) showed an incomplete hard-cleft palate deformity. The patient was 8 months of age, with the scan taken under sedation for evaluation of an undiagnosed musculoskeletal syndrome. Both of these scans had a 1-mm slice thickness, with an interslice distance of 0.488 mm.
The software Mimics 17 (Materialise, Leuven, Belgium) allows users to view each slice of an MRI scan in axial, coronal, and sagittal planes, and to segment out regions or tissues of interest. Within Mimics, the thresholding tool was used to designate areas of the MRI image with gray scale values representing the soft tissue and bone structures. Manual segmentation tools were used to edit the threshold selection to remove the lower jaw and tongue, so that the cleft was visible. Three-dimensional models of the segmented regions were created using Mimics modeling tools. For these 2 models, a simple 2-part virtual model was created—a lower jaw with tongue and the rest of the head separately.
Creation of Artificial Case 3
In an effort to experiment with the capabilities of the Mimics software, we chose to create a model (Case 3) with a complete cleft deformity for 3D printing. To this end, the incomplete hard-cleft palate deformity scan (Case 1) was duplicated and segmented again. In this instance, we purposefully segmented out and removed the posteriorly fused palatal tissue—thus producing an artificial or digitally created complete cleft deformity.
Once satisfied with the 3D rendering, the monochrome 3D model is then exported from Mimics as a stereolothographic file for coloring using specialist software 3D-Coat (Pilgway, Ukraine), where the model was realistically colored to define his or her skin, tongue, palate, and lips. Final edits, including hollowing the models to reduce their weight, and volume of consumables used in their printing were performed using Geomagic (3D Systems, Rock Hill, S.C.,). The finished product was exported as a PLY file to the 3D printer (ZCorp Z650 or Projet 4500 [3D Systems, USA]). The digital models were also 3D printed in a flexible material to give a more realistic soft-tissue feel. To minimize the amount of 3D printing material needed, the segmented models were cut using Geomagic to show just the cleft area and upper lip, omitting the tongue, lower jaw, and remainder of the face and head. The model surface was thickened to 3 mm to allow for printing. The models were printed on a multiproperty 3D printer, the Connex J750 (Stratasys, Eden Prairie, Minn.,). These models consisted of a blend of Stratasys’ UV curing PolyJet Vero (Stratasys, USA) hard plastic in a mix of magenta and yellow, and their clear rubber-like Tango Plus (Stratasys, USA) material to produce models with a hardness of Shore 40 (Shore A scale).
RESULTS
Case 1 was an incomplete soft-cleft deformity (Fig. 1). Case 2 was an incomplete hard-cleft deformity (Fig. 2). Case 3 shows the artificially created complete soft- and hard-palate deformity (Fig. 3). Each model clearly showed an element of cleft lip deformity. The final 3D printed models showed the cleft palate deformities very clearly. This was especially the case following our decision to digitally subtract the lower jaw. The scans were also able to show the structure of the nasopharynx and oropharynx and the relation of the cleft deformities. The resolution of the MRI was such that the individual palatal musculature was unable to be identified and digitally segmented, and as a result the model shows a smooth continuation between the soft palate and hard palate. In regard to the oral cavity itself, the dental alveoli could not be demonstrated. The alveolar process appears smooth and edentulous.
Fig. 1.
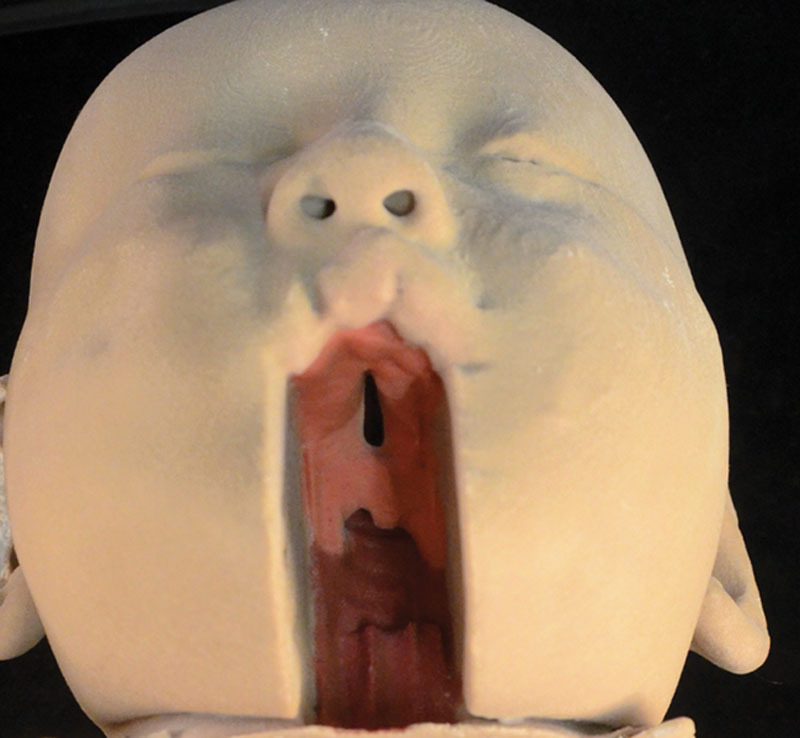
Image of the 3D print of Case 1 showing an incomplete soft-cleft deformity.
Fig. 2.
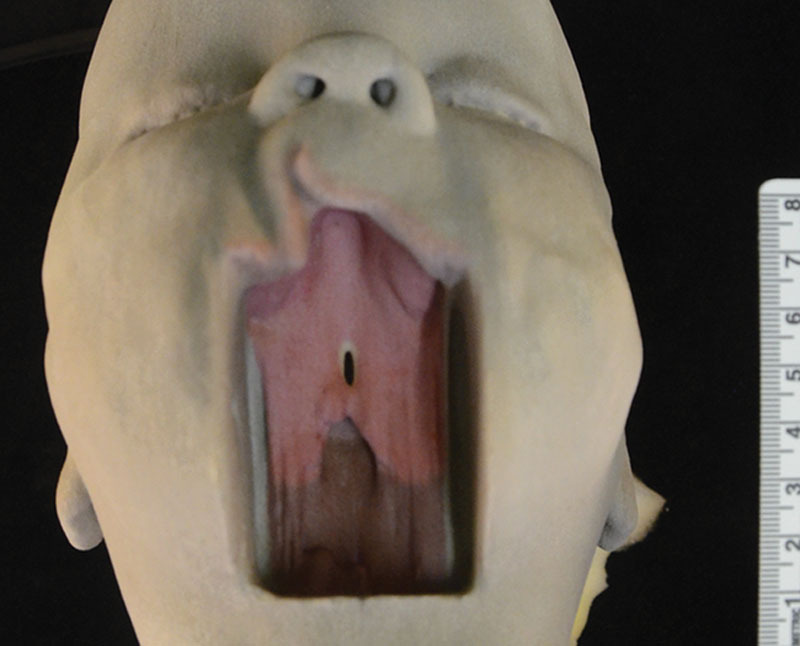
Image of the 3D print of Case 2 showing an incomplete hard-cleft deformity. Scale shown here is the same for Figures 1–3.
Fig. 3.
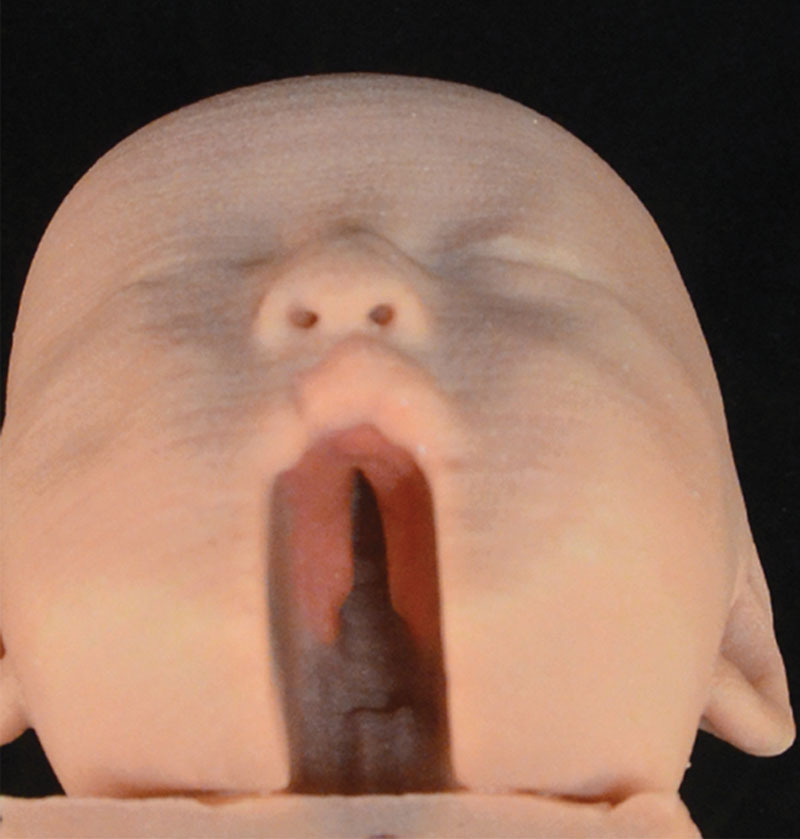
Image of the 3D print of the digitally created Case 3 (complete soft- and hard-palate deformity).
The soft 3D printed models were printed at a Shore hardness of 40, which allows them to deform under slight pressure with enough elasticity to spring back into shape, similar to real soft tissue (Fig. 4). These models could printed only be in a single color because of limitations in the available colors of the soft 3D printing material, though the accurate geometry meant that the anatomy was clearly visible.
Fig. 4.
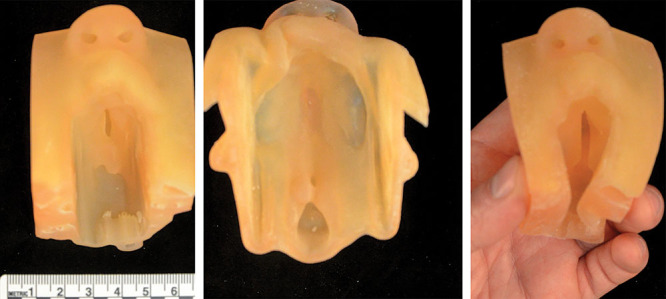
The palate only of the 3 cases shown in Figures 1–3 printed to scale on the Connex J750 as described in Methods.
DISCUSSION
Current Uses in Surgery
Three-dimensional printing is impacting surgery and patient management in a wide variety of ways, including design of bespoke implants as well as presurgical planning,11,12 creation of scaffolds for soft-tissue defects,12 and creation of prosthetics.12 Moreover, a recent systematic review of the literature has shown that 3D prints have been created internationally for presurgical planning in orthopedic surgery, craniomaxillofacial reconstruction, breast reconstruction,13 and neurosurgery.14
In regard to cleft palate pathology, 3D virtual modeling is being used for reproduction of palatal musculature mechanisms in adults15 as well as to simulate velopharyngeal closure for research purposes.16 The creation of some conservative methods of treatment for cleft palate, the use of distraction osteogenesis, has also been evaluated and put into practice using 3D modeling.17 None of the above uses have gone so far as to print a full reproduction of a patient with cleft palate.
Limitations
The present study not only has shown that it is possible to re-create the 3D pathology of cleft palate and lip deformities from digital MRI data suitable for 3D printing, but also has shown that with the advent of new multimaterial printing technology it is feasible to create an accurate soft compliant model of this abnormality, which could augment the training of pediatric surgeons who deal with these complex cases. The resolution of the MRI scan plays a major factor in determining the quality of the final 3D print, and any low-quality images will not result in a satisfactory 3D model. To create a more accurate representation of the patient pathology being modeled, high-resolution scans are optimal. Similarly the 3D printer being used must be able to print at such a high resolution, so as to preserve the accuracy of the radiographic data. As 3D printing is a relatively novel technology within the medical field, the required resolution for an accurate print of pediatric anatomy has not yet been established. Commercial 3D printers are able to print up to 100 μm isometric resolutions, which allows for much greater quality than current imaging techniques. A standard 64-slice CT scanner will have a scan thickness ranging between 0.15 and 0.5 mm, with an interslice distance measuring 0.4–1.0 mm.18 MRI scans are generally set at a greater thickness, reducing the quality of the scans. A standard MRI sequence of the spine will produce slices of 3 mm thickness, with slices of up to 5 mm thickness considered acceptable. In pediatrics, MRIs of the craniofacial area are sequenced at 3-mm thickness slices—as the structures requiring visualization are generally small.19 The 1-mm slice thickness of the MRI scans although sufficient to reproduce topographical details of the palate were unfortunately of insufficient detail and tissue resolution for us to properly re-create the underlying musculature of the palate.
Although CT scanning could produce satisfactory images of bone (and possibly of the palatal musculature), the necessity of a CT scan for treatment of cleft palate would be a clinical decision, which may be questionable. Cleft palate is a condition that is clinically evaluated and assessed with limited need for diagnostic imaging after birth. As a result, acquisition of suitable medical imaging data for the production of further cleft palate models may prove difficult. Initially we set out to locate scans in the hospital archives, which demonstrated an incomplete cleft palate, a complete cleft palate, and a submucosal cleft palate with bifid uvula. Although many imaging data sets available to us had satisfactory resolution for clinical diagnosis, they were not of sufficient scan resolution for high-quality 3D printing. Even in our cases that were suitable, none of the models produced had a distinctive uvula, and it is unsure whether this is due to the patients themselves having congenitally absent uvulae, or whether the MRIs were unable to pick up the small amount of uvula tissue.
Although primary dentition should have erupted at this age, both cases had delayed eruption of teeth. The delayed eruption of Case 1 may have been due to a Joubert syndrome–related disorder, such as oral–facial–digital syndrome.20 Case 2 at 8 months would be expected to have developed primary mandibular central incisors; however, the presence of cleft palate and the yet undiagnosed syndrome may have contributed to delayed eruption. In either case, the appearance of an absence of teeth may be due to a delayed eruption associated with their oral cleft.21 The appearance may also be due to a congenital absence of dentition, which has been demonstrated to be associated with cleft palate and lip deformities.22
The whole head prints shown in Figures 1–3 are printed on single-property multicolor 3D printers (ZCorp Z650 and Projet 4500). These models are “hard” and do not give any satisfactory tactile representation of what the hard palate, soft palate, and surrounding tissues would feel like in the patients. The recent development of multiproperty printers allows printing in hard and soft rubber-like materials. We have exploited this technology to print a palate with deformation behavior,23 much akin to human tissue. Ideally it would have been valuable to print hard tissues, such as bone, in a different Shore hardness; however, this was not possible as bone could not be segmented from the MRI scans. We are currently exploring multicolor multimaterial printing technology which would have great potential to recreate realistic looking 3D prints that also had multiple properties to match those of soft and hard human tissues. Although initially the cost of the hardware, namely the 3D printers themselves, may appear as an obstacle to their widespread use in surgery, it should be noted that there are a number of 3D printing services (eg, 3D Systems Quickparts) that can print on demand with short turnaround times. The cost of the prints shown in this study ranged from Aus$210 (Z650 Printer) to $258 (Projet 4500) for the complete heads to $59 for the cropped soft-palate model (Connex J750). These are consumable costs only. The costs of purchasing printers vary with models from a few thousand dollars to several hundreds of thousands. If we factor in cost recovery of the printer purchase, technical staff time, and consumables, the above costs rise to $335, $516, and $140, respectively.
Potential Uses for Education
One of the primary aims of this study was to create 3D printed models as teaching aids for undergraduate medical students and surgical trainees. The use of 3D printing in medical education has been described previously, especially in relation to surgical education.24 Pathological findings on imaging data have previously been printed as isolated specimens, with a view to better visualizing pathology for educational purposes.25 The potential for 3D printed models to be used for enhanced surgical education is slowly being tested, with a recent study using basic 3D printed models to simulate burr hole creation and tissue biopsy showing some success in surgical workshops.26 The possibility of developing bespoke pathological models for complication-free, realistic simulated surgery is exciting and warrants further development and research. Although 2D and 3D visualizations on a computer screen may be suitable for the experienced surgeon, we propose that trainees would benefit far more from physical models that they would be able to handle, dissect, and practice surgery upon.
These models could also be used to assist in developing the doctor–patient relationship in the context of pre- and postoperative clinics. With the example of cleft palate repair, 3D models showing examples of the cleft palate defect and its relations to the rest of the body would allow both a visual and physical representation of the defect for parents and other family members to better understand the physical nature of the condition. Such a model would allow easier explanation of the complexities of the defect, and could give further clarification as to the symptomatology displayed by children with cleft palate. The intricate proximity of the cleft defect to the Eustachian tube and its relation to the nasopharynx could be displayed, providing greater understanding to those caring for the symptomatic child. With further development of these models, it would be feasible to consider a situation where surgeons would be able to show a preoperative and postoperative example of a child with cleft palate to family members. This would allow explanation of the procedure of palatoplasty, its complications, and its recovery course to be made easier with such a visual aid.
Creation of accurate, cheap 3D models of pathology would also assist educative efforts overseas. In a number of countries, financial constraints and the inability to acquire cadavers for dissection for various reasons, including cultural accessibility, ethical accessibility, and lack of accessibility, create major hindrances, reducing anatomical education to the study of 2D sketches in medical textbooks or artificial plastic models. Ongoing development of 3D modeling will allow for the creation of a new form of teaching anatomy/pathology laboratory. By virtue of the nature of 3D printing technology, trainees and staff would no longer need to be exposed to hazardous preservation chemicals such as formalin/formaldehyde, which are known cancerogenic agents.27
It is our belief that as 3D printing technology develops and new, cheaper materials are incorporated into the process (eg, soft tissue–like/biosynthetic materials), there is a very real possibility that models such as these could be used for realistic simulation for surgical training.
ACKNOWLEDGMENTS
We would like to thank Mr. Christopher Bennett of the Monash Health Cleft & Facial Anomalies Clinic, Department of Plastics & Reconstructive Surgery for his invaluable advice and guidance on this topic.
Footnotes
Presented at ACLAPA 2016—Brisbane, Queensland, Australia.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported by Monash University Centre for Human Anatomy Education—3D printing equipment and software. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Mastroiacovo P, Maraschini A, Leoncini E, et al. Prevalence at birth of cleft lip with or without cleft palate: data from the International Perinatal Database of Typical Oral Clefts (IPDTOC). Cleft Palate Craniofac J. 2011;1(48):66–81. doi: 10.1597/09-217. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka SA, Mahabir RC, Jupiter DC, et al. Updating the epidemiology of isolated cleft palate. Plast Reconstr Surg. 2013;131:650e–652e. doi: 10.1097/PRS.0b013e3182827790. [DOI] [PubMed] [Google Scholar]
- 3.Shkoukani MA, Chen M, Vong A. Cleft lip – a comprehensive review. Front Pediatr. 2013;53(1):1–10. doi: 10.3389/fped.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzel EB, Basile P, Koltz PF, et al. Current surgical practices in cleft care: cleft palate repair techniques and postoperative care. Plast Reconstr Surg. 2009;124:899–906. doi: 10.1097/PRS.0b013e3181b03824. [DOI] [PubMed] [Google Scholar]
- 5.Martin L, Delbridge L, Martin J, et al. Trainee surgery in teaching hospitals: is there a cost? Aust N Z J Surg. 1989;59:257–260. doi: 10.1111/j.1445-2197.1989.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 6.Aitken RJ. Lost opportunity cost of surgical training in the Australian private sector. ANZ J Surg. 2012;82:145–150. doi: 10.1111/j.1445-2197.2011.05968.x. [DOI] [PubMed] [Google Scholar]
- 7.Sasor SE, Flores RL, Wooden WA, et al. The cost of intraoperative plastic surgery education. J Surg Educ. 2013;70:655–659. doi: 10.1016/j.jsurg.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 8.McMenamin PG, Quayle M, McHenry CR, et al. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat Sci Ed. 2014;7:479–486. doi: 10.1002/ase.1475. [DOI] [PubMed] [Google Scholar]
- 9.Lim KH, Loo ZY, Goldie SJ, et al. Use of 3D printed models in medical education: a randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat Sci Educ. 2015;9:213–221. doi: 10.1002/ase.1573. [DOI] [PubMed] [Google Scholar]
- 10.Kyle K, VanKoevering TJ, Morrison SP, et al. Antenatal three-dimensional printing of aberrant facial anatomy. Pediatrics. 2015;136(5):1382–1385. doi: 10.1542/peds.2015-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chae MP, Rozen WM, McMenamin PG, et al. Emerging applications of bedside 3D printing in plastic surgery. Front Surg. 2015;2:25. doi: 10.3389/fsurg.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamali P, Dean D, Skoracki R, et al. The current role of three-dimensional printing in plastic surgery. Plast Reconstr Surg. 2016;137:1045–1055. doi: 10.1097/01.prs.0000479977.37428.8e. [DOI] [PubMed] [Google Scholar]
- 13.Bauermeister AJ, Zuriarrain A, Newman MI. Three-dimensional printing in plastic and reconstructive surgery: a systematic review. Ann Plast Surg. 2015 doi: 10.1097/SAP.0000000000000671. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Waran V, Menon R, Pancharatnam D, et al. The creation and verification of cranial models using three-dimensional rapid prototyping technology in field of transnasal sphenoid endoscopy. Am J Rhinol Allergy. 2012;26:e132–e136. doi: 10.2500/ajra.2012.26.3808. [DOI] [PubMed] [Google Scholar]
- 15.Perry JL, Kuehn DP. Three-dimensional computer reconstruction of the levator veli palatini muscle in situ using magnetic resonance imaging. Cleft Palate Craniofac J. 2007;44:421–423. doi: 10.1597/06-137.1. [DOI] [PubMed] [Google Scholar]
- 16.Inouye JM, Pelland CM, Lin KY, et al. A computational model of velopharyngeal closure for simulating cleft palate repair. J Craniofac Surg. 2015;26:658–662. doi: 10.1097/SCS.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 17.Ghasemianpour M, Ehsani S, Tahmasbi S, et al. Distraction osteogenesis for cleft palate closure: a finite element analysis. Dent Res J (Isfahan) 2014;11:92–99. [PMC free article] [PubMed] [Google Scholar]
- 18.Kalender WA. X-ray computed tomography. Phys Med Biol. 2006;51:29–43. doi: 10.1088/0031-9155/51/13/R03. [DOI] [PubMed] [Google Scholar]
- 19.Saunders DE, Thompson C, Gunny R, et al. Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr Radiol. 2007;37:789–797. doi: 10.1007/s00247-007-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parisi M, Glass I.Pagon RA, Adam MP, Ardinger HH.Joubert syndrome and related disorders. In: GeneReviews® [Internet] July 9, 2003Seattle, WA: University of Washington, Seattle; 1993–2015.[Updated April 11, 2013]Available at: http://www.ncbi.nlm.nih.gov/books/NBK1325/ [Google Scholar]
- 21.Peterka M, Tvrdek M, Müllerová Z. Tooth eruption in patients with cleft lip and palate. Acta Chir Plast. 1993;35:154–158. [PubMed] [Google Scholar]
- 22.Palubis JE. Cleft lip and palate with delayed eruption and congenital absence of teeth. Birth Defects Orig Artic Ser. 1971;7:265–266. [PubMed] [Google Scholar]
- 23.Bickel B, Bächer M, Otaduy MA, et al. Design and fabrication of materials with desired deformation behaviour. ACM Transactions on Graphics (TOG) 2010;29(4):63. [Google Scholar]
- 24.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5:335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 25.Knox K, Kerber CW, Singel SA, et al. Rapid prototyping to create vascular replicas from CT scan data: making tools to teach, rehearse, and choose treatment strategies. Catheter Cardiovasc Interv. 2005;65:47–53. doi: 10.1002/ccd.20333. [DOI] [PubMed] [Google Scholar]
- 26.Waran V, Narayanan V, Karuppiah R, et al. Injecting realism in surgical training-initial simulation experience with custom 3D models. J Surg Educ. 2014;71:193–197. doi: 10.1016/j.jsurg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Raja DS, Sultana B. Potential health hazards for students exposed to formaldehyde in the gross anatomy laboratory. J Environ Health. 2012;74:36–40. [PubMed] [Google Scholar]


