Summary:
In the pursuit of success in sports, some athletes are not deterred by health risks associated with the (mis)use of black market preparations of dubious origin as performance-enhancing agents. Several studies published in the recent years demonstrated that anabolic-androgenic steroids, but also stimulants and growth hormones, are misused by numerous recreational athletes from all over the world. Trenbolone is an anabolic steroid routinely used in the finishing phase of beef production to improve animal performance and feed efficiency. A 35-year-old male patient presented to our plastic surgery clinic after self-intramuscular administration of Trenbolone to the superior gluteal area bilaterally, which led to a full-thickness defect in a cone-like distribution. The wounds underwent surgical debridement and were treated locally with mafenide acetate irrigation and wound dressings. Closure was achieved by secondary intention healing. In this report, we discuss the first documented case of full-thickness skin and subcutaneous tissue necrosis after black market anabolic steroid injection. This illustrates a plastic complication and resolution of a widespread but seldom reported problem.
In the pursuit of success in sports, some athletes are not deterred by health risks associated with the (mis)use of black market preparations of dubious origin as performance-enhancing agents.1–3 In the black market, pharmaceuticals are commonly distributed without prescription and in some cases even without clinical approval. Moreover, clandestine laboratories frequently yield drug products of low quality.4 Several studies published in the recent years demonstrated that anabolic-androgenic steroids (AAS), but also stimulants and growth hormones, are misused by numerous recreational athletes from all over the world.5–9 Every year, the World Anti-Doping Agency (WADA) publishes a list comprising both prohibited substances and methods, which is mandatory for all competing athletes and their trainers, physiotherapists, and physicians.10
The prevalence of these drugs is hard to assess, but the proportion of different substances used can be inferred from different products confiscated at German airports for prohibited drugs in 2014. The identified chemically pure raw material comprised 259 kg of AAS, which were 83.7% of the total positive findings comprised of AAS and 21% of which were findings of Trenbolone.11
Trenbolone is an anabolic steroid routinely used in the finishing phase of beef production to improve animal performance and feed efficiency.12
We present a case of a 35-year-old male patient who self-injected Trenbolone intramuscularly to the superior gluteal area bilaterally. This led to a full-thickness defect in a cone-like distribution.
CASE PRESENTATION
A 35-year-old healthy man was referred to our emergency room by his physician because of painful skin necrosis over the left buttock approximately 5 × 4 cm and painful skin necrosis with purulent discharge on the right approximately 6.5 × 4 cm (Fig. 1).
Fig. 1.
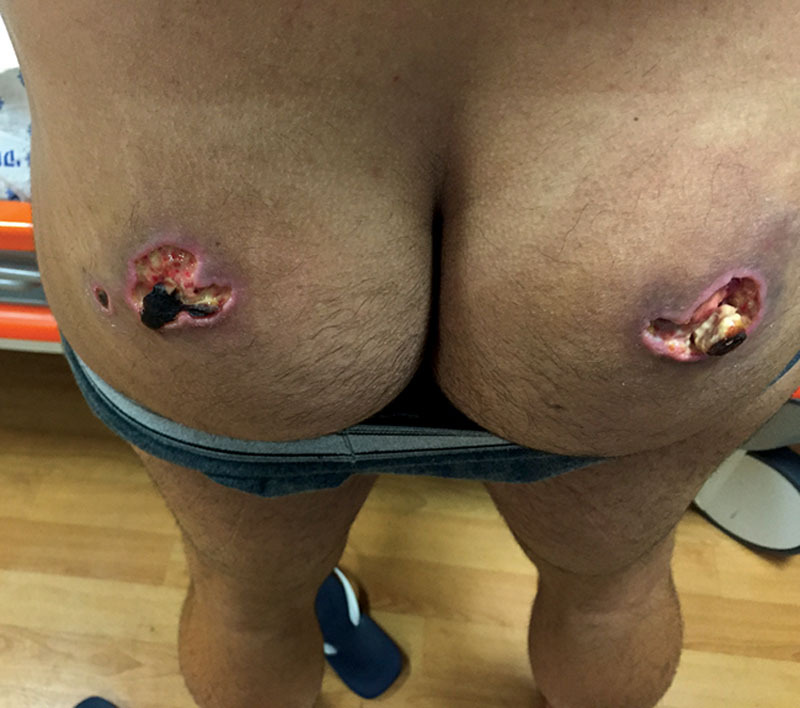
A 35-year-old healthy male referred by his physician due to painful skin necrosis approximately 5 × 4 cm and painful skin necrosis with purulent discharge on the right approximately 6.5 × 4 cm.
The patient stated that he is a recreational “body builder” and uses illicit substances to rapidly gain muscle mass. He has been using testosterone and various anabolic steroids for the past 4 years and 3 weeks before his referral changed his regimen to include a new steroid, Trenbolone. He recalled feeling pain upon injection, which led to him injecting more slowly and in an alternating pattern to both gluteus maximus muscles. The patient recalled persistent tenderness and induration in the injection sites followed by “darkening of the skin,” extreme pain, and secretion.
The patient was prescribed a course of first-generation cephalosporin, followed by a course of amoxicillin and clavulanic acid by his physician with no improvement and thereafter was referred to our center.
In the Plastic and Reconstructive Surgery Department, he underwent surgical wound debridement. The necrosis seemed to involve the skin, subcutaneous fat, and a small portion of the gluteus maximus muscle. Wound cultures were positive for Staphylococcus aureus and treated locally with mafenide acetate irrigation and wound dressings. Wound closure options included surgical closure by skin graft, local flaps, or healing by secondary intention. Because of the patient’s concern of further scarring and donor site morbidity, the wound was designated for healing by secondary intention. The patient was discharged with clean wounds and a hydrocolloid dressing 7 days after being admitted to the department. On ambulatory follow-up, the wound healed well with good granulation tissue filling the wound and peripheral epithelialization was observed shrinking the wound on the left to 3.5 × 3 cm and the wound on the right to 5 × 3 cm by 3 weeks after discharge (Fig. 2). At 6 weeks after discharge, wound dressings were changed to polyurethane (Fig. 3) and complete epithelialization was observed by 2 months after discharge (Fig. 4). No complications were noted.
Fig. 2.
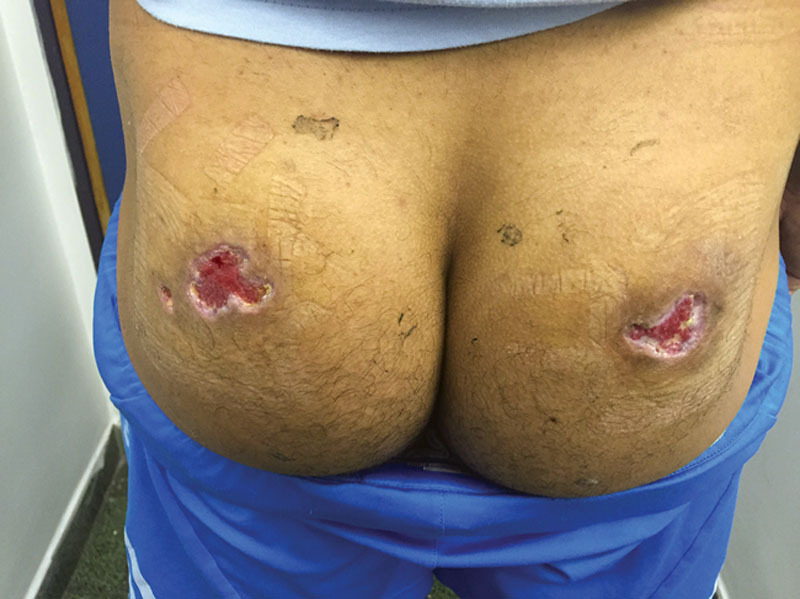
On ambulatory follow-up, the wound healed well with good granulation filling the wound and peripheral epithelialization shrinking the wound on the left to 3.5 × 3 cm and the wound to the right to 5 × 3 cm by 3 weeks after discharge.
Fig. 3.
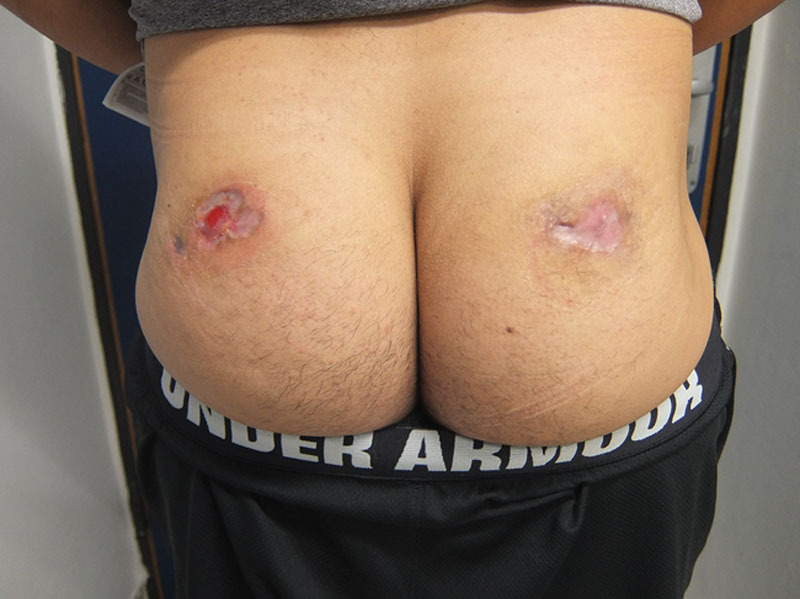
At 6 weeks, wound dressings were changed to polyurethane. Almost complete epithelialization 1.5 months after discharge.
Fig. 4.
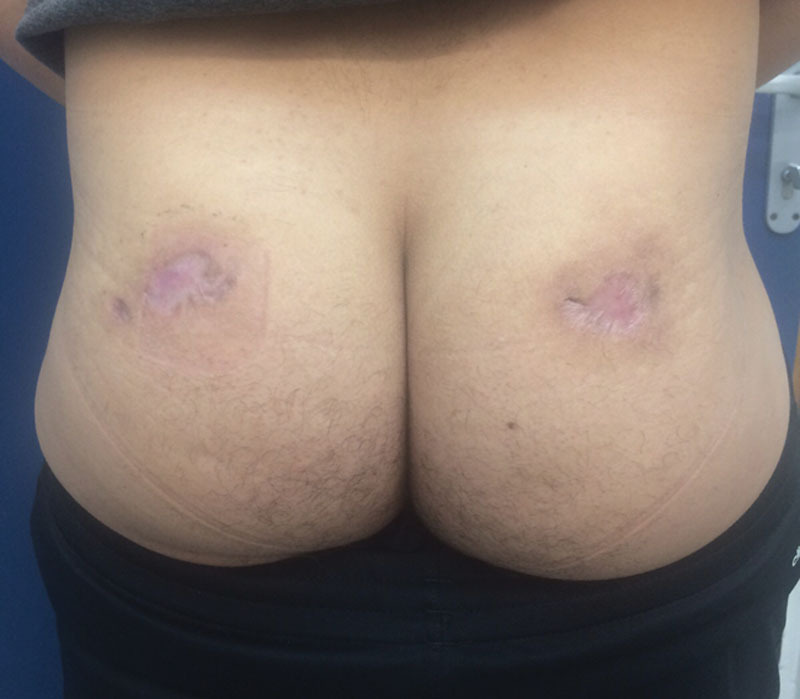
Complete epithelialization by 2 months after discharge.
DISCUSSION
Intramuscular Trenbolone is illegally used for rapid muscle mass accumulation by “body builders.” Tissue necrosis is a rare but serious complication of intramuscular injections.
Tissue necrosis caused by intramuscular drug injection was first observed in the 1920s by Freudenthal and Nicolau after administration of bismuth salts for syphilis treatment, and has been referred to since then as Nicolau’s syndrome.13 The syndrome typically begins as an immediate intense pain and pallor at the injection site, followed by erythema that evolves within hours into a livedoid bluish reticular patch, which becomes hemorrhagic and then necrotic. The necrosis may involve the skin, subcutaneous tissue, and muscular layer. Demarcation of the necrotic area appears after several days, resulting in a thick eschar that either may slough off and leave an underlying ulcer or may require surgical debridement. Recovery usually occurs over a few months, often leaving an atrophic scar.14 Complications, such as neurological injury, extensive necrosis, limb ischemia, sepsis due to superimposed infection, and even death in children, have been reported. In addition to bismuth salts, injection of several other drugs has been reported to cause necrosis, including nonsteroidal anti-inflammatory drugs, local anesthetics, corticosteroids, antihistamines, penicillin and other types of antibiotics, interferon, vitamin B complexes, iodine, and several vaccine preparations.14,15
The pathogenesis of postinjection necrosis is not completely understood; however, damage to an end artery by massive inflammatory reaction induced by intra-arterial or para-arterial drug injection seems to be the leading hypothesis. Allergic, immunologic, and mechanical vascular occlusion theories have been disproved.16,17
Several studies have mentioned clinical improvement with prompt administration of anticoagulation treatment (eg, subcutaneous heparin), intravenous steroids (eg, intravenous betamethasone, dexamethasone, or methyl prednisolone), and vasoactive therapy (eg, pentoxifylline).5 However, data regarding treatment of acute Nicolau’s syndrome are available only from sporadic case reports, and no established guidelines exist. There may not be any alternative to debridement and reconstructive surgery after necrosis and ulceration have evolved.
CONCLUSIONS
Because of the prevalent misuse of performance-enhancing drugs and the illegal nature of this practice, we believe professional and recreational athletes presenting with full-thickness wounds warrant a high index of suspicion of AAS misuse. The plastic reconstructive approach is standard in that the wound should be debrided and any infection, local or systemic, treated followed by reconstruction with the addition of using the experience as a strong deterrent from future drug abuse.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid by authors.
REFERENCES
- 1.Thevis M, Schrader Y, Thomas A, et al. Analysis of confiscated black market drugs using chromatographic and mass spectrometric approaches. J Anal Toxicol. 2008;32:232–240. doi: 10.1093/jat/32.3.232. [DOI] [PubMed] [Google Scholar]
- 2.Kohler M, Thomas A, Geyer H, et al. Confiscated black market products and nutritional supplements with non-approved ingredients analyzed in the Cologne Doping Control Laboratory 2009. Drug Test Anal. 2010;2:533–537. doi: 10.1002/dta.186. [DOI] [PubMed] [Google Scholar]
- 3.Thevis M, Geyer H, Thomas A, et al. Trafficking of drug candidates relevant for sports drug testing: detection of non-approved therapeutics categorized as anabolic and gene doping agents in products distributed via the Internet. Drug Test Anal. 2011;3:331–336. doi: 10.1002/dta.283. [DOI] [PubMed] [Google Scholar]
- 4.Fotiou F, Aravind S, Wang PP, et al. Impact of illegal trade on the quality of epoetin alfa in Thailand. Clin Ther. 2009;31:336–346. doi: 10.1016/j.clinthera.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Rich JD, Dickinson BP, Feller A, et al. The infectious complications of anabolic-androgenic steroid injection. Int J Sports Med. 1999;20:563–566. doi: 10.1055/s-1999-8841. [DOI] [PubMed] [Google Scholar]
- 6.Raschka C, Chmiel C, Preiss R, et al. [Recreational athletes and doping–a survey in 11 gyms in the area of Frankfurt/Main]. MMW Fortschr Med. 2013;155:57. doi: 10.1007/s15006-013-1052-4. [DOI] [PubMed] [Google Scholar]
- 7.Woerdeman J, de Hon O, Levi M, et al. [Anabolic androgenic steroids in amateur sports in the Netherlands]. Ned Tijdschr Geneeskd. 2010;154:A2004. [PubMed] [Google Scholar]
- 8.Striegel H, Simon P, Frisch S, et al. Anabolic ergogenic substance users in fitness-sports: a distinct group supported by the health care system. Drug Alcohol Depend. 2006;81:11–19. doi: 10.1016/j.drugalcdep.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Simon P, Striegel H, Aust F, et al. Doping in fitness sports: estimated number of unreported cases and individual probability of doping. Addiction. 2006;101:1640–1644. doi: 10.1111/j.1360-0443.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- 10.WADA. The 2014 Prohibited List. Available at: http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/2014/WADA-prohibited-list-2014-EN.pdf. Accessed April 24, 2014.
- 11.Krug O, Thomas A, Walpurgis K, et al. Identification of black market products and potential doping agents in Germany 2010-2013. Eur J Clin Pharmacol. 2014;70:1303–1311. doi: 10.1007/s00228-014-1743-5. [DOI] [PubMed] [Google Scholar]
- 12.Duckett SK, Pratt SL. Meat science and muscle biology symposium—anabolic implants and meat quality. J Anim Sci. 2014;92(1):3–9. doi: 10.2527/jas.2013-7088. [DOI] [PubMed] [Google Scholar]
- 13.Köhler LD, Schwedler S, Worret WI. Embolia cutis medicamentosa. Int J Dermatol. 1997;36:197. doi: 10.1046/j.1365-4362.1997.00233.x. [DOI] [PubMed] [Google Scholar]
- 14.Faucher L, Marcoux D. What syndrome is this? Nicolau syndrome. Pediatr Dermatol. 1995;12:187–190. doi: 10.1111/j.1525-1470.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 15.Corazza M, Capozzi O, Virgilit A. Five cases of livedo-like dermatitis (Nicolau’s syndrome) due to bismuth salts and various other non-steroidal anti-inflammatory drugs. J Eur Acad Dermatol Venereol. 2001;15:585–588. doi: 10.1046/j.1468-3083.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 16.Lie C, Leung F, Chow SP. Nicolau syndrome following intramuscular diclofenac administration: a case report. J Orthop Surg (Hong Kong) 2006;14:104–107. doi: 10.1177/230949900601400123. [DOI] [PubMed] [Google Scholar]
- 17.Luton K, Garcia C, Poletti E, et al. Nicolau syndrome: three cases and review. Int J Dermatol. 2006;45:1326–1328. doi: 10.1111/j.1365-4632.2006.02674.x. [DOI] [PubMed] [Google Scholar]


