Abstract
The purpose of this study is to evaluate the efficacy of the enhancement of docetaxel by pulsed focused ultrasound (pFUS) in combination with radiotherapy (RT) for treatment of prostate cancer in vivo. LNCaP cells were grown in the prostates of male nude mice. When the tumors reached a designated volume by MRI, tumor bearing mice were randomly divided into 7 groups (n=5): (1) pFUS alone; (2) RT alone; (3) docetaxel alone; (4) docetaxel + pFUS; (5) docetaxel + RT; (6) docetaxel + pFUS + RT; and (7) control. MR guided pFUS treatment was performed using a focused ultrasound treatment system (InSightec ExAblate 2000) with a 1.5T GE MR scanner. Animals were treated once with pFUS, docetaxel, RT or their combinations. Docetaxel was given by i.v. injection at 5 mg/kg before pFUS. RT was given 2 Gy after pFUS. Animals were euthanized 4 weeks after treatment. Tumor volumes were measured on MRI at 1 and 4 weeks post-treatment. Results showed that triple combination therapies of docetaxel, pFUS and RT provided the most significant tumor growth inhibition among all groups, which may have a potential for the treatment of prostate cancer due to an improved therapeutic ratio.
Keywords: MRgFUS, docetaxel, RT, prostate cancer, in vivo
1. Introduction
Focused ultrasound (FUS) has clinically emerged as a noninvasive therapy technique for localized prostate cancer and other solid malignancies (Kennedy 2005). For clinical treatment, FUS is predominantly being used for thermal ablation in targeted tissues by continuous deposition of focused acoustic energy. Pulsed FUS (pFUS) uses non-thermal effects at low duty cycles that alter the tissue properties. Recent studies have suggested that pFUS exposure may alter vascular or cell membrane permeability to enhance drug delivery in tumors in animal models (Bednarski et al. 1997, Nelson et al. 2002, Dittmar et al. 2005, Yuh et al 2005, Frenkel V et al 2006a, Hancock et al. 2009 and Chen et al 2010). Enhancement of drug delivery to the tumor target by pFUS exposures and its effect on tumor growth inhibition in vivo has been reported by other investigators (Dittmar et al 2005, Dromi et al 2007 and Poff et al 2008).
Radiation therapy (RT) is one of the primary treatments for prostate cancer. Although there has been a reduction in failure rates with increased RT dose using sophisticated planning and delivery techniques, local persistence of disease remains in many cases (Hanks et al 1998, Pollack et al 2002, Jacob et al 2004). Androgen deprivation (AD) is one of the most common additional treatments for advanced prostate cancer. Chemotherapy has been shown to prolong median survival for hormone refractory disease (Tannock et al 2004, Petrylak et al 2004). Docetaxel has become the standard first-line chemotherapy for the treatment of advanced hormone refractory prostate cancer (Armstrong and George 2010).
In our laboratory, we have developed techniques for prostate tumor implantation orthotopically. In a previous study, we developed MR guided focused ultrasound (MRgFUS) treatment techniques for a small animal model (nude mouse) using a clinical patient treatment device (InSightec ExAblate 2000) together with a 1.5 T MR scanner (Signa Excite HD, GE Healthcare, Milwaukee, WI, USA) for MR guidance during treatment. We performed in vivo experiments to investigate the use of MRgFUS for the enhancement of chemotherapy in prostate tumors grown in nude mice using [3H]-docetaxel. Our experimental data showed that the [3H]-docetaxel concentration in tumors treated with MRgFUS was significantly increased compared with those without the MRgFUS treatment (Chen et al 2010). The purpose of this study is to investigate whether the enhancement of the docetaxel uptake using MR guided pFUS combined with RT will improve the tumor response in vivo.
2. Methods and Materials
2.1 Cell Culture and Tumor Model
Human prostate cancer LNCaP cells were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM)-F12 medium, containing 10% fetal bovine serum (FBS), 1% L-glutamine, and 1% penicillin-streptomycin as described previously (Mu et al 2008). Male athymic Balb/c nude mice (6 weeks old) were purchased from Harlan (Indianapolis, IN). All animal studies were carried out in compliance with approval of both the institutional animal care and use committee (IACUC) and the institutional radiation safety committee. Aseptic techniques were used for injection and implantation of LNCaP cells in the prostates of nude mice as described previously (Mu et al 2008, Stoyanova et al 2007, Chen et al 2010). Nude mice were anesthetized using methoxyflurane. A lower midline incision was made approximately 1.5 cm above the presumed location of the bladder. The seminal vesicles were gently brought out through the incision and LNCaP cells (1 × 106) in 25 μl of PBS (phosphate buffered saline) were injected into the dorsal prostate lobes using a 30-gauge 1-inch needle. The incision was sealed by suturing the muscle layer (suture size-4.0 silk) and 2-3 wound clips for the skin layer.
2.2 MR Imaging
Tumor volume measurement
Beginning at 3 weeks after the tumor implantation the tumor volume was monitored weekly by MRI in a vertical wide-bore magnet equipped with a Bruker DRX 300 console at a field strength of 7 Tesla. The standard MR imaging protocol was provided by the small animal imaging facility at Fox Chase Cancer Center (FCCC), as described previously (Stoyanova et al 2007). Briefly, the tumor volumes were determined by outlining tumors using the Paravision software supplied with the spectrometer, and then summing the volumes from all the sections. The images were made with a two dimensional multi-slice spin echo pulse sequence. Repetition times were in the range of 400-600 milliseconds, the echo time was 13.2 milliseconds, the slice thickness was 0.75mm, and the in-plane resolution was 0.1mm. Signal averaging over two acquisitions brought the scan time to less than 4 minutes. This protocol allowed us to visualize tumors with volumes as small as 5 mm3. Animal preparation and scout image acquisition brought the total MR imaging time to approximately 10 minutes/animal. Prior to imaging, the mouse received an injection of 0.2 ml of the commercial contrast agent Magnevist (Berlex Industries-Montville, New Jersey) diluted 10:1 with a physiological concentration of saline. During imaging the mouse was anesthetized with a mixture of 1% isofluorane in oxygen. When the tumor volume reached a designated size (see below) treatments were initiated.
MRI for FUS
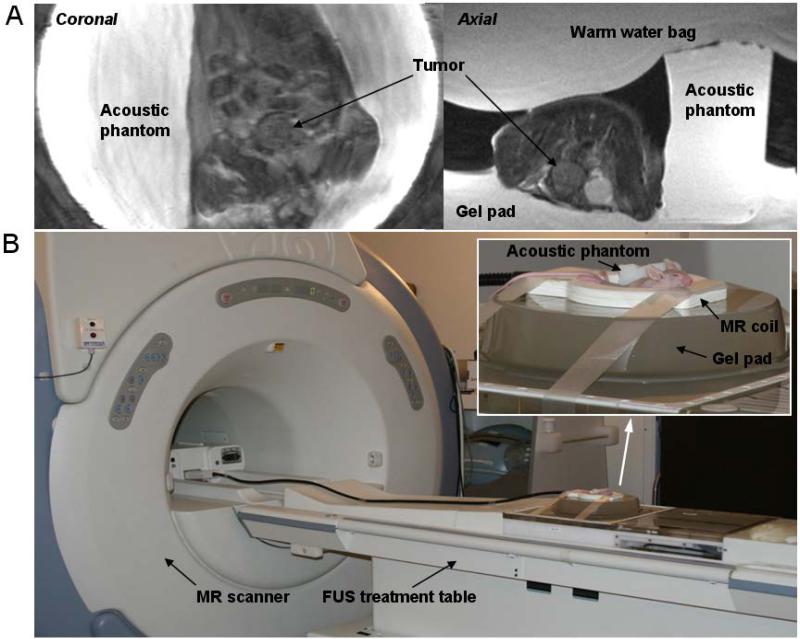
The “optimal” MR imaging protocol for MRgFUS treatment with the 1.5 T scanner used in this study was based on our previous study (Chen et al 2010). The protocol provides a high quality image to identify the prostate tumor in a nude mouse within approximately 1 h anesthesia time for the entire treatment procedure. The scans were first performed for localization (3 dimensional) using a fast spin echo (FSE) image sequence at low image quality with a 0.13 minute scan time and then for the coronal image sequence with a 7 minute scan time to achieve high quality imaging for tumor target identification. The MR parameters were: T2-weighted coronal FSE sequence; TR/TE = 2150/102 ms; Bandwidth: 10.4 kHz; FOV = 9.0 × 9.0 cm; Matrix: 384 × 384; NEX = 4; slice thickness: 2.0 mm/0.0 sp; frequency direction: SI and the spatial resolution: 0.23 mm. Based on the coronal image, an axial FSE sequence was performed with a 4.25 minute scan time. Figure 1A shows an example of the high-quality images used for tumor delineation and treatment planning in real time for this study.
Figure 1.
A: MR images showing prostate tumors in a coronal view and an axial view obtained on a 1.5 T MR scanner. B: The FUS treatment table with a 1.5 T MR scanner and the animal setup for the pFUS treatment.
2.3 Experimental setup
An ExAblate 2000 FUS system (InSightec-Tx-Sonics, Haifa, Israel, and Dallas, TX) together with a 1.5 T GE MR scanner was used for the pFUS treatment (Fig. 1B). The system was installed in the Department of Radiation Oncology at FCCC in 2006. This treatment system was approved by the FDA for treating uterine fibroids clinically. In our department it is being used for treatment of painful bone metastases (Chen et al. 2009), for preclinical investigations of prostate and breast cancer ablation under the local Institutional Review Board (IRB) approval (Ma et al. 2009).
Quality control (QC) for the FUS treatment unit was performed according to the procedures provided by the vendor to check the transducer output, the focal spot and the electronic motion system before animal treatments as described previously (Chen et al 2010). Mice were anesthetized by intraperitoneal injection of a mixture of Ketamine (60 mg/kg) and Ace-promazine (2.5 mg/kg). Figure 1B shows the animal setup for pFUS treatments. A gel pad was placed on the treatment table in line with the transducer. Degassed water was used for the interface between the treatment table and the gel pad for the acoustic coupling. Mice were carefully placed in the hole (approximately 5cm × 5cm × 1cm) of the gel pad, which was filled with degassed water. A 3-inch surface coil was placed around the animal to receive the MR signals. A small (4cm × 2cm × 2cm) acoustic phantom provided by the vendor was placed beside the mouse for verification of the location of the focal spot prior to animal sonication (Fig. 1). The acoustic phantom was manufactured by ATS Labs Inc. (Bridgeport, CT) with acoustic properties similar to those of human soft tissue (the attenuation coefficient: 0.503 dB/cm/MHz; speed of sound: 1538 MPS; estimated specific heat: 2.684 cal/gram). The phantom was not used for the validation of thermometry as the thermal conductivity of the phantom was not available. A surgical glove filled with warm water was placed on top of the mouse to protect the animal from hypothermia.
2.4 Pulsed focused ultrasound treatment
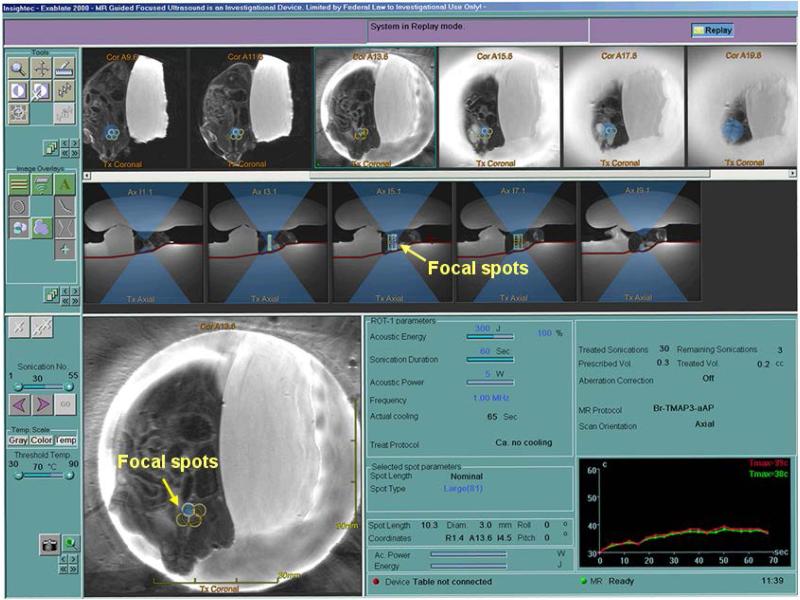
Pulsed-FUS treatment was performed using a method described in detail in a previous publication (Chen et al 2010). Briefly, both coronal and axial MR images were loaded on the focused ultrasound treatment work station. The tumor was contoured, the skin surface was delineated, and a treatment plan was generated. Prior to focused ultrasound treatment, the effective focal spot was verified using the small acoustic phantom beside the animal using MR thermometry. Animals were treated using the following parameters: 1 MHz; 5W acoustic power for 60 sec with 50% duty cycle (0.1s power on and 0.1s power off) per sonication, depending on the experimental design (see below). The time averaged acoustic focal intensity was approximately 220 W/cm2 estimated using the acoustic power and the beam cross section area. The treatment parameters were derived from our acoustic phantom studies as described by Chen et al (2010). The whole tumor volumes were covered with multiple focal spots depending on the tumor sizes. Figure 2 shows real-time treatment planning for the pFUS treatment. The tumor target was covered by multiple focal spots on both the coronal and axial MR images. The temperature was monitored in real-time (~ 3 sec delay) by MR thermometry.
Figure 2.
A FUS treatment plan with multiple focal spots (arrows) covering the tumor target on both coronal and axial images. The real-time temperature was monitored by MR thermometry.
2.5 Study design
Study 1
This is a pilot study aimed to determine a reasonable docetaxel dose and the FUS treatment scheme. When the tumor volume reached 45 ± 8.5 mm3 on MRI, mice were randomly assigned to 4 groups (n=5): (1) pFUS alone; (2) pFUS + docetaxel; (3) docetaxel alone; and (4) control. For groups 1 and 2, each mouse was treated with pFUS under general anesthesia for 2 fractions (one treatment per week for two consecutive weeks). For groups 2 and 3, each animal received docetaxel (Taxotere; sanofi-aventis U.S. LLC, Bridgewater, NJ) by tail vein injection at 10 mg/kg for 2 fractions (one injection per week for two consecutive weeks). For group 2 the docetaxel was injected immediately after the pFUS treatment. For the control group, a sham FUS treatment was given. Animals were allowed to survive for 4 weeks. The tumor volumes were measured by MR imaging at 1 and 4 weeks after the treatment.
Study 2
Based on the results from study 1, both the docetaxel dose and the pFUS treatment scheme were adjusted to reduce the systemic toxicities. When the tumor volume reached 36 ± 5.9 mm3 on MRI, mice were randomly divided into 7 groups (n=5): (1) pFUS alone; (2) RT alone; (3) docetaxel only; (4) docetaxel + pFUS; (5) docetaxel + RT; (6) docetaxel + pFUS + RT; and (7) control. Animals receiving the pFUS treatment were only treated once. Animals receiving the docetaxel treatment were also treated once and the dose was reduced to 5 mg/kg. The docetaxel injection was performed before the pFUS and RT treatment was performed immediately after the pFUS. For the RT treatment, animals were restrained under general anesthesia in the supine position with tape in a jig and irradiated on a Cesium 137 irradiator (model 81-14; J.L. Shepherd and Associates, San Fernando, CA). A collimator was used to treat the prostate while protecting the lung, abdomen, and legs (Stoyanova et al 2007). Animals were allowed to survive for 4 weeks. Tumor volumes were measured by MRI at 1 and 4 weeks after the treatment.
2.6 Data analysis and statistics
The relative tumor volume (TV) for each animal was calculated as a ratio of the tumor volume at 1 and 4 weeks after treatment to the tumor volume on the treatment day. Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS). The one-way ANOVA least significance difference test (LSD) was used to determine the significance among experimental groups, and P < 0.05 was considered significant.
3. Results
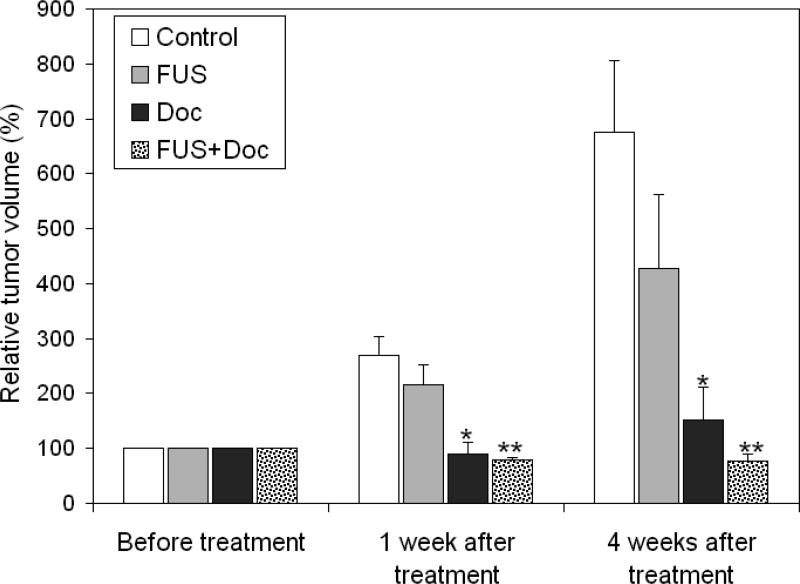
Our first pilot study (study 1) was designed to find a reasonable docetaxel dose and the pFUS treatment scheme for our investigation. Figure 3 shows the relative TV change at 1 week and 4 weeks after the pFUS and docetaxel treatment. Tumor growth delay was observed for the group receiving pFUS alone at 1 week and 4 weeks after the treatment; the average tumor volume was 28.3% and 39% smaller compared to that of the control group, respectively. The tumor growth delay was more significant for the group receiving docetaxel only; the average tumor volume at 1 week after treatment was smaller than that prior to the treatment (P = 0.0013) and it only increased slightly 4 weeks post treatment (P = 0.0028). It should be mentioned that the tumor volume measured by MRI within a short period of time (e.g., 1 week) after the treatment might not accurately reflect the actual number of surviving tumor cells because both chemo/radiotherapy would thin out tumor cells randomly in the tumor volume rather than changing the tumor bulk volume/shape. The tumor volume at 4 weeks after treatment would be a better indicator of the total viable tumor cells when it grew significantly greater than that prior to the treatment. The combination of pFUS and docetaxel showed excellent tumor control; the average tumor volume was smaller than that prior to the treatment both 1 week and 4 weeks after treatment. However, their overwhelming cell killing power, especially that by docetaxel, also drowned out the potential synergistic effect of pFUS and docetaxel.
Figure 3.
Relative tumor volume at 1 and 4 weeks after the two-week treatment with docetaxel (10 mg/kg, 2 fractions), pFUS (2 fractions) or the combination of the two, relative to the average tumor volume prior to the treatment. Error bars are standard deviation of the mean. * and **, p < 0.05 compared with the control and FUS groups (one way ANOVA, LSD test).
Although docetaxel alone at a dose of 10mg/kg for two consecutive weeks resulted in a significant delay in tumor growth, the associated toxicities were also severe to the small animals (the average body weight of the nude mice was 25g). Results from study 1 showed that the animals did not tolerate the two-week treatment very well; the docetaxel-treated mice suffered severe weight loss (>10% body weight) and an approximately 30% mortality. The pFUS-treated mice also lost weight (about 10%) during the two-week treatment (recovered 4 weeks after treatment), which was probably due to the effect of the general anesthesia since the animals could not have normal intake for the entire treatment day. There was a concern that severe treatment toxicities might adversely affect the experimental accuracy on the tumor growth. The second study was therefore designed to reduce the treatment toxicities and mortality.
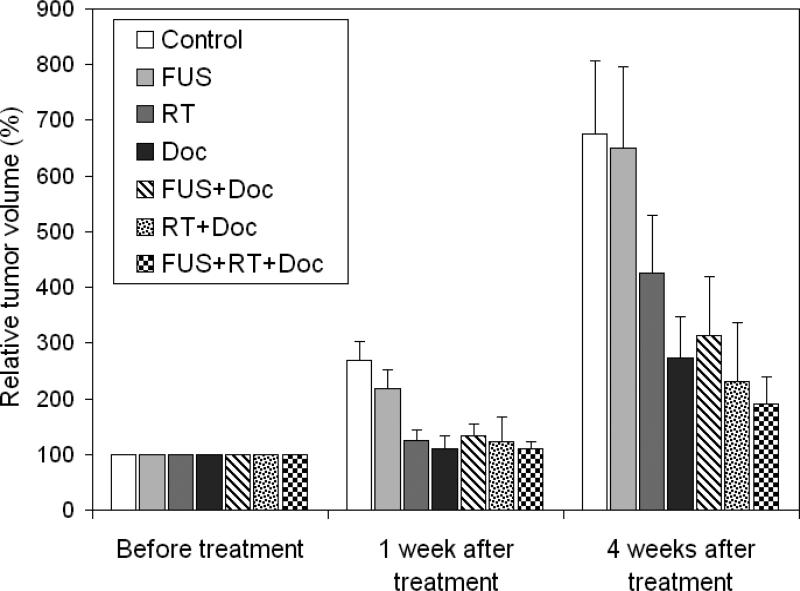
In study 2, the docetaxel dose was lowered to 5 mg/kg (mouse body weight) with only one injection to reduce the side effects of chemotherapy. The pFUS treatment was given once only to minimize the possible effect due to general anesthesia. The docetaxel injection was given immediately before the FUS/RT treatment to enhance drug penetration/absorption, assuming that the docetaxel concentration was still at or near its peak value when pFUS altered the permissibility of the vessel wall and the cell membrane. Figure 4 shows the relative tumor volume based on the experimental results from the second study. The relative tumor volume and the associated uncertainty are given in Table 1. Animals treated with pFUS alone showed a small (about 4%) reduction in the average tumor volume at 4 weeks after the treatment compared to the control group, but it was not statistically significant (P = 0.45). The tumor growth delay was more than 37% for the RT alone group 4 weeks after a 2Gy irradiation, which is a typical daily dose for standard-fractionation radiation therapy. The tumoricidal effect of docetaxel at 5mg/kg was still significant; the average tumor volume 4 weeks post treatment was about 60% smaller than that of the control group. The level of inhibition achieved with dual combination therapy of docetaxel + FUS or of docetaxel + RT was not statistically different from that with docetaxel alone (P=0.39 and 0.37 respectively). The tumor volume for the group receiving triple combination therapy of docetaxel + pFUS + RT appeared to be the lowest compared to all treatment groups (Table 1). Further experiments are needed to quantify the therapeutic ratio of dual or triple combination therapies of docetaxel with FUS and/or RT in terms of the dose level and fractionation scheme.
Figure 4.
Relative tumor volume at 1 and 4 weeks after the single-fraction treatment of docetaxel (5 mg/kg), pFUS, RT and dual- or triple combination therapies of the three modalities, relative to the average tumor volume prior to the treatment. The error bars are standard deviation of the mean.
Table 1.
Relative tumor volumes at 1 and 4 weeks after treatments.
| Group | Relative tumor volume* (Mean ± SEM**) | |
|---|---|---|
| 1 week | 4 weeks | |
| Control | 2.69 ± 0.34 | 6.76 ± 1.30 |
| FUS | 2.19 ± 0.33 | 6.50 ± 1.46 |
| RT | 1.25 ± 0.18 | 4.26 ± 1.04 |
| Doc | 1.11 ± 0.23 | 2.73 ± 0.73 |
| FUS + Doc | 1.34 ± 0.20 | 3.13 ± 1.06 |
| RT + Doc | 1.24 ± 0.44 | 2.31 ± 1.06 |
| FUS + RT + Doc | 1.10 ± 0.13 | 1.91 ± 0.47 |
Ratio of tumor volume after the treatment to that before the treatment.
SEM: standard deviation of the mean.
4. Discussion and Summary
In our previous study, a technique was established that is capable of performing pFUS on nude mice with implanted prostate tumors using a clinical treatment equipment. The results from our in vivo studies demonstrated the pFUS enhancement of the docetaxel delivery. The purpose of this study is to investigate if the increased uptake of docetaxel would result in tumor growth inhibition in vivo.
Although some effects of pFUS on tumor growth inhibition were observed using the described treatment parameters as compared to the control group, our results did not yield statistically significant differences between them with the current small sample numbers (n=5). This was consistent with the findings of other solid tumor model studies (Dittmar et al 2005, Dromi et al. 2007, Proff et al 2008 and Frenkel et al. 2006). However, one study showed that mechanical FUS with different treatment parameters might induce anti-tumor immunity and therefore reduce tumor growth in a murine tumor model (Hu et al 2007).
The pFUS exposure with an acoustic power of 5W used in this study generated a temperature elevation less than 5 °C in targeted tumor tissues (to ensure a temperature of < 42 °C for potential future human applications) (Chen et al 2010). The MR proton resonance frequency shift sequence (machine built-in software) was used for monitoring the temperature changes during the pFUS treatment (Fig. 2). Our prior results have shown that the 3H-docetaxel delivery in implanted tumors can be enhanced by pFUS using the same treatment parameters.
Increasing experimental and theoretical results have indicated that pulsed FUS can enhance the permeability of target tissues or change the structure of extracellular matrix to improve drug or gene delivery (i.e., Frenkel et al. 2006b). A recent study showed that pFUS generated acoustic radiation forces produced in the targeted tissue may have a role in enhancing delivery (Hancock et al. 2009). The mechanisms for producing the observed enhancement are not well understood. It is thought mainly due to the nonthermal effects of ultrasound – mechanical streaming and cavitation although larger increases in temperature could occur within microenvironments of the cell as a result of cavitation. The increased extravasation of the blood was observed in our previous study using the same ultrasonic parameters as for this study. Our results suggested that the increase drug delivery in tumor was a result of the increased blood vessel permeability. The possible mechanisms have been discussed in our previous published paper (Chen et al 2010). However, the precise mechanism is little known and needs to be further investigated. Extensive histological studies are being conducted on prostate tumors treated with pFUS to answer these questions.
Radiation is one of the most effective treatment modalities for prostate cancer. Docetaxel is one of the few effective drugs for the treatment of advanced hormone refractory prostate cancer and/or metastatic disease clinically. Docetaxel inhibits microtubule formation and downregulates antiapoptotic protein Bcl-2 expression (Engels et al 2005). Furthermore, the combination of docetaxel and bortezomib as a chemotherapeutic drug sensitizes Bcl-2 overexpressing human prostate cancer cells to radiation effects by modulating the expression of key members of the Bcl-2 family (Cao et al 2008). In the present study, we chose to test the efficacy of pFUS, docetaxel, and RT alone and dual- or triple-combination therapies using an orthotopic prostate cancer model. Our results showed that both docetaxel and RT alone caused a significant increase in tumor growth inhibition compared with the control group. The strong effect of docetaxel on tumor growth was observed with both double injections of 10 mg/kg and a single injection of 5 mg/kg. However, the radiation-sensitizing effect of docetaxel was not evident when docetaxel and RT were given together (Cao et al 2008). When pFUS was given prior to the injection of 10mg/kg docetaxel, there was a further delay in tumor growth compared to treating with pFUS or docetaxel alone (Fig. 3). However, when docetaxel was injected at a lower dose of 5 mg/kg prior to the pFUS treatment, there was no further tumor growth inhibition for the limited number of animals investigated (Fig 4). The lowest level of tumor growth was seen when all three treatments, pFUS, docetaxel and RT were given together. Our results suggested that the in-situ dose of docetaxel might be very uncertain between individual mice, which may have an impact on the determination of tumor growth inhibition especially when combined with other therapies. The variation of the blood flow among individual animals may have contributed to the uncertainty in the measured drug-dose response. The standard deviation of the tumor volume was also higher than expected, which may also have an impact on the drug-dose response. A larger sample may help reduce the overall experimental uncertainty. Because docetaxel alone at both dose levels of 5 mg/kg and 10 mg/kg already resulted in significant tumor cell killing, additional antitumor effects from pFUS or RT may become less pronounced, at least with our current number of animals used. A similar study showed pFUS enhanced tumor growth inhibition with a chemotherapeutic drug, botezomib, at a low dose level; drug alone had no effect on the tumor growth (Poff et al 2008).
In summary, we have performed experiments to investigate the effectiveness of the increased uptake of docetaxel by pFUS in combination with RT in prostate tumor control in vivo. Although previous studies have demonstrated the antitumor efficacy of pFUS in combination with a variety of therapeutic agents at different settings and using different tumor models by other investigators, the results from this study did not show significant synergistic effects between docetaxel, pFUS and RT. However, this study was performed with a small sample and only a single animal tumor model. Future studies are being designed to continue investigating the drug enhancement effect of pFUS by focusing on optimal pFUS treatment parameters and chemo/gene dose levels and fractionation schemes.
Acknowledgments
We would like to thank Dr. Harvey Hensley for his technical assistance with MRI using the 7T MR scanner, the small animal imaging facility and the animal care facility at Fox Chase Cancer Center. We would also like to thank InSightec, especially Dr. Arik Hananel, Ms Osnat Dogadkin, Mr. Amit Sokolov, Javier Grinfeld MSc and Dr. Jessica Foley for their excellent technical support. This study was supported by the Focused Ultrasound Surgery Foundation and DOD (PC073127).
Footnotes
The materials in this paper have been partially presented at the AAPM 2010
References
- Armstrong AJ, George DJ. Optimizing the use of docetaxel in men with castration-resistant metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:108–116. doi: 10.1038/pcan.2009.62. [DOI] [PubMed] [Google Scholar]
- Bednarski MD, Lee JW, Callstrom MR, Li KC. In vivo target-specific delivery of macromolecular agents with MR-guided focused ultrasound. Radiology. 1997;204:263–268. doi: 10.1148/radiology.204.1.9205257. [DOI] [PubMed] [Google Scholar]
- Cao W, Shiverick KT, Namiki K, Sakai Y, Porvasnik S, Urbanek C, Rosser CJ. Docetaxel and bortezomib downregulate Bcl-2 and sensitize PC-3-Bcl-2 expressing prostate cancer cells to irradiation. World J Urol. 2008;26:509–516. doi: 10.1007/s00345-008-0289-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Ma C-M, Richardson T, Freedman G, Konski A. Treatment of Bone Metastasis Using MR Guided Focused Ultrasound. Medical Physics. 2009;36:2486. (Abstract) [Google Scholar]
- Chen L, Mu Z, Hachem P, Ma C-M, Wallentine A, Pollack A. MR Guided Focused Ultrasound: enhancement of intratumoral uptake [3H]-docetaxel in vivo. Physics in Medicine and Biology. 2010;55:7399–7410. doi: 10.1088/0031-9155/55/24/001. [DOI] [PubMed] [Google Scholar]
- Dittmar KM, Xie J, Hunter F, Trimble C, Bur M, Frenkel V, Li KC. Pulsed high-intensity focused ultrasound enhances systemic administration of naked DNA in squamous cell carcinoma model: initial experience. Radiology. 2005;235:541–546. doi: 10.1148/radiol.2352040254. [DOI] [PubMed] [Google Scholar]
- Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bup M, Poff J, Xie J, Libutti SK, IBUTTI SK, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels FK, Sparreboom A, Mathot RA, Verweij J. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer. 2005;93:173–177. doi: 10.1038/sj.bjc.6602698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel V, Etherington A, Greene M, Quijano J, Xie IEJ, Hunter F, Dromi S, Li KC. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol. 2006a;13:469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Frenkel V, Li KC. Potential role of pulsed-high intensity focused ultrasound in gene therapy. Future Oncol. 2006b;2:111–119. doi: 10.2217/14796694.2.1.111. [DOI] [PubMed] [Google Scholar]
- Hancock HA, Smith LH, Cuesta J, Durrani AK, Angstadt M, Palmeri ML, Kimmel E, Frenkel V. Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound Med Biol. 2009;35:1722–1736. doi: 10.1016/j.ultrasmedbio.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks GE, Hanlon AL, Schultheiss TE, Pinover WH, Movsas B, Epstein BE, Hunt MA. Dose escalation with 3D conformal treatment: five year outcomes, treatment optimization, and future directions. Int J Radiat Oncol Biol Phys. 1998;41:501–510. doi: 10.1016/s0360-3016(98)00089-3. [DOI] [PubMed] [Google Scholar]
- Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Hanlon AL, Horwitz EM, Movsas B, Uzzo RG, Pollack A. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer. 2004;100:538–43. doi: 10.1002/cncr.11927. [DOI] [PubMed] [Google Scholar]
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- Ma C-MCL, Freedman G, et al. MR guided Focused ultrasound for high risk and recurrent breast cancer. In: Dössel O, Schlegel WC, editors. WC, IFMBE Proceedings 25/VI. Springer; Heidelberg, Germany: 2009. pp. 140–43. [Google Scholar]
- Mu Z, Hachem P, Hensley H, Stoyanova R, Kwon HK, Hanlon AL, Agrawal S, Pollack A. Antisense MDM2 enhances the response of androgen insensitive human prostate cancer cells to androgen deprivation in vitro and in vivo. Prostate. 2008;68:599–609. doi: 10.1002/pros.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JL, Roeder BL, Carmen JC, et al. Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Research. 2002;62:7280–7283. [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, JR., Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohhli M, Benson MC, Small EJ, Raghavan D, Crawford E. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Poff JA, Allen CT, Traughber B, Colunga J, Xie Z, Chen BJ, Wood C, Waes V, Li KC, Frenkel V. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008;248:485–491. doi: 10.1148/radiol.2482071674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A, Zagars GK, Antolak JA, Kuban DA, Rosen Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. International journal of radiation oncology, biology, physics. 2002a;54:677–85. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- Stoyanova R, Hachem P, Hensley H, Khor LY, Mu Z, Hammond ME, Agrawal S, Pollack A. Antisense-MDM2 sensitizes LNCaP prostate cancer cells to androgen deprivation, radiation, and the combination in vivo. International journal of radiation oncology, biology, physics. 2007;68:1151–60. doi: 10.1016/j.ijrobp.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Yuh EL, Shulman SG, Mehta SA, Xie J, Chen L, Frenkel V, Bednarski MD, Li KC. Delivery of systemic chemotherapeutic agent to tumors by using focused ultrasound: study in a murine model. Radiology. 2005;234:431–7. doi: 10.1148/radiol.2342030889. [DOI] [PubMed] [Google Scholar]