Abstract
The effects of coastal acidification on the growth and toxicity of the saxitoxin-producing dinoflagellate Alexandrium fundyense were examined in culture and ecosystem studies. In culture experiments, Alexandrium strains isolated from Northport Bay NY, USA, and the Bay of Fundy, Canada, grew significantly faster (16 -190%; p<0.05) when exposed to elevated levels of pCO2 (~ 800- 1900μatm) compared to lower levels (~390μatm). Exposure to higher levels of pCO2 also resulted in significant increases (71 – 81%) in total cellular toxicity (fg STX eq. cell−1) in the Northport Bay strain, while no changes in toxicity were detected in the Bay of Fundy strain. The positive relationship between pCO2 enhancement and elevated growth was reproducible using natural populations from Northport; Alexandrium densities were significantly and consistently enhanced when natural populations were incubated at 1500 μatm pCO2, a value at the upper range of those recorded in Northport Bay, 390 – 1500 µatm. During natural Alexandrium blooms in Northport Bay, pCO2 concentrations increased over the course of a bloom to more than 1700μatm and were highest in regions with the greatest Alexandrium abundances, suggesting Alexandrium may be further exacerbating acidification or be especially adapted to these extreme, acidified conditions. The co-occurrence of Alexandrium blooms and elevated pCO2 represents a previously unrecognized, compounding environmental threat to coastal ecosystems. The ability of elevated pCO2 to enhance the growth and toxicity of Alexandrium indicates that acidification promoted by eutrophication or climate change can intensify these, and perhaps other, harmful algal blooms.
Introduction
It has recently been recognized that eutrophication resulting from anthropogenic nutrient loading can contribute to the acidification of coastal systems (Borges and Gypens 2010; Cai et al. 2011; Melzner et al. 2013). While atmospheric CO2 levels are estimated to rise beyond 800 ppm by 2100 (I.P.C.C. 2007), many estuaries are already experiencing CO2 levels exceeding these projected climate change scenarios (Talmage and Gobler 2009; Cai et al. 2011; Hofmann et al. 2011; Barton et al. 2012; Melzner et al. 2013). These high CO2 and low pH conditions can change nitrification rates (Beman et al. 2011; Fulweiler et al. 2011), hydrolytic enzyme activity (Yamada and Suzumura 2010; Maas et al. 2013), and alter trace metal chemistry (Millero et al. 2009; Hoffmann et al. 2012) all of which can alter nutrient cycles and in turn affect algal communities. Given the important role that marine phytoplankton play in food webs and carbon cycling, further research on the effects of ocean acidification on phytoplankton is needed.
During the past decade there have been multiple studies investigating the effects of ocean acidification (increased pCO2 and decreased pH) on individual phytoplankton species as well as the composition of natural phytoplankton communities (Riebesell et al. 2000; Lefebvre et al. 2012; Nielsen et al. 2012 and references therein). One group of phytoplankton that may be strongly affected by acidification is harmful algae. Among Pseudo-nitzschia spp., increasing pCO2 concentrations can increase cellular growth rates and concentrations of its toxin, domoic acid (Sun et al. 2011; Tatters et al. 2012). Other marine HABs, such as Karlodinium veneficum and Heterosigma akashiwo have displayed significantly faster growth rates under elevated levels of pCO2 (Fu et al. 2008; Fu et al. 2010). Contrastingly, using acid additions to manipulate pH, other studies have reported that multiple coastal phytoplankton strains (including P. minimum and K. veneficum) are unaffected by large changes in pH (7.0 to 8.4; Berge et al. 2010). Clearly, more research on the effects of CO2 on HAB taxa is needed given the wide range of effects that has already been observed for this group.
One group of harmful algae that seems particularly sensitive to elevated pCO2 concentrations is that comprised of the saxitoxin-producing dinoflagellate species in the genus Alexandrium (Flores-Moya et al. 2012; Fu et al. 2012; Kremp et al. 2012; Tatters et al. 2013a; Van De Waal et al. 2014). Alexandrium species from Europe (A. minutum, Flores-Moya et al., 2012; A. ostenfeldii, Kremp et al., 2012) and the west coast of North America (A. catenella; Fu et al., 2012, Tatters et al., 2013) have displayed strain-specific increases in growth and/or toxicity when exposed to elevated pCO2. While A. fundyense strains from the east coast of North America have caused paralytic shellfish poisoning (PSP) for more than fifty years (Martin and Richard 1996), the responses of this species to elevated pCO2 are poorly known. Given that dinoflagellates possess form II RubisCO, which has a low affinity for CO2 (Morse et al. 1995; Rost et al. 2006; Reinfelder 2011) and is the key enzyme facilitating CO2 fixation, Alexandrium and other dinoflagellates may flourish within a high CO2 environment (Fu et al. 2012). Furthermore, high pCO2 (low pH) environments may change cellular toxin levels of Alexandrium by altering biosynthesis rates (Fu et al. 2012) and/or causing pH-induced toxin conversions (Laycock et al. 1995). Hence, it is important to assess the effects of elevated CO2 on the growth and toxicity of North American strains of Alexandrium given that many coastal systems within this region are currently experiencing levels of elevated pCO2 (Talmage and Gobler 2009) as a result of cultural eutrophication (Nixon 1995; Heisler et al. 2008).
Here we report on the effects of elevated CO2 on the growth and toxicity of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. We present a series of culture experiments using two strains of Alexandrium (from NY, USA, and the Bay of Fundy, Canada) with differing toxin profiles to assess the effects of pCO2 on the growth and toxicity of Alexandrium. In addition, we examined the temporal and spatial dynamics of Alexandrium densities, water chemistry, plankton communities, and pCO2 concentrations in a coastal system. Finally, natural phytoplankton communities were artificially subjected to varying levels of pCO2 to assess changes in Alexandrium densities and toxicity as well as the total phytoplankton community during bloom events.
Methods
Culture experiments
Culture experiments were performed to assess the effects of different CO2 levels on Alexandrium growth and toxicity (toxin content, toxin profiles, and cellular toxicity). Experiments were performed using two Alexandrium strains (clone NPB8 isolated from Northport Bay, NY, USA and clone CCMP 2304 isolated from the Bay of Fundy, Canada) with differing toxin profiles (Maranda et al. 1985; Anderson et al. 1990; Anderson et al. 1994), affording a comparison of changes in toxin composition due to changes in pCO2. Stock cultures were maintained at 20°C using f/2 (-Si) media (Guillard and Ryther 1962) made from filtered coastal Atlantic Ocean water (40.7969°N, 72.4606°W; salinity = 32-33) supplemented with 2% antibiotic solution (stock solution, Thermo Scientific HyClone Penicillin (10,000 U ml−1) Streptomycin (10,000 μg ml−1) in 0.85% NaCl) under 100 µmol quanta m−2 s−1.
Experiments were designed to assess how current, eutrophication-induced coastal acidification may affect the development of Alexandrium blooms. To assess the effects of CO2 on Alexandrium growth and toxicity, cultures were subjected to a control level of pCO2 (390μatm; Mauna Loa Observatory by the Earth Systems Research Laboratory NOAA) as well as elevated levels observed in local coastal systems with Alexandrium blooms (800- 1900 μatm; this study) using a gas proportionator system (Cole Parmer® Flowmeter system, multitube frame) that mixed ambient air with 5% CO2 gas at a net flow rate of 300 ± 5 mL min−1 (Talmage and Gobler 2009). Experiments with each strain were repeated 3 - 4 times over the course of two years; within each experiment, treatments were run in triplicate or quadruplicate and incubated at 20°C under 100 µmol quanta m−2 s−1. A subset of these experiments, n = 2 for each strain, were analyzed for toxin profile and content, and then were values converted to cellular toxicity.
Experimental cultures were grown semi-continuously (Feng et al. 2008) being diluted to 400 cells mL−1 every three days to maintain cells in exponential growth phase and to minimize pH fluctuations associated with the photosynthetic consumption of CO2. Stock media (f/2 -Si) with 2% antibiotic solution was bubbled at the proper CO2 level to ensure that, upon diluting cultures to starting densities for each time point, cells were inoculated into media set to the proper CO2 and pH level. For each continuous culture transfer, culture aliquots were preserved in Lugol’s iodine and quantified using a Multisizer 3 Coulter Counter (Beckman Coulter, USA) to determine the dilution needed for each experimental flask. Enumeration of cells via the Multisizer and a microscope differed by ≤5% and each method yielded a relative standard deviation of 5-10%. Cellular growth rates were calculated at each time point. Aliquots of culture were pelletized using centrifugation, 1500 xg for 11 minutes, and the supernatant aspirated without disturbing the pellet in preparation for extraction and HPLC-FLD analysis (high performance liquid chromatography coupled with fluorescence detection).
Experiments were performed to match the duration of bloom events and thus lasted two to four weeks (Anderson 1997; Hattenrath et al. 2010; Hattenrath-Lehmann and Gobler 2011). Measurements of pH within cultures (Table 1) were made throughout each experiment using an Orion 3-star Plus electrode (± 0.001) calibrated prior to each use using NBS traceable standards. Measurements using this pH meter were highly similar to and never significantly different from scale corrected (Dickson 1993) spectrophotometric pH measurements made using m-cresol purple as described by Dickson et al. (2007). Total dissolved inorganic carbon (DIC) concentrations in cultures were measured using an EGM-4 Environmental Gas Analyzer (PP Systems) system that quantifies DIC levels after separating the gas phase from seawater via acidification and using a Liqui-Cel Membrane (Membrana; Talmage and Gobler 2009). This instrument provided a methodological precision better than ± 5% for replicated measurements of total dissolved inorganic carbon. The levels of DIC and pH within Dr. Andrew Dickson’s (University of California San Diego, Scripps Institution of Oceanography) certified reference material (Batch 102 and 123) were measured during every analytical run as a quality assurance measure; analysis of samples proceeded only after complete recovery of those standards was obtained. pCO2 levels were calculated using measured levels of DIC, pH (NBS scale), temperature, and salinity, as well as the first and second dissociation constants of carbonic acid in seawater according to Roy et al. (1993) using the program CO2SYS (http://cdiac.ornl.gov/ftp/co2sys/).
Table 1.
pH, dissolved inorganic carbon (DIC, μmol L−1), calculated alkalinity (TA), calculated pCO2 (μatm), and length of two- level CO2 culture experiments (days).
| Northport Bay, NPB8 | Bay of Fundy, CCMP2304 | |||
|---|---|---|---|---|
| Parameter | Ambient | High CO2 | Ambient | High CO2 |
| Experiment #1 | Experiment #5 | |||
| pH | 8.084 (0.002) | 7.657 (0.002) | 8.123 (0.005) | 7.842 (0.004) |
| pCO2 (μatm) | 417 (8) | 1191 (22) | 436 (4) | 895 (5) |
| Total DIC (μmol L−1) | 1643 (33) | 1693 (22) | 1893 (5) | 1969 (9) |
| Alkalinity (TA) | 1869 (35) | 1773 (22) | 2143 (8) | 2105 (10) |
| Length of experiment (days) | 15 | 15 | 24 | 24 |
| Experiment #2 | Experiment #6 | |||
| pH | 8.062 (0.007) | 7.744 (0.01) | 8.054 (0.007) | 7.582 (0.005) |
| pCO2 (μatm) | 440 (40) | 1132 (41) | 436 (5) | 1617 (14) |
| Total DIC (μmol L−1) | 1639 (122) | 1972 (34) | 1597 (13) | 1941 (5) |
| Alkalinity (TA) | 1855 (127) | 2082 (33) | 1796 (16) | 1994 (7) |
| Length of experiment (days) | 27 | 27 | 12 | 12 |
| Experiment #3 | Experiment #7 | |||
| pH | 8.026 (0.001) | 7.516 (0.006) | 8.009 (0.001) | 7.495 (0.011) |
| pCO2 (μatm) | 441 (13) | 1679 (3) | 496 (5) | 1922 (20) |
| Total DIC (μmol L−1) | 1509 (49) | 1731 (25) | 1629 (11) | 1888 (26) |
| Alkalinity (TA) | 1692 (53) | 1768 (27) | 1811 (11) | 1917 (29) |
| Length of experiment (days) | 15 | 15 | 12 | 12 |
| Experiment #4 | ||||
| pH | 8.046 (0.001) | 7.589 (0.006) | ||
| pCO2 (μatm) | 483 (8) | 1536 | ||
| Total DIC (μmol L−1) | 1734 (26) | 1890 | ||
| Alkalinity (TA) | 1937 (27) | 1946 | ||
| Length of experiment (days) | 12 | 12 | ||
Values represent means and (SD).
Toxin analysis
Cell pellets in pre-weighed tubes were resuspended in 500 µL or 1,000 µL of 0.05M acetic acid, weighed, and freeze-thawed three times to aid in cell rupture. Cell suspensions were then sonified (Branson, Model S-250D), on ice, using a microtip at 40% for one minute. Samples were centrifuged at 3,000 g for five minutes at room temperature and supernatants were passed through an Oasis HLB solid phase cartridge (Waters, 3cc, 60mg) to remove interfering compounds after the cartridge was equilibrated with 3 mL of methanol and 3 mL of Milli-Q water, following the manufacturer’s instructions. The eluate was transferred to a filter unit (Amicon Ultra 0.5 10,000 MW, regenerated cellulose) and centrifuged for 15 minutes at 12,000 g. Samples were stored frozen at -20 °C prior to HPLC-FLD analysis at which time the extracts were thawed, mixed and analyzed by HPLC for saxitoxins using the three-step isocratic elution method of Oshima (1995) with post-column derivatization, as modified in Anderson et al. (1994). Twelve congeners were quantified against reference standards (National Research Council, Canada): saxitoxin; neosaxitoxin; decarbamoyl saxitoxin; gonyautoxins 1, 2, 3, 4, 5 (or B1); decarbamoyl gonyautoxins 2, 3; toxins C1 and C2. Toxicities (in fg STX equivalent cell−1) were calculated from molar composition data using congener-specific conversion factors (mouse units/µmol toxin) published in Oshima (1995) and epimer pairs were then pooled. In several instances, non-detects were reported as DL/2 (i.e. half the method detection limit) instead of “0” to avoid artificial changes to toxin profiles where the lack of a congener’s presence was due to lower detection limits. To qualify for this adjustment, data met the following criteria: 1) ≥ half of the replicates showed the congener present, and 2) the congener was present in other experiments and/or pellets of a high density culture of that same strain. Differences in growth rates and toxin levels among treatments within experiments were elucidated by means of a one-way ANOVA, using Sigma Stat software embedded within Sigma Plot 11.0. Data not meeting the assumptions of normality were log transformed.
Field study
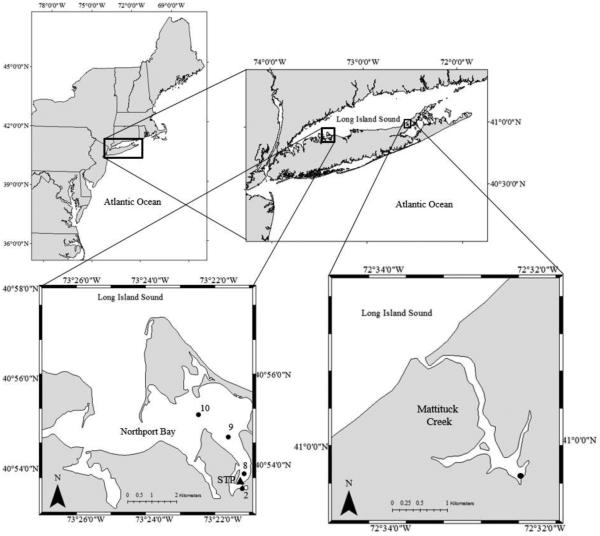
Field samples were collected on a weekly basis from March through June during 2011 and 2012. Samples were collected from a site in Northport Harbor, NY, USA (40.8916°N, 73.3572°W; site 2, Fig. 1; Hattenrath et al., 2010), which is a shallow (2 - 4m), well mixed, eutrophic system within the southeastern portion of the Northport-Huntington Bay complex, located on the southern shore of Long Island Sound. Additionally, in 2012 a cruise was conducted across multiple sites (Fig.1) to assess the spatial extent of these blooms. Further samples were collected from Mattituck Creek, NY, USA (40.9942°N, 72.5381°W), a tributary 50 km east of Northport Bay that also tidally exchanges with Long Island Sound and experiences annual Alexandrium blooms.
Figure 1.
Northeast US and the Long Island embayments Northport Bay and Mattituck Inlet. Black circles indicate sampling sites.
At each site, concentrated water samples were made by sieving 2L of water through a 200 μm mesh (to eliminate large zooplankton) and then onto a 20 μm sieve that was backwashed into a 15mL centrifuge tube. Alexandrium fundyense densities were enumerated using a highly sensitive molecular probe procedure described by Anderson et al. (2005). Briefly, aliquots of phytoplankton concentrates (formalin and then methanol preserved) were hybridized with an oligonucleotide probe specific for the NA1 North American (Group I) ribotype Alexandrium fundyense/catenella/tamarense with Cy3 dye conjugated to the 5’ terminus (5’-/5Cy3/AGT GCA ACA CTC CCA CCA-3’). Cells were enumerated using a Nikon epifluorescence microscope with a Cy3™ filter set (Anderson et al. 2005).
Samples for chlorophyll a and bacterial enumeration were collected from Northport Harbor (Fig.1). For the determination of chlorophyll a, water was filtered in triplicate using glass fiber filters (GF/F; nominal pore size 0.7μm) and measured using standard fluorometric techniques described in Welschmeyer (1994). Whole water samples were preserved in 10% buffered formalin (0.5% v/v final), stored at -80°C, and analyzed flow cytometrically to quantify the abundance of heterotrophic bacteria. Samples were stained with SYBR Green I and heterotrophic bacteria were quantified using a FACScan (BD®) flow cytometer (Jochem 2001).
To quantify the pCO2 concentrations present during Alexandrium blooms, two types of in situ measurements were made in Northport Bay. In 2011, pCO2 levels were measured during the Alexandrium bloom in Northport Harbor via the stationary deployment of a HydroC™/CO2 probe (Contros, Kiel, Germany) that makes in situ measurements every 5 seconds using infrared technology. This instrument has been shown to provide measurements of CO2 in multiple coastal systems consistent with levels determined from discrete measurements of dissolved inorganic carbon and pH using standard methods (Act 2010; Fiedler et al. 2012; Baumann et al. submitted). To groundtruth measurements made by the HydroC™/CO2 probe during this study, total dissolved inorganic carbon (DIC) samples were collected from the same depth in the water column that the probe was deployed (0.5 m) using a Van Dorn bottle. Water was transferred without bubbling to a 300 mL borosilicate bottle and preserved using a saturated mercuric chloride solution added as 0.03% of the sample volume and kept at 4°C until analysis of pH and DIC and determination of carbonate chemistry as described above for laboratory experiments.
The spatial distribution of pCO2, chlorophyll a, and salinity during Alexandrium blooms was assessed in May 2012 during a horizontal transect cruise through Northport Bay (Fig. 1). The HydroC™/CO2 probe and a YSI 6920v2 sonde (YSI Inc., Yellow Springs, OH) equipped with salinity and chlorophyll a fluorescence sensors were affixed to a bracket mounted on the side (towards the stern) at a depth of 0.5m on a small vessel that proceeded below wake speed (~1 m s−1) to minimize turbulent mixing around sensors. Prior to the cruise, the time signatures of the HydroC™/CO2 probe and the YSI sonde were aligned with a GeoChron Blue GPS device (SparkFun™ Electronics, Boulder, CO) to link measurements in space and time. Maps of these measured parameters were generated using ARC GIS 10 (Esri, Redlands, CA).
Incubations of natural populations
To assess how short term changes in CO2 levels that occurred during this study may affect the growth and toxin production of Alexandrium fundyense as well as competing phytoplankton, Northport Bay water was subjected to three levels of CO2 (~390, ~750, and ~1500 μatm; 13 and 22 May 2011) under controlled laboratory conditions. An additional experiment was conducted on 27 May 2011 using water from Mattituck Creek, NY, USA, (Fig 1). To reduce algal biomass levels and thus permit better control of carbonate chemistry and further algal growth, triplicate 2.5L bottles were filled with 1.25L whole seawater and 1.25L of 0.2 μm filtered seawater made via gravity filtration with a sterile, 0.2 µm capsule filter (Pall© Port Washington, NY). Bottles were amended with f/80 nutrients (with a N:Si ratio of 1:1) and incubated in front of a bank of fluorescent lights (100 µmol quanta m−2 s−1) at the temperature of the bloom water (~16°C) for 4-6 days at the Stony Brook Southampton Marine Science Center. A gas proportionator system was used to deliver ambient air (390 μatm) and premixed CO2 gas (750, 1500 μatm; Praxair) to seawater treatments at a net flow rate of 300 ± 5 mL min−1 which was continuously delivered to the bottom of the experimental bottles using airstones (Table 2; Rose et al. 2009). This delivery rate turned over the volume of experimental bottles >100 times daily, ensuring that desired CO2 concentrations and pH levels were maintained (Talmage and Gobler 2009). Multiple pH measurements were made throughout the experiment using both Oakton® (± 0.01) and Orion 3-star plus (± 0.001) electrodes calibrated prior to each use using NBS traceable standards (Table 2). pH measurements made via the Orion and Oakton® probes were highly correlated to each other (r2= 0.99) and highly similar to and not significantly different from scale corrected spectrophotometric pH measurements (Dickson 1993; Dickson et al. 2007).
Table 2.
pH, dissolved inorganic carbon (DIC, μmol L−1), calculated alkalinity (TA), calculated pCO2 (μatm) and length of incubation (days) during field experiments conducted in the spring of 2011.
| Parameter | ~390 μatm | ~750 μatm | ~1500 μatm |
|---|---|---|---|
| 13-May | |||
| pH | 8.22 (0.01) | 8.06 (0.20) | 7.72 (0.04) |
| pCO2 (μatm) | 348 (10) | 543 (226) | 1199 (44) |
| Total DIC (μmol L−1) | 1873 (46) | 1846 (65) | 1939 (126) |
| Alkalinity (TA) | 2056 (49) | 1972 (145) | 1961 (137) |
| length of incubation (days) | 4 | 4 | 4 |
| 22-May | |||
| pH | 8.52 (0.60) | 7.88 (0.04) | 7.64 (0.01) |
| pCO2 (μatm) | 235 (193) | 916 (113) | 1409 (19) |
| Total DIC (μmol L−1) | 1496 (210) | 2131 (81) | 1888 (14) |
| Alkalinity (TA) | 1851 (161) | 2196 (71) | 1888 (13) |
| length of incubation (days) | 6 | 6 | 6 |
| 27-May | |||
| pH | 8.03 (0.01) | 7.81 (0.02) | 7.58 (0.01) |
| pCO2 (μatm) | 438 (27) | 767 (81) | 1439 (108) |
| Total DIC (μmol L−1) | 1454 (74) | 1547 (92) | 1714 (108) |
| Alkalinity (TA) | 1544 (75) | 1588 (88) | 1704 (106) |
| length of incubation (days) | 4 | 4 | 4 |
Values represent means and (SD).
Table 3.
Cellular toxicity of saxitoxin derivatives (fg STX eq. cell−1) from culture experiments conducted with the Northport Bay (NPB8) Alexandrium strain.
| Saxitoxin derivatives fg STX eq cell −1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1, C2 | Neo | dcSTX | STX | GTX1, 4 | GTX5 | dcGTX2, 3 | GTX2, 3 | Total | |
| Experiment #2 | |||||||||
| 390μatm | 519 (90) | 165 (136) | n.d. | 112 (25) | 343 (6) | 141 (73) | n.d. | 133 (13) | 1253 (21) |
| 1200μatm | 626 (67) | 286 (101) | n.d. | 161 (83) | 706 (90)* | 197 (29) | n.d. | 165 (34) | 2141 (383)* |
| Experiment #4 | |||||||||
| 390μatm | 188 (47) | 76 (45) | n.d. | 34 (10) | 334 (84) | 45 (11) | 5 (1) | 84 (20) | 765 (213) |
| 1500μatm | 355 (205) | 228 (139) | n.d. | 47 (36) | 750 (252)* | 76 (44) | 8 (6) | 108 (56) | 1383 (395)* |
Values represent the mean (SD) of triplicate or quadruplicate measurements. Asterisks indicate significant differences (p<0.05) between treatments and the control (~390μatm).
Upon termination of the experiment, A. fundyense cells were enumerated and cell pellets from 1L of water were collected, extracted and the toxin content quantified via HPLC-FLD, as described above. Size fractionated chlorophyll a (GF/F and 20μm polycarbonate filters, see Field study) and Lugol’s iodine samples were preserved and analyzed to assess changes in the plankton community. Plankton cells larger than 10 µm were identified to at least genus level and grouped as dinoflagellates and diatoms using a 1mL Sedgewick-Rafter slide under a compound microscope. Differences among treatments were assessed using a One-Way ANOVA using Sigma Stat software embedded within Sigma Plot 11.0.
Results
Culture experiments
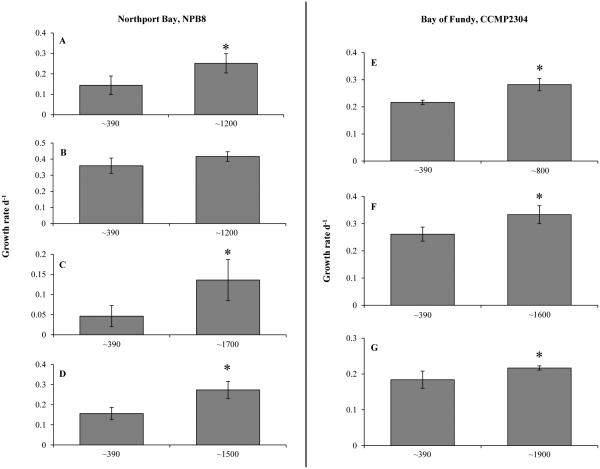
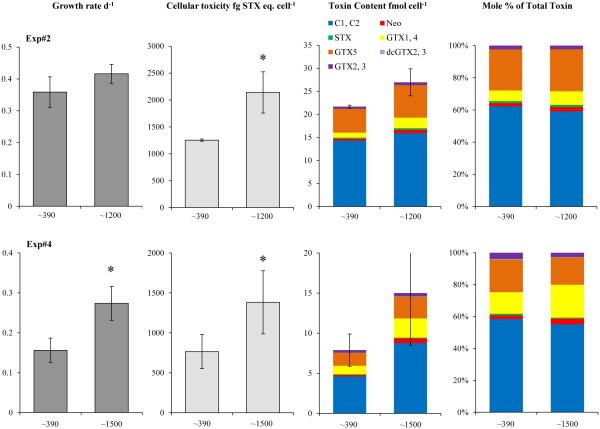
Carbon dioxide concentrations altered the growth and toxicity of the Northport Bay and Bay of Fundy strains of Alexandrium fundyense. Both Alexandrium strains, isolated from Northport Bay (NPB8) and Bay of Fundy (CCMP2304), had significantly higher growth rates (16 -190%) when exposed to elevated levels of pCO2 (~ 800- 1900μatm, Table 1) compared to the control (~390μatm; Fig. 2). These growth rate enhancements were statistically significant (p<0.05) in six of the seven experiments performed with one of four experiments with NPB8 being the single exception (Fig. 2). In addition, the total cellular toxicity (fg STX eq. cell−1) of the Northport Bay strain was significantly higher (71-81%) in cultures exposed to elevated pCO2 compared to the control (p<0.05; Exp. #2, 4; Fig. 3). This increase in the overall cellular toxicity in the higher pCO2 treatment was largely driven by the enhanced production of a more toxic derivative, GTX1,4, as reflected by this derivative’s increased toxin content and greater percent molar composition (Fig. 3). The toxin content of all other derivatives increased under elevated pCO2 as well; however, the high potency of GTX 4,1 relative to other derivatives (TEF values reported in Oshima 1995) and the switch in the profile to include relatively more of this derivative, ultimately led to a significantly more toxic Northport Bay strain. In contrast, the total cellular toxicity of the Bay of Fundy strain was not consistently or significantly altered by pCO2, with elevated pCO2 levels resulting in both small increases and decreases in the toxin content and molar composition of each derivative within the two experiments (Fig. 4; Table 4).
Figure 2.
Growth rates (d−1) of two Alexandrium strains (Northport Bay, NPB8 and Bay of Fundy, CCMP2304) under two levels of CO2 (as in Table 1). Bars are means while error bars represent the SD of triplicate or quadruplicate measurements. Panels A-G represents experiments 1-7, respectively.
Figure 3.
Growth rates (d−1), cellular toxicity (fg STX eq. cell−1), toxin content (fmol cell−1) and percent molar toxin composition of the Northport Bay (NPB8) Alexandrium isolate under two levels of CO2 (as in Table 1). Bars are means while error bars represent the SD of triplicate or quadruplicate measurements.
Figure 4.
Growth rates (d−1), cellular toxicity (fg STX eq. cell−1), toxin content (fmol cell−1) and percent molar toxin composition of the Bay of Fundy (CCMP2304) Alexandrium isolate under two levels of CO2 (as in Table 1). Bars are means while error bars represent the SD of triplicate or quadruplicate measurements.
Table 4.
Cellular toxicity of saxitoxin derivatives (fg STX eq. cell−1) from culture experiments conducted with the Bay of Fundy (CCMP2304) Alexandrium strain.
| Saxitoxin derivatives fg STX eq cell−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1, C2 | Neo | dcSTX | STX | GTX1, 4 | GTX5 | dcGTX2, 3 | GTX2, 3 | Total | |
| Experiment #5 | |||||||||
| 390μatm | 275 (50) | 590 (1) | n.d. | 1646 (309) | 74 (18) | 21 (8) | 4 (2) | 447 (81) | 2861 (791) |
| 800μatm | 202 (77) | 397 (65) | n.d. | 1226 (185) | 80 (22) | 18 (5) | 5 (1) | 328 (105) | 2255 (453) |
| Experiment #6 | |||||||||
| 390μatm | 178 (47) | 842 (153) | n.d. | 1682 (306) | 729 (134) | 9 (2) | 6 (2) | 1414 (291) | 4860 (913) |
| 1600μatm | 221 (38) | 964 (254) | n.d. | 1608 (261) | 1113 (297) | 7 (1) | 8 (2) | 1599 (351) | 5521 (1190) |
Values represent the mean (SD) of triplicate or quadruplicate measurements. Asterisks indicate significant differences (p<0.05) between treatments and the control (~390μatm).
The temporal and spatial dynamics of pCO2 during Alexandrium blooms
During spring 2011, Alexandrium was detectable in the water column of Northport Bay from late March through late May, with peak densities occurring on 9 May (25,300 cells L−1) and a smaller secondary peak (6,600 cells L−1) on 16 May (Fig 5A). Total phytoplankton biomass was significantly lower during the Alexandrium bloom (3- 24 May; 3.3 ± 0.9 μg chlorophyll a L−1) compared to before (28 March –29 April) and after (1- 6 June) the bloom (11.5 ± 2.1 μg chlorophyll a L−1; Fig. 5A; p<0.01, Mann-Whitney Rank Sum test). Heterotrophic bacterial abundances were higher (6.8 ± 0.9 × 106 cells mL−1) during the bloom compared to before and after (4.4 ± 1.0 × 106 cells mL−1) but not significantly so (t-test, p>0.05; Fig. 5B). During the Alexandrium bloom, autonomously recorded pCO2 concentrations displayed daily fluctuations but gradually increased from 235μatm (7 May) to 1799μatm (21 May; Fig. 5B). The first peak of the Alexandrium bloom coincided with lower pCO2 levels (9 May; 350 – 560μatm), while the secondary peak (16 May) occurred during elevated pCO2 levels (590 – 1000μatm; Fig. 5A,B). The levels of pCO2 measured by the probe were slightly lower (3 - 22%) than levels measured via the discrete DIC samples, but concentrations measured using both of these methodologies were highly correlated (R=0.96; p=0.10). Finally, pCO2 levels determined within discrete samples were inversely correlated with chlorophyll a concentrations (R= -0.77; p=0.15).
Figure 5.
Northport Harbor, NY, USA, 2011: A) Log Alexandrium densities (cells L−1) and total chlorophyll a (μg L−1). B) pCO2 (μatm) as measured by a HydroC™/CO2 (Contros) probe and from discrete dissolved inorganic carbon (DIC) and pH measurements, and heterotrophic bacteria (cells mL−1 × 106).
During spring 2012, Alexandrium was found in Northport Bay from mid-March to late May with peak densities reaching 23,000 cells L−1 on 7 and 15 of May (Fig. 6A). Heterotrophic bacterial abundances (peak= 5.6 × 106 cells mL−1) gradually increased over the course of, and peaked in unison with, the Alexandrium bloom (Fig. 6B). pCO2 concentrations (as measured from discrete DIC samples) measured before and during the peak of the Alexandrium bloom were elevated and ranged from 896 to 1260 μatm (Fig. 6B). Similar to 2011, phytoplankton biomass was lower during the peak of the Alexandrium bloom (30 April- 16 May; 4.3 ± 0.3 μg chlorophyll a L−1) compared to before (15 March –24 April) and after (21- 29 May) the bloom (9.7 ± 1.9 μg chlorophyll a L−1; Fig. 6A).
Figure 6.
A) Log Alexandrium densities (cells L−1) and total chlorophyll a (μg L−1). B) pCO2 (μatm) as determined from discrete dissolved inorganic carbon (DIC) and pH samples and heterotrophic bacteria (cells mL−1 × 106) for Northport Harbor, NY, USA during 2012.
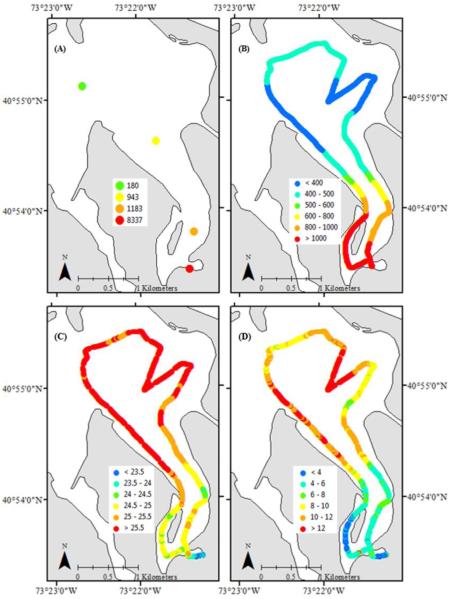
During the peak of the Alexandrium bloom (16 May 2012), a cruise was conducted to assess the spatial distribution of Alexandrium densities, pCO2 concentrations, salinity, and chlorophyll a concentrations across Northport Bay (Fig. 7). Alexandrium densities ranged from 180 – 8,300 cells L−1 with the highest densities occurring in Northport Harbor (site 2) and gradually decreasing towards Northport Bay (site 10; Fig. 7A). A transect from Northport Harbor into Northport Bay (and back) measured pCO2 concentrations from 360 – 1230μatm with the highest levels (>1,000μatm) of pCO2 confined to the Northport Harbor region and lower levels towards the Bay (<500μatm; Fig. 7B). In contrast, salinity was lower in the Harbor region (~24) and increased (25.7) towards the Bay (Fig. 7C). Chlorophyll a concentrations ranged from 1- 19 μg L−1 and were generally lower in the Harbor (<9 μg L−1) and higher in the Bay (Fig. 7D). Across the region, pCO2 levels were inversely correlated with salinity (R=-0.85, p<0.001) and chlorophyll a concentrations (R= -0.83, p<0.001) while chlorophyll a was positively correlated with salinity (R=0.86, p<0.001). Similarly, Alexandrium densities were highly correlated with pCO2 levels (R=1.00, p=0.08).
Figure 7.
Maps of A) Alexandrium densities (cells L−1), B) pCO2 (μatm) as measured by a HydroC™/CO2 (Contros) probe, and C) salinity and D) chlorophyll a (μg L−1) as measured by a YSI 6920v2 probe, from a horizontal transect conducted in Northport Bay in May of 2012. Points in (A) represent individual samples/sites where cruise tracks in (B-D) represent multiple data points taken in close proximity via probes.
Incubations of natural populations
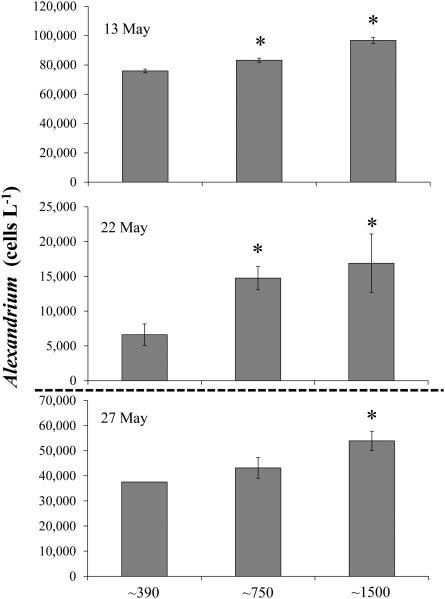
Altering levels of pCO2 caused significant alterations in the phytoplankton communities in experiments conducted during Alexandrium blooms in Northport Bay and Mattituck Creek (Fig. 8). Compared to ambient pCO2 levels, elevated pCO2 concentrations significantly enhanced Alexandrium densities (10 - 123% and 27 - 155%, for ~750 and ~1500μatm, respectively; p<0.01) during all experiments conducted except for 27 May when the increase at ~1500μatm was statistically significant but the increase at ~750μatm was not (Fig. 8). The effect of elevated pCO2 levels on the cellular toxicity of Alexandrium, however, was less consistent (Table 5). While the total toxin content and cellular toxicity increased 35% under the highest pCO2 level (1500μatm) during the first Northport Bay experiment (13 May, Experiment #5, Fig. 4), this pattern was reversed in the later experiment (22 May, Experiment #6, Fig. 4). Elevated pCO2 levels resulted in both increases and decreases in each derivative’s contribution to the total cellular toxicity (Table 5) and variations in the percent molar toxin composition due to changes in pCO2 were negligible (data not shown). Higher pCO2 levels resulted in both increases and decreases (in some cases significant; p<0.05) in different components of the phytoplankton community (diatoms, dinoflagellates, chlorophyll a size fractions; Table 6). The most significant and consistent observation was that Alexandrium densities increased with higher pCO2 concentrations.
Figure 8.
Alexandrium densities (cells L−1) at the end of field incubations during which Northport Bay (13 and 22 May) and Mattituck Creek (27 May) water was subjected to varying levels of CO2: ~390, ~750 and ~1500 μatm (Table 2). Bars are means while error bars represent the standard deviation of triplicate bottles. Dotted line represents the two different systems used for experiments.
Table 5.
Toxicity of saxitoxin derivatives (fg STX eq. cell−1) from field experiments conducted during the spring of 2011.
| Saxitoxin derivatives fg STX eq cell−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1, C2 | Neo | dcSTX | STX | GTX1, 4 | GTX5 | dcGTX2, 3 | GTX2, 3 | Total | |
| 13-May | |||||||||
| 390μatm | 1093 (280) | 349 ( 372) | n.d. | 845 (215) | 107 (73) | 587 (155) | n.d. | 243 (71) | 3072 (999) |
| 750μatm | 809 (162) | 609 (446) | n.d. | 504 (100) | 372 ( 380) | 606 (431) | n.d. | 154 (5) | 2362 (639) |
| 1500μatm | 1542 (532) | 559 (106) | n.d. | 1014 (126) | 582 (119) | 731 (84) | n.d. | 329 (24) | 4757 (852) |
| 22-May | |||||||||
| 390μatm | 1944 (815) | 243 (70) | n.d. | 690 (372) | 203 (101) | 641 (123) | n.d. | 562 (36) | 4095 (1052) |
| 750μatm | 1456 (517) | 104 (15) | n.d. | 941 (533) | 90 (24) | 513 (209) | n.d. | 712 (259) | 3786 (1470) |
| 1500μatm | 1916 (544) | 87 (28) | n.d. | 749 (195) | 178 (126) | 328 (286) | n.d. | 459 (401) | 4110 (320) |
| 27-May | |||||||||
| 390μatm | 1319 (323) | 157 (64) | n.d. | 256 (114) | 606 (425) | 287 (123) | n.d. | 363 (24) | 2815 (880) |
| 750μatm | 1635 (574) | 30 (1) | n.d. | 227 (55) | 397 (285) | 305 (62) | n.d. | 433 (89) | 3016 (921) |
| 1500μatm | 1251 (315) | 23 (3) | n.d. | 262 (42) | 524 (355) | 325 (40) | n.d. | 448 (113) | 2827 (784) |
Values represent the mean (SD). Asterisks indicate significant differences (p<0.05) between treatments and the control (~390μatm). n.d. = not detected.
Table 6.
Diatom and non-Alexandrium sp. densities (cells mL−1), and size fractionated chlorophyll a (μg L−1) from field experiments conducted during the spring of 2011.
| Total Dinoflagellates (cells mL−1) |
Total Diatoms (cells mL−1) |
Total Chlorophyll a
(μg L−1) |
<20 μm Chlorophyll a
(μg L−1) |
>20 μm Chlorophyll a
(μg L−1) |
|
|---|---|---|---|---|---|
| 13-May | |||||
| 390μatm | 33 (7) | 72390 (3649) | 69 (31) | 23 (4) | 46 (27) |
| 750μatm | 20 (3) | 43540 (7192)* | 87 (25) | 27 (4) | 60 (25) |
| 1500μatm | 30 (9) | 57477 (6791)* | 113 (3) | 31 (8) | 82 (5) |
| 22-May | |||||
| 390μatm | 29 (3) | 161800 (2050) | 67 (15) | 46 (10) | 21 (5) |
| 750μatm | 29 (6) | 110030 (16989)* | 58 (6) | 47 (3) | 11 (4)* |
| 1500μatm | 34 (3) | 168833 (5618) | 47 (4) | 38 (6) | 9 (2)* |
| 27-May | |||||
| 390μatm | 171 (13) | 2110 (786) | 5 (1) | 3 (1) | 2 (0) |
| 750μatm | 154 (26) | 2626 (669) | 11 (10) | 5 (5) | 6 (5) |
| 1500μatm | 157 (18) | 14067 (1916)* | 14 (8) | 6 (4) | 7 (5) |
Values are mean (SD). Asterisks indicate significant differences (p<0.05) between treatments and the control (~390μatm).
Discussion
This is the first study to assess the effects of acidification on the growth and toxicity of North American strains of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. The growth of two Alexandrium strains from North America, as well as field populations from two New York estuaries, were significantly enhanced by elevated pCO2. Similarly, Northport Bay cultures became significantly more toxic, producing more of the potent derivative GTX4,1, when exposed to elevated pCO2. This link between acidification and toxicity appears to be strain dependent, however, as we were unable to detect a consistent effect of pCO2 on the potency or toxin content of the Bay of Fundy culture or on field populations of Alexandrium from Northport Bay. In an ecosystem setting, the levels of pCO2 measured during blooms were within the range found to enhance Alexandrium growth experimentally, suggesting Alexandrium growth rates may be stimulated by elevated pCO2 levels in situ. These findings provide new perspective regarding the causes and impacts of HABs caused by Alexandrium and perhaps other harmful algae.
Growth of Alexandrium fundyense under varying levels of CO2
Elevated pCO2 (low pH) levels have been shown to increase the growth rates of multiple HABs. Using acid additions to manipulate pH, Hwang and Lu (2000) found that a culture of Alexandrium minutum grew maximally at a pH of 7.5. Using similar methodology, Flores-Moya et al. (2012) found that cultures of Alexandrium minutum grown at pH of 7.5 at 25°C had significantly higher growth rates compared to those at pH 8 at 20°C. Kremp et al. (2012) reported a significant enhancement in Alexandrium ostenfeldii growth rates at elevated pCO2 (750ppm) in one of eight strains examined. Recently, Tatters et al. (2013a) reported significantly higher growth rates in Alexandrium catenella when exposed to 750ppm compared to 380ppm. Similarly, the growth rate of other HABs such as Pseudo-nitzschia multiseries and Pseudo-nitzschia fraudulenta (diatoms), Karlodinium veneficum (dinoflagellate) and Heterosigma akashiwo (raphidophyte) increased significantly with elevated pCO2 (Fu et al. 2008; Fu et al. 2010; Sun et al. 2011; Tatters et al. 2012). In contrast, higher pCO2 levels had no effect on the growth rate of cultures of the dinoflagellate Prorocentrum minimum (Fu et al. 2008), and K. veneficum and P. mimimum cultured at pH 7.0 to 8.5 (achieved via acid additions) (Berge et al. 2010). Whether due to strain- or species- specific differences (Burkholder and Glibert 2009; Pitcher 2012), or potential differences in experimental methodology (acid addition v bubbling CO2), the above research suggests that increasing pCO2 affects HAB species in different ways. Regardless of methodology, of the species and strains tested thus far, species within the genus Alexandrium (A. fundyense, A. minutum, A. ostenfeldii and A. catenella) have consistently displayed enhanced growth rates when exposed to elevated levels of pCO2 (low pH; Hwang and Lu 2000; Flores-Moya et al. 2012; Kremp et al. 2012; Tatters et al. 2013a, this study) with the exception of a strain of A. tamarense (Alex 2), for which growth rates decreased by up to 25% (Van De Waal et al. 2014). While some of these prior studies were short-term experiments (weeks), Tatters et al. (2013b) recently reported that the effects of elevated CO2 on coastal phytoplankton strains observed after two weeks persisted after one year of maintenance under the same condition, suggesting these short term changes may be indicative of expected longer term alterations.
Dinoflagellates evolved ~350 million years ago when atmospheric CO2 concentrations were high (~3000ppm; Beardall and Raven 2004) and possess a low CO2 affinity form of RubisCO (form II; Morse et al. 1995; Rost et al. 2006; Reinfelder 2011). Some species possess carbon concentrating mechanisms (CCMs) including the ability to transport bicarbonate (HCO3−), and/or either extra- or intracellular carbonic anhydrase which converts HCO3− to CO2 (Reinfelder 2011; Fu et al. 2012). Among the few marine dinoflagellates that have been assessed thus far, there is a wide range of CCM capabilities. Heterocapsa oceanica and Amphidinium carterae are highly dependent on free CO2 given their limited capacity for bicarbonate uptake (Dason et al. 2004), whereas Prorocentrum minimum, Heterocapsa triquetra, and Ceratium lineatum possess HCO3− transport coupled with internal carbonic anhydrase capabilities (Rost et al. 2006). This may partly account for the invariant growth of P. minimum under a range of pCO2 levels (Fu et al. 2008; Berge et al. 2010). While there are no studies regarding CCMs in Alexandrium, the positive growth response of strains within this genus suggest that if they do possess CCMs, they are not effective enough to prevent slowed growth under current pCO2 levels.
While it has been suggested that diatoms may not benefit from increasing CO2 levels given that they possess highly efficient CCMs, and that algae such as coccolithophores and dinoflagellates with less efficient CCMs and/or low CO2 affinities may benefit from living in a high CO2 world (Reinfelder 2011), exceptions to this dogma abound (Fu et al. 2008; Berge et al. 2010; Sun et al. 2011). This taxonomic variability in response was echoed in the present study, as Alexandrium appeared to benefit from higher levels of pCO2 during field studies and incubations of natural populations; however, the responses of diatom and other dinoflagellate populations varied. These varied responses may have been due to differential CO2 requirements (use of free CO2 vs HCO3−) of individual species present during each experiment (Fu et al. 2012). Given that our(??) experiments were conducted at different time points over the duration of Alexandrium blooms, the community structure of each experiment differed along with the effects of CO2 on competing phytoplankton. Its seems likely that assessing impacts of varying CO2 on natural plankton communities will require species- or even strain-specific evaluations and should account for concurrent changes in grazing pressure as well (Rose et al. 2009).
Toxicity of Alexandrium fundyense under varying levels of CO2
Some harmful algae synthesize more toxin when exposed to elevated levels of pCO2, perhaps as a means to divert excess carbon and maintain internal elemental balance (Fu et al. 2012). Fu et al. (2010) found that increasing pCO2 increased cellular toxin production in the dinoflagellate, Karlodinium veneficum, with higher pCO2 levels increasing the production of the more potent karlotoxin form, KmTx-1, while decreasing production rates of KmTx-2. Domoic acid quotas in the diatom, Pseudo-nitzschia multiseries, were significantly higher at elevated pCO2 (730ppm) compared to the lowest pCO2 level (220ppm; Sun et al. 2011), while toxin quotas for Pseudo-nitzschia fraudulenta increased at higher pCO2 but not significantly (Tatters et al. 2012). Flores-Moya et al.’s (2012) assessment of pH effects on the toxicity of Alexandrium minutum were inconclusive, and Kremp et al. (2012) found that while total toxins in Alexandrium ostenfeldii were relatively unaffected by elevated pCO2, the STX fraction significantly increased. Tatters et al. (2013a), however, found that the total toxicity of Alexandrium catenella more than doubled when grown at 750ppm CO2 compared to 380ppm. In addition to these differences among species of Alexandrium, our observations demonstrate that the effects of pCO2 on the toxicity of Alexandrium fundyense are strain-specific, as cellular toxicity was significantly and consistently enhanced (70- 80%) at higher pCO2 levels in the Northport Bay strain while the Bay of Fundy strain displayed more variability and no consistent pattern of increased toxicity. While the most abundant toxin in the Northport Bay strain was the epimer pair C1,C2, the cellular toxicity was driven mainly by the more potent derivative, GTX1,4, which became a larger percentage of the toxin composition, increased in toxin content, and was the only derivative whose contribution to the total toxicity significantly increased (almost doubled) with increasing pCO2 (Table 3). Interestingly, Tatters et al. (2013a) also demonstrated that concentrations of GTX1,4 doubled in high pCO2 treatments, suggesting a biochemical pathway may be involved in this composition shift that is common to both A. fundyense and A. catenella. In contrast, Van De Waal et al. (2014) found that increased pCO2 levels decreased cellular PST (paralytic shellfish poisoning toxin) content and cellular toxicity in two strains (Alex 2 and 5) of Alexandrium tamarense from the North Sea. Changes in cellular toxicity for Alex2 were driven by toxin content while changes in Alex5 were driven by changes in toxin composition (i.e. a shift towards less toxic derivatives; Van De Waal et al. 2014). Given that these studies demonstrated vast differences in toxicity patterns among different species and strains of Alexandrium, more research is clearly warranted.
While the precise mechanism controlling the changes in the toxicity of HABs under varying levels of pCO2 has not been identified, there are several plausible explanations. Drawing from terrestrial systems and observed increases in secondary metabolites with higher pCO2 in plants, Fu et al. (2012) suggested that algal toxin synthesis could increase via the shunting of excess fixed carbon toward toxin synthesis. Changes in toxicity may also be related to changes in the intracellular pH of phytoplankton (Suffrian et al. 2011) which can alter toxin biosynthesis by changing enzyme activity (Yamada and Suzumura 2010; Fu et al. 2012). While changes in intracellular pH may also cause transformations of saxitoxin congeners with low pH environments converting less potent N-sulfocarbamoyl toxins to the more potent carbamate toxins, as has been demonstrated with weak acid hydrolysis (Laycock et al. 1995), this phenomenon was not observed during this study. Furthermore, while pCO2 significantly increased the total cellular toxicity of the Northport Bay strain as well as individual derivatives of both strains of Alexandrium, the differences in toxicity seen in the same derivative (STX, GTX5) between the two strains under nutrient replete conditions are more suggestive of a genetically controlled modification of toxicity rather than a chemical one (i.e. hydrolysis). In Alexandrium, where the gene pathway responsible for saxitoxin biosynthesis has been characterized, including several putatively identified genes involved in the modification of the saxitoxin parent compound (Kellmann et al. 2008; Stüken et al. 2011; Neilan et al. 2013), how acidification affects toxicity at the transcriptional or post-translational (chemical) level has rarely been evaluated (Van De Waal et al. 2014). The mechanisms controlling changes in cellular toxicity under elevated pCO2 clearly warrants further study for all toxin producing HABs.
Acidification, eutrophication and A. fundyense interactions
During this study, Alexandrium blooms were observed to occur in nearshore regions with levels of pCO2 not predicted for the open ocean until the next century (e.g. >1,000 µatm; I.P.C.C. 2007). Concentrations of pCO2 progressively increased during the course of an Alexandrium bloom and were higher in regions with the highest Alexandrium densities. Furthermore, distinct and consistent changes in the microbial and phytoplankton community were observed, with Alexandrium blooms being associated with lower chlorophyll a and increased bacterial abundances. The consistently lower chlorophyll a levels associated with the bloom may have been a consequence of allelochemical production which has been reported for Alexandrium spp. (Tillmann et al. 2009) including North American strains of A. fundyense (Hattenrath-Lehmann and Gobler 2011). Allelochemicals have been shown to inhibit or lyse co-occurring phytoplankton (Tillmann et al. 2009; Hattenrath-Lehmann and Gobler 2011) and thus may result in the release of organic matter from allelopathically affected phytoplankton, enhanced bacterial respiration, and ultimately, increased pCO2 concentrations (Agusti and Duarte 2013). In this regard, Alexandrium may indirectly influence pCO2 levels in its surrounding environment. Other HABs with allelopathic properties (Prince et al. 2008; Tang and Gobler 2010) or associated with elevated bacterial and/or organic matter levels (Gobler and Sanudo-Wilhelmy 2003; Gasol et al. 2005) may also have the potential to co-occur with elevated pCO2 concentrations. Many studies have demonstrated that variation in pCO2 is tightly coupled to temporal variation in primary and bacterial production (Frankignoulle et al. 1998; Algesten et al. 2004; Borges et al. 2008). We suggest that Alexandrium, and HABs in general, may indirectly contribute to changes in estuarine pCO2 by causing alterations in organic matter cycling and bacterial production.
Further evidence of the association of Alexandrium blooms with elevated levels of pCO2 came from the spatial survey which detected elevated Alexandrium densities and pCO2 levels in the southern region of Northport Bay along with lower chlorophyll a concentrations and salinities. This spatial distribution of Alexandrium is consistent with prior surveys of this region and have been linked to nitrogen loading from wastewater (Hattenrath et al. 2010). The lower salinities found in Northport Harbor are likely associated with intense groundwater discharge in this region (Young et al. 2013) which has the potential to be a significant source of pCO2 (Basterretxea et al. 2010). The elevated Alexandrium densities and pCO2 concentrations in the Harbor as well as the salinity gradient between the Bay and Harbor are indicative of a long residence time in the Harbor region which may create positive feedback with regard to pCO2 concentrations within the system. Low flushing rates would retain nutrients (from point and non-point sources) and phytoplankton which would initially stimulate primary production and subsequently lower pCO2 concentrations. However, without a removal mechanism (i.e. flushing) coupled with the constant input of nutrients wastewater, stagnant algal productivity would ultimately increase the organic loads to sediments and increase bacterial respiration, all of which would enhance pCO2 levels in the Harbor and overall make Northport Harbor a net heterotrophic system (Frankignoulle et al. 1998; Algesten et al. 2004; Borges et al. 2008). Our experimental results demonstrate that these higher pCO2 concentrations can promote the growth and toxicity of Alexandrium in this system.
A vast body of research has documented the potential for ocean acidification to negatively impact an array of ocean organisms (Doney et al. 2009; Baumann et al. 2012; Gazeau et al. 2013). While HABs are also known for their negative effects on marine life, only one study has assessed the impacts of acidification and HABs, reporting that the alga Aureococcus anophagefferens acted synergistically with acidification to cause near complete mortality in bivalve larvae (Talmage and Gobler 2012). Given the co-occurrence of HABs and acidification reported here, and the likely co-occurrence in other coastal systems, a comprehensive assessment of the effects of concurrent acidification and HABs such as Alexandrium on marine animals is needed to more fully understand their ecosystem impacts.
Anthropogenic nutrient loading and coastal acidification are processes associated with cultural eutrophication (Nixon 1995; Borges and Gypens 2010; Cai et al. 2011) and are factors that promote many HABs around the world (Anderson et al. 2008; Heisler et al. 2008; Hallegraeff 2010). While HABs may directly or indirectly exacerbate eutrophication-enhanced acidification, acidification can in turn increase the growth and toxicity of HABs. Given the large scale ecosystem effects that these interactions could have, this is certainly an area of study that warrants further investigation, especially in coastal regions where acidification occurs seasonally (Cai et al. 2011) and is intensified at estuarine salinities (Hu and Cai 2013; Melzner et al. 2013) where HABs are often a recurrent problem.
Acknowledgements
We extend our sincere gratitude and appreciation to Dr. Stephanie C. Talmage for logistical assistance and advice. We thank John Carroll for field assistance and Dr. Hans Dam for generously providing culture NPB8. We owe a great debt of gratitude to Peter Houmere for the generous use of his facility during this study. Funding for C. Gobler and co-workers was provided by New York Sea Grant (R/CMB-38-NYCT) and NOAA’s Monitoring and Event Response to Harmful Algal Blooms (MERHAB) program (NA11NOS4780027). Funding for D. Anderson and J. Smith was provided through the Woods Hole Center for Oceans and Human Health, National Science Foundation (NSF) Grants OCE- 1128041 and OCE-1314642; and National Institute of Environmental Health Sciences (NIEHS) Grant 1-P50-ES021923-01. This is MERHAB contribution number xxxxxxxx.
References
- Act . Performance demonstration statement of the Contros HydroC™ CO2 analyzer. Alliance for Coastal Technologies, University of Maryland Chesapeake Biological Laboratory; 2010. p. 26. UMCES Technical Report Series Ref. No.[UMCES] CBL 10-091. [Google Scholar]
- Agusti S, Duarte CM. Phytoplankton lysis predicts dissolved organic carbon release in marine plankton communities. Biogeosciences. 2013;10:1259–1264. [Google Scholar]
- Algesten G, Wikner J, Sobek S, Tranvik LJ, Jansson M. Seasonal variation of CO2 saturation in the Gulf of Bothnia: Indications of marine net heterotrophy. Glob. Biogeochem. Cycle. 2004;18:7. [Google Scholar]
- Anderson D, Kulis D, Doucette G, Gallagher J, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeastern United States and Canada. Mar. Biol. 1994;120:467–478. [Google Scholar]
- Anderson D, Kulis D, Sullivan J, Hall S. Toxin composition variations in one isolate of the dinoflagellate Alexandrium fundyense. Toxicon. 1990;28:885–893. doi: 10.1016/0041-0101(90)90018-3. [DOI] [PubMed] [Google Scholar]
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeastern US. Limnol. Oceanogr. 1997;42:1009–1022. [Google Scholar]
- Anderson DM. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae. 2008;8:39–53. doi: 10.1016/j.hal.2008.08.017. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep-Sea Research Part Ii-Topical Studies in Oceanography. 2005;52:2467–2490. [Google Scholar]
- Barton A, Hales B, Waldbusser GG, Langdon C, Feely RA. The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: Implications for near-term ocean acidification effects. Limnol. Oceanogr. 2012;57:698–710. [Google Scholar]
- Basterretxea G. Submarine Groundwater Discharge to the Coastal Environment of a Mediterranean Island (Majorca, Spain): Ecosystem and Biogeochemical Significance. Ecosystems. 2010;13:629–643. others. [Google Scholar]
- Baumann H, Talmage SC, Gobler CJ. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Chang. 2012;2:38–41. [Google Scholar]
- Baumann H, Wallace R, Tagliaferri T, Gobler CJ. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal and interannual time scales. submitted. [Google Scholar]
- Beardall J, Raven JA. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia. 2004;43:26–40. [Google Scholar]
- Beman JM. Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc. Natl. Acad. Sci. U. S. A. 2011;108:208–213. doi: 10.1073/pnas.1011053108. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge T, Daugbjerg N, Andersen BB, Hansen PJ. Effect of lowered pH on marine phytoplankton growth rates. Marine Ecology Progress Series. 2010;416:79–91. [Google Scholar]
- Borges AV, Gypens N. Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnol. Oceanogr. 2010;55:346–353. [Google Scholar]
- Borges AV, Ruddick K, Schiettecatte LS, Delille B. Net ecosystem production and carbon dioxide fluxes in the Scheldt estuarine plume. BMC Ecology. 2008;8:15. doi: 10.1186/1472-6785-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder JM, Glibert PM. The importance of intraspecific variability in harmful algae-Preface to a collection of topical papers. Harmful Algae. 2009;8:744–745. [Google Scholar]
- Cai W-J. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011;4:766–770. others. [Google Scholar]
- Dason JS, Huertas IE, Colman B. Source of inorganic carbon for photosynthesis in two marine dinoflagellates. J. Phycol. 2004;40:285–292. [Google Scholar]
- Dickson AG. pH buffers for sea-water media based on the total hydrogen-ion concentration scale. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 1993;40:107–118. [Google Scholar]
- Dickson AG, Sabine CL, Christian JR. Guide to best practices for ocean CO2 measurements. PICES Special Publication; 2007. pp. 1–191. [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean Acidification: The Other CO2 Problem. Annual Review of Marine Science. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Feng Y. Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae) Eur. J. Phycol. 2008;43:87–98. others. [Google Scholar]
- Fiedler B, Fietzek P, Vieira N, Silva P, Bittig HC, Körtzinger A. In Situ CO2 and O2 measurements on a profiling float. Journal of Atmospheric and Oceanic Technology. 2012;30:112–126. [Google Scholar]
- Flores-Moya A. Effects of adaptation, chance, and history on the evolution of the toxic dinoflagellate Alexandrium minutum under selection of increased temperature and acidification. Ecol. Evol. 2012;2:1251–1259. doi: 10.1002/ece3.198. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankignoulle M. Carbon dioxide emission from European estuaries. Science. 1998;282:434–436. doi: 10.1126/science.282.5388.434. others. [DOI] [PubMed] [Google Scholar]
- Fu FX, Place AR, Garcia NS, Hutchins DA. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microb. Ecol. 2010;59:55–65. [Google Scholar]
- Fu FX, Tatters AO, Hutchins DA. Global change and the future of harmful algal blooms in the ocean. Marine Ecology Progress Series. 2012;470:207–233. [Google Scholar]
- Fu FX, Zhang YH, Warner ME, Feng YY, Sun J, Hutchins DA. A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae. 2008;7:76–90. [Google Scholar]
- Fulweiler RW, Emery HE, Heiss EM, Berounsky VM. Assessing the Role of pH in Determining Water Column Nitrification Rates in a Coastal System. Estuaries Coasts. 2011;34:1095–1102. [Google Scholar]
- Gasol JM, Garces E, Vila M. Strong small-scale temporal bacterial changes associated with the migrations of bloom-forming dinoflagellates. Harmful Algae. 2005;4:771–781. [Google Scholar]
- Gazeau F. Impacts of ocean acidification on marine shelled molluscs. Mar. Biol. 2013;160:2207–2245. others. [Google Scholar]
- Gobler CJ, Sanudo-Wilhelmy SA. Cycling of colloidal organic carbon and nitrogen during an estuarine phytoplankton bloom. Limnol. Oceanogr. 2003;48:2314–2320. [Google Scholar]
- Guillard RR, Ryther JH. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea Cleve. Can. J. Microbiol. 1962;8:229. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Hallegraeff GM. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J. Phycol. 2010;46:220–235. [Google Scholar]
- Hattenrath-Lehmann TK, Gobler CJ. Allelopathic inhibition of competing phytoplankton by North American strains of the toxic dinoflagellate, Alexandrium fundyense: Evidence from field experiments, laboratory experiments, and bloom events. Harmful Algae. 2011;11:106–116. [Google Scholar]
- Hattenrath TK, Anderson DM, Gobler CJ. The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) estuary. Harmful Algae. 2010;9:402–412. [Google Scholar]
- Heisler J. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae. 2008;8:3–13. doi: 10.1016/j.hal.2008.08.006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LJ, Breitbarth E, Boyd PW, Hunter KA. Influence of ocean warming and acidification on trace metal biogeochemistry. Marine Ecology Progress Series. 2012;470:191–205. [Google Scholar]
- Hofmann GE. High-Frequency Dynamics of Ocean pH: A Multi-Ecosystem Comparison. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028983. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XP, Cai WJ. Estuarine acidification and minimum buffer zone-A conceptual study. Geophys. Res. Lett. 2013;40:5176–5181. [Google Scholar]
- Hwang DF, Lu YH. Influence of environmental and nutritional factors on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. Toxicon. 2000;38:1491–1503. doi: 10.1016/s0041-0101(00)00080-5. [DOI] [PubMed] [Google Scholar]
- I.P.C.C. Summary for Policymakers. Cambridge University Press; 2007. Intergovernmental Panel on Climate Change. [Google Scholar]
- Jochem FJ. Morphology and DNA content of bacterioplankton in the northern Gulf of Mexico: analysis by epifluorescence microscopy and flow cytometry. Aquat. Microb. Ecol. 2001;25:179–194. [Google Scholar]
- Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008;74:4044–4053. doi: 10.1128/AEM.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremp A. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecol. Evol. 2012;2:1195–1207. doi: 10.1002/ece3.245. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock MV, Kralovec J, Richards R. Some in vitro chemical interconverconversions of paralytic shellfish poisoning (PSP) toxins useful in the preparation of analytical standards. Journal of marine biotechnology. 1995;3:121–125. [Google Scholar]
- Lefebvre SC. Nitrogen source and pCO2 synergistically affect carbon allocation, growth and morphology of the coccolithophore Emiliania huxleyi: potential implications of ocean acidification for the carbon cycle. Glob. Change Biol. 2012;18:493–503. others. [Google Scholar]
- Maas EW. Effect of ocean acidification on bacterial abundance, activity and diversity in the Ross Sea, Antarctica. Aquat. Microb. Ecol. 2013;70:1–15. others. [Google Scholar]
- Maranda L, Anderson DM, Shimizu Y. Comparison of toxicity between populations of Gonyaulax tamarensis of eastern North American waters. Estuarine, Coastal and Shelf Science. 1985;21:401–410. [Google Scholar]
- Martin JL, Richard D. In: Shellfish toxicity from the Bay of Fundy, eastern Canada: 50 years in retrospect. Yasumoto T, Oshima Y, Fukuyo Y, editors. Intergovernmental Oceanographic Commission of UNESCO; 1996. pp. 3–6. [Google Scholar]
- Melzner F. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 2013;160:1875–1888. others. [Google Scholar]
- Millero FJ, Woosley R, Ditrolio B, Waters J. Effect of Ocean Acidification on the Speciation of Metals in Seawater. Oceanography. 2009;22:72–85. [Google Scholar]
- Morse D, Salois P, Markovic P, Hastings JW. A nuclear-encoded form-II RUBISCO in dinoflagellates. Science. 1995;268:1622–1624. doi: 10.1126/science.7777861. [DOI] [PubMed] [Google Scholar]
- Neilan BA, Pearson LA, Muenchhoff J, Moffitt MC, Dittmann E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013;15:1239–1253. doi: 10.1111/j.1462-2920.2012.02729.x. [DOI] [PubMed] [Google Scholar]
- Nielsen LT, Hallegraeff GM, Wright SW, Hansen PJ. Effects of experimental seawater acidification on an estuarine plankton community. Aquat. Microb. Ecol. 2012;65:271–285. [Google Scholar]
- Nixon SW. Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia. 1995;41:199–219. [Google Scholar]
- Oshima Y. Post-column derivatization HPLC methods for paralytic shellfish toxins. In: Hallegraeff GM, Anderson DM, Cembella AD, editors. Manual on harmful marine microalgae. IOC of UNESCO; 1995. pp. 81–95. [Google Scholar]
- Pitcher GC. Harmful algae—The requirement for species-specific information. Harmful Algae. 2012;14:1–4. [Google Scholar]
- Prince EK, Myers TL, Naar J, Kubanek J. Competing phytoplankton undermines allelopathy of a bloom-forming dinoflagellate. Proc. R. Soc. B-Biol. Sci. 2008;275:2733–2741. doi: 10.1098/rspb.2008.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfelder JR. Carbon Concentrating Mechanisms in Eukaryotic Marine Phytoplankton. In: Carlson CA, Giovannoni SJ, editors. Annual Review of Marine Science, Vol 3. Annual Review of Marine Science. Annual Reviews; 2011. pp. 291–315. [DOI] [PubMed] [Google Scholar]
- Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- Rose JM. Effects of increased pCO2 and temperature on the North Atlantic spring bloom. II. Microzooplankton abundance and grazing. Marine Ecology Progress Series. 2009;388:27–40. others. [Google Scholar]
- Rost B, Richter KU, Riebesell U, Hansen PJ. Inorganic carbon acquisition in red tide dinoflagellates. Plant Cell Environ. 2006;29:810–822. doi: 10.1111/j.1365-3040.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- Roy RN. The dissociation-constants of carbonic-acid in seawater at salinities 5 to 45 and temperatures 0C to 45C. Mar. Chem. 1993;44:249–267. others. [Google Scholar]
- Stüken A, Orr RJ, Kellmann R, Murray SA, Neilan BA, Jakobsen KS. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS One. 2011;6:e20096. doi: 10.1371/journal.pone.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffrian K, Schulz KG, Gutowska MA, Riebesell U, Bleich M. Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytol. 2011;190:595–608. doi: 10.1111/j.1469-8137.2010.03633.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Hutchins DA, Feng YY, Seubert EL, Caron DA, Fu FX. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011;56:829–840. [Google Scholar]
- Talmage SC, Gobler CJ. The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica) Limnol. Oceanogr. 2009;54:2072–2080. [Google Scholar]
- Talmage SC, Gobler CJ. Effects of CO2 and the harmful alga Aureococcus anophagefferens on growth and survival of oyster and scallop larvae. Marine Ecology Progress Series. 2012;464:121–U147. [Google Scholar]
- Tang YZ, Gobler CJ. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Marine Ecology Progress Series. 2010;406:19–31. [Google Scholar]
- Tatters AO, Flewelling LJ, Fu F, Granholm AA, Hutchins DA. High CO2 promotes the production of paralytic shellfish poisoning toxins by Alexandrium catenella from Southern California waters. Harmful Algae. 2013a;30:37–43. [Google Scholar]
- Tatters AO, Fu FX, Hutchins DA. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzshia fraudulenta. PLoS One. 2012;7:7. doi: 10.1371/journal.pone.0032116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatters AO. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Philos. Trans. R. Soc. B-Biol. Sci. 2013b;368:14. doi: 10.1098/rstb.2012.0437. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann U, Alpermann TL, Da Purificacao RC, Krock B, Cembella A. Intra-population clonal variability in allelochemical potency of the toxigenic dinoflagellate Alexandrium tamarense. Harmful Algae. 2009;8:759–769. [Google Scholar]
- Van De Waal DB, Eberlein T, John U, Wohlrab S, Rost B. Impact of elevated pCO2 on paralytic shellfish poisoning toxin content and composition in Alexandrium tamarense. Toxicon. 2014;78:58–67. doi: 10.1016/j.toxicon.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Welschmeyer NA. Fluorometric analysis of chlorophyll-a in the presence of chlorophyll-b and pheopigments. Limnol. Oceanogr. 1994;39:1985–1992. [Google Scholar]
- Yamada N, Suzumura M. Effects of seawater acidification on hydrolytic enzyme activities. J. Oceanogr. 2010;66:233–241. [Google Scholar]
- Young C, Kroeger KD, Hanson G. Limited denitrification in glacial deposit aquifers having thick unsaturated zones (Long Island, USA) Hydrogeol. J. 2013;21:1773–1786. [Google Scholar]