Abstract
Objectives
To understand the gradient between rectal (RT) and brain (BT) temperature in children after severe traumatic brain injury (TBI). We hypothesized that the BT-RT gradient will be influenced by the child’s body surface area (BSA), and that this relationship will persist over physiological temperature ranges.
Design
Retrospective review of a prospectively-collected Pediatric Neurotrauma Registry.
Setting
Academic, University-based Pediatric Neurotrauma program.
Patients
Consecutive children (n = 40) with severe TBI (Glasgow Coma Scale [GCS] < 8) who underwent BT monitoring (7/03 – 12/08) were studied after informed consent was obtained. A subset of children (n = 24) were concurrently enrolled in a randomized, controlled clinical trial of early-moderate hypothermia for neuroprotection.
Interventions
Data extraction of multiple clinical variables, including demographic data, BSA, and RT and BT at recorded at hourly intervals.
Measurements and Main Results
Paired BT and RT measurements (in °C, n = 4369) were collected hourly and compared using Pearson correlations. Patients were stratified according to body surface area (BSA in M2; < 1.0, 1.0 – 1.99, 2.0 – 2.99 and > 3) and based on BT (≤ 34.0, 34.1 – 36.0, 36.1 – 38, ≥ 38.1). BSA and BT were compared between groups using Pearson correlations with correction for repeated measures. Mean BT-RT difference was calculated for stratified BT ranges. Overall, BT and RT were highly correlated (r = 0.86, P < 0.001). During brain hyperthermia, BT-RT was similar to that reported in previous studies with BT higher than RT (1.75 ± 0.4; r = 0.54). Surprisingly, this relationship was reversed during brain hypothermia (BT-RT = -1.87± 0.8; r = 0.37), indicating a reversal of the brain-systemic temperature gradient. When stratified for BSA, the correlation between RT and BT remained strong (r = 0.78, 0.91, 0.79 and 0.95, respectively, P < 0.001). However, the correlation between BT and RT was substantially decreased when stratified for BT (r = 0.37, 0.58, 0.48, 0.54, P < 0.001). In particular, during moderate brain hypothermia (BT ≤ 34), the correlation between BT and RT was weakest, indicating the greatest variability during this condition which is often targeted for therapeutic trials.
Conclusions
BT and RT are generally well-correlated in children with TBI. This relationship is different at the extremes of the physiological temperature range, with the temperature gradient reversed during brain hypo- and hyperthermia. Given that studies showing neuroprotection from hypothermia in animal models of brain injury generally target BT, our data suggest the possibility that if BT were the therapeutic target in clinical trials this would result in somewhat higher systemic temperature and potentially fewer side effects. This relationship may be exploited in future clinical trials to maintain brain hypothermia (for neurological protection) at slightly higher systemic temperatures (and potentially fewer systemic side effects).
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in the United States and abroad, both in adults and children [1–3]. Targeted temperature management is becoming more important in neurocritical care for a number of reasons. Several studies have demonstrated that systemic hyperthermia can be detrimental at critical times after TBI and other acute brain injuries [4–8] and aggressive treatment of hyperthermia is now standard of care. Early, therapeutic hypothermia is being investigated for efficacy in adults and children after TBI [9–12] and systemic hypothermia has been recommended as an optional rescue therapy for intractable intracranial hypertension in TBI [13].
With this increasing interest and attention to temperature management, understanding the relationship between temperature measurements in various sites is important to make optimal therapeutic decisions on temperature management. Rectal (RT), esophageal, bladder, jugular venous, axillary, inguinal, tympanic, and pulmonary artery temperatures have been advocated as possible targets in various studies [14]. Recently, brain temperature (BT) monitoring has become more common and may represent a more CNS-relevant target for targeted temperature management as either controlled normothermia or induced hypothermia for neuroprotection. The technical capability to monitor BT has existed for more than 2 decades by using a variety of commercially-available, intraparenchymal devices [15–17]. These devices are generally used in conjunction with catheters that measure brain tissue oxygen tension (PbO2). Use of PbO2 monitoring in adults after TBI or subarachnoid hemorrhage is becoming more common [18–24] and reports of small series of children after TBI have emerged [25].
Relatively few studies have explored the relationship between RT and BT. While Henker and colleagues demonstrated that brain temperature ranged from 0.3 – 1.9 °C greater than bladder and rectal temperature measurements in 8 adults after TBI, Childs and colleagues found a much smaller difference (mean = 0.04°C) [26, 27]. Rossi and colleagues demonstrated a difference between brain and pulmonary artery catheter temperature (PAT) measurements of 0.16°C during periods of normothermia but an increase in this gradient to 0.41°C during periods of fever (measured by pulmonary artery temperature). However, virtually all patients in this study had periods of systemic hyperthermia (mean PAT = 38.1°C; mean BT = 38.4°C) [28].
The relationship between systemic temperature and brain temperature over a wide range of temperatures has not been studied in children. We theorized that many factors, including normal differences in heat generation between individual subjects, changes in body surface area: head circumference changes that occur as a part of normal development, and alterations in brain metabolism could influence the relationship between BT and RT. Therefore, we hypothesized that a temperature gradient between BT and RT would be consistent across the normal range of physiological temperatures and would be influenced by BSA (due to intrinsic changes in brain metabolism
Methods
Children with severe TBI (GCS score ≤ 8) were prospectively enrolled in a Pediatric Neurotrauma Center database that was approved by the Institutional Review Board of the University of Pittsburgh. Parental consent was obtained for all subjects. Consecutive children (n = 40) with severe TBI within the database who underwent BT monitoring (7/03 – 12/08) were studied.
Treatment Protocol
The management of severe TBI at our institution utilizes a clinical protocol that has been previously described [11] and based on published Guidelines [29]. Briefly, intracranial pressure (ICP) monitoring was initiated when GCS score was ≤ 8 and a step-wise protocol to treat intracranial hypertension was initiated. Both an external ventricular drain and BT and brain tissue oxygen (PbO2) monitor (Licox, Integra LifeSciences, Plainsboro, NJ) were inserted in all patients studied. The BT monitor was placed at the grey-white matter junction in the right frontal cortex. Placement of the BT monitor was not directed toward contused tissue or placed directly into the obviously necrotic tissue based on CT scan findings. An ICP-directed protocol was used that included first-tier therapies (sedation with fentanyl, neuromuscular blockade with vecuronium and continuous cerebrospinal fluid drainage at 3 cm above midbrain), second-tier therapies (mannitol [0.25 – 0.5 mg/kg/dose] and/or hypertonic saline [3%]), third-tier therapies (pentobarbital as a bolus and/or continuous infusion) and rescue maneuvers (decompressive craniectomy with duraplasty, moderate hypothermia) as necessary. Some children were enrolled in an interventional study evaluating the role of early-moderate hypothermia as a neuroprotectant after severe TBI [11]. BT measurements were obtained directly from the Licox device. RT measurements were performed using commercially-available probes for the Gaymar Medi-Therm III surface cooling system (Gaymar, Orchard Park, NY) in accordance with the manufacturer’s instructions. Temperature management for all patients was guided by rectal temperature measurements. During periods of induced hypothermia as part of a clinical study, rectal temperature was targeted at 32 – 33°C. During periods of induced hypothermia for intractable intracranial hypertension, rectal temperature was decreased at the discretion of the clinical care team. For children receiving standard therapy, systemic hyperthermia (RT > 38°C) was avoided by various maneuvers at the discretion of the clinical care team. BT was never used as a clinical target during this study.
Data Collection and Analysis
BT and RT (all in °C) were recorded by the bedside nurse at least hourly for the duration of the study. BT monitoring generally began within 6 hours of injury and collection of this data was obtained from insertion up to 168 hours, as several groups have questioned the accuracy of this monitoring system over prolonged periods of time [30]. Only time points with paired recordings for both BT and RT were studied for comparison and measurements were obtained after a calibration period for the BT device was completed (1 h). Demographic information including sex, age, mechanism of injury, initial GCS and outcome was also collected for analysis. Overall comparison of BT and RT was performed using Pearson correlations with correction for repeated measures. Children were stratified based on body surface area (BSA, derived from height and weight and a previously published formula) as well as brain temperature in separate analyses. BSA stratification included (in M2); < 1.0, 1.0 – 1.99, 2.0 – 2.99 and > 3 and comparisons between BT and RT at each stratification level was determined using Pearson correlations. Similarly, data were stratified based on BT with groups including BT ≤ 34.0ºC; 34.1–36.0ºC, 36.1–38.0ºC, ≥ 38.1ºC. Pearson correlations were performed for data in these groups. Differences between brain and rectal temperature measurements were determined for each set of paired values. These were stratified by BT range as described above, and the average difference for each patient in each temperature range was determined. Mean difference values from all patients in each temperature range were pooled and averaged to determine overall mean difference within each temperature range. Data are expressed as mean ± SEM unless otherwise indicated.
Results
The Pediatric Neurotrauma Center database contained information from 162 children with TBI within the time frame under study (7/03 – 12/08). Forty children who underwent BT monitoring were included for this analysis and a total of 4369 paired BT-RT measurements were analyzed. There was a predominance of males (28/40; 70%) and those with favorable outcome (24/40; 60%) at 3 months as measured by GOS. Twenty children were enrolled in trials of early hypothermia (n = 9 in normothermic arm, n = 11 in hypothermic arm) and 20 received standard temperature management. The average age of children enrolled was 8.9 y ± 0.8 years (range 0.1 – 17.2), average admission GCS score was 6.7 ± 0.3 (range 3 – 15) and average BSA was 1.5 M2 ± 0.1 (median = 1.1M2). Injury mechanisms included fall, motor vehicle crash, pedestrian vs motor vehicle, and assault. Penetrating injuries were not included.
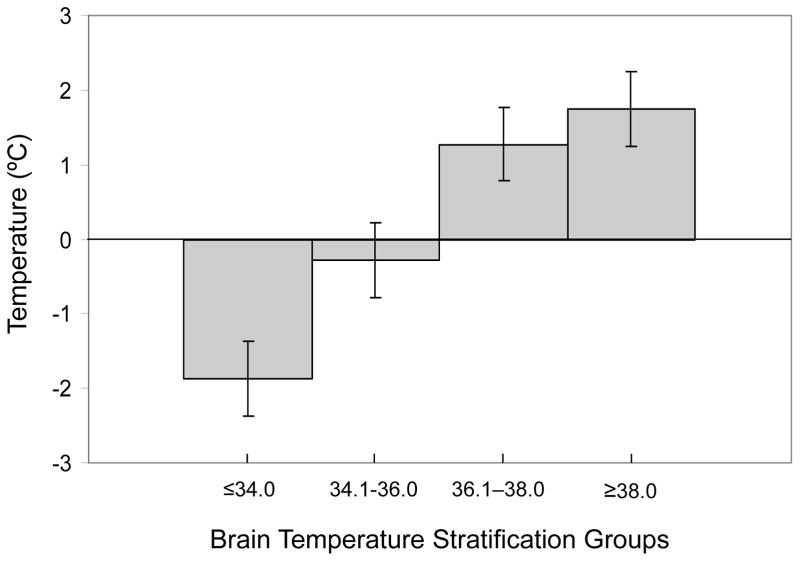
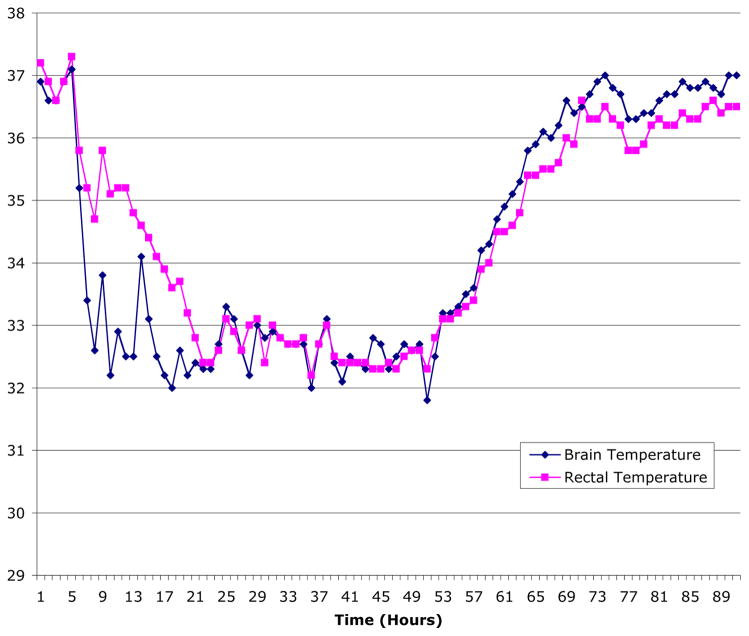
Overall, BT and RT were highly correlated (r = 0.86, p < 0.001, Figure 1), indicating that RT variance was responsible for 73.9% of the variance of BT. The average temperature difference for all paired measurements was 0.12°C. In individual children, the range of mean BT-RT differences over the entire study period was between –1.58 and 1.92°C, with variance in RT associated with 13.6 – 33.6% of the variance in BT. The difference between BT and RT was temperature dependent (see Figure 1). Specifically, during episodes brain hyperthermia (BT ≥ 38.1), the difference between BT and RT was 1.75 ± 0.4 (n = 213 observations), indicating the brain was warmer than the body. Conversely, during periods of the greatest degree of brain hypothermia (BT ≤ 34), the BT-RT gradient was actually reversed (BT-RT = −1.87± 0.4, n = 618 observations). In intermediate ranges of hypothermia the trend persisted with a negative BT-RT gradient, while in normothermic brain temperature ranges the BT-RT gradient was modestly positive (for BT 34.1– 36.0, BT – RT = −0.2 ± 0.2, n = 1470; for BT 36.1 – 38.0, BT – RT = 1.3 ± 0.3, n = 2068). An individual case demonstrating this phenomenon is presented in Figure 2.
Figure 1.
Differences between brain and rectal temperatures vary based on brain temperature (BT < 34, BT-RT = −1.87± 0.4, n = 618; BT 34.1– 36.0, BT – RT = −0.2 ± 0.2, n = 1470; BT 36.1 – 38.0, BT −RT = 1.3 ± 0.3, n = 2068; BT ≥ 38.1, BT − RT = 1.75 ± 0.4, n = 213.
Figure 2.
Plot of brain temperature (in blue) and rectal (in red) temperature over time in a child who underwent induced hypothermia, demonstrating the temperature dependent nature of the BT=RT relationship.
When stratified based on BSA, correlations between BT and RT remained significant but were similar between stratification groups (BSA < 1.0, 1.0 – 1.99, 2.0 – 2.99 and > 3, r = 0.78, 0.91, 0.79 and 0.95, respectively, p < 0.001 for all correlations). However, when stratified based on BT, the correlation between BT and RT were substantially weaker but remained statistically significant (BT ≤ 34, 34.0 – 36.0, 36.1 – 38.0, ≥ 38.1 r = 0.37, 0.58, 0.48, 0.54, respectively, p < 0.001).
Discussion
This is the first report of the relationship between BT and RT in children after TBI to our knowledge. Overall, we found that BT and RT show a relatively high degree of correlation and this relationship was not related to body size. Surprisingly, we found that the relationship between BT and RT was temperature-dependent – specifically that at extremes of temperature, BT becomes more exaggerated with a relative decrease in BT during hypothermia and a relative increase in during hyperthermia. We also observed that the correlation between BT and RT was weakest during hypothermia. With ongoing interest in targeted temperature management as a potential treatment for patients with brain injury, precise understanding of the relationship between brain and core body temperature measurements has taken on even greater importance.
Importance of Temperature after Brain Injury and the Relationship between BT and RT
Control of BT after TBI is considered to be important for neurocritical care and has been studied in experimental as well as clinical studies. Using a variety of TBI models, several groups have reported increases in contusion volume, increased extravasation of blood, and histological damage with hyperthermia [4, 5]. Other studies of experimental TBI have demonstrated improved survival and cognitive/motor performance with therapeutic cooling in adult rats [31] and decreased cerebral edema in immature rats [32].
Less well studied is the relationship between BT and RT in animals. Most have found a relatively constant gradient of systemic to brain temperature. For example, Ao and colleagues found that BT was persistently greater (< 1°C) than RT throughout the temperature ranges in dogs after TBI [33]. The deleterious effects of hyperthermia on outcome are presumably related to increased cerebral metabolism at a time of compromised cerebral blood flow and increases in ICP [34]. Hyperthermia is a relatively common occurrence after clinical TBI, developing in up to 44% of adult TBI victims within 2 days of injury, and up to 73% of patients within the first week [16]. Kilpatrick and colleagues found that hyperthermia is also associated with increased length of stay in the intensive care unit [35]. To date, while the deleterious consequences of hyperthermia after TBI in humans have not been reported and the Brain Trauma Foundation makes no recommendations regarding temperature measurement or control after TBI in adults [36] or children [29], a consensus of opinion is forming regarding the deleterious effects of hyperthermia on outcome after TBI, presumably from increased ICP [34]. Our data suggests that systemic hyperthermia may underestimate the degree of brain hyperthermia in children, in some cases up to 2ºC. While the current Pediatric guidelines [29] recommend avoidance of hyperthermia, our new evidence emphasizes the danger of even mild systemic hyperthermia and raise the consideration that BT should be monitored rather than RT. The relationship between BT and other core temperature measurement sites also needs to be studied if the optimal site of measurement as a surrogate for BT is desired.
The existing data on the relationship between BT and RT are exclusively collected from adult victims of TBI and generally shows a slight increase in BT throughout the systemic temperature ranges. Henker a series of 8 patients with severe TBI and found that the difference between BT and RT ranged between 0.1 and 2.0°C [26]. The differences were greatest at temperatures outside of the normal range. Childs and colleagues found no significant difference between BT and RT in 20 adults after TBI, but also observed reversal of the BT-RT gradient in some patients and concluded that BT could not be predicted consistently from body temperature [22]. Rumana and colleagues studied BT-RT differences in 30 adult TBI patients and found that there was a difference of 2°C between BT and RT [17]. Furthermore, they found that the difference between measurements increased as cerebral perfusion pressure decreased and that differences decreased in the setting of barbiturate coma. They concluded that direct measurement of BT is likely important, as BT might be higher than measured body temperature. Only Soukup and colleagues have reported that this relationship might have a dichotomous pattern, in that the BT-RT difference during periods of hypothermia were slightly lower than during periods of normothermia (−0.2 ± 0.6 vs. 0.0 ± 0.5) [37]. Our data demonstrate a much more robust and dichotomous temperature dependence of BT on RT. We hypothesized that the BSA could explain this difference between adults and children after TBI, but we failed to confirm that hypothesis. While it is possible that our methods of cooling utilized (intravenous iced saline, surface cooling with blankets, cooled ventilator gases) may affect the brain-rectal temperature differences, our data collection system for this retrospective study was inadequate to answer this important question. However, our data do demonstrate that the discrepancy between BT and RT is greatest during induction of hypothermia and rewarming, which are critical periods associated with important morbidities in clinical studies [10].
Clinical Implications
The concept that hyperthermia is deleterious to the injured brain is endorsed by many recent reviews [38, 39] and appears to be a prudent step in the management of the brain injured patient. We have observed that, while BT and RT measurements are highly correlated across the clinically significant temperature range, the relationship is temperature dependent. Hyperthermia, as measured via RT, may significantly underestimate the degree of hyperthermia in the injured pediatric brain. We believe that our data adds further support for the avoidance of systemic hyperthermia because the concomitant increase in brain temperature appears to portend an even greater risk to the injured brain. Moreover, avoidance of systemic hyperthermia in children might have important implications for the treatment of other, non-traumatic disease processes such as meningoencephalitis, status epilepticus, and hypoxic-ischemic encephalopathy, where BT is not currently being measured. Although we recognize that this issue is more controversial in CNS insults other than TBI or ischemia, we speculate that if others replicate our findings of the BT-RT gradient in children suffering from these diseases, then seemingly mild systemic fevers could underestimate the degree of cerebral hyperthermia and possibly unfavorably influence outcome.
Our data also indicate that systemic hypothermia as assessed by RT leads to a greater than predicted degree of brain hypothermia. In the past, almost all studies evaluating the efficacy of hypothermia as a neuroprotectant use systemic temperatures within their clinical protocols, while few have targeted BT. Tokutumi et al found that intracranial hypertension decreased at BT of 35 – 36°C and that further decreases in BT below this threshold had little effect [40]. In our study, this could be achieved by decreasing systemic temperature only slightly below normothermia (perhaps 35.5 – 36.5°C). If it were proven that BT was linked to neurological outcome more closely than systemic temperature, then children undergoing such therapies might achieve these BT goals at more modest RT reductions. This may have a beneficial effect on minimizing the complications of systemic hypothermia for this critically-ill patient population.
Limitations
There are several limitations to the current study. We used data collected from nursing flow sheets of children after TBI which could be subject to bias of nurses charting patterns or theoretical inaccuracies of the FDA-approved devices themselves. However, since temperature was emphasized during the clinical care of these patients (as many were enrolled within a hypothermia trial), we believe that the human and technical errors (from the devices) were minimal. Second, from our data extraction methods we could not determine if children were undergoing active cooling (as part of a clinical trial or due to intracranial hypertension crisis) or if they had spontaneously decreased temperatures due to severe injuries. Fuentas et al reported a dissociation of the BT-RT relationship in adults who became brain dead and this theoretically could complicate our analysis [41]. However, we had only 2 deaths within the 40 children studied; thus this relationship could only have a limited effect on the interpretation of our data. No specific calibration or zeroing of the measurement devices was performed at the time of rectal and brain temperature probe placement, owing to the urgency of the situation and because of infection concern. Nevertheless, as the devices were not calibrated and zeroed in a standard manner, there exists the possibility for discrepancy in measurements based on device-to-device variability. Finally, it was impossible within our retrospective review to determine the methods applied to individual patients to cool or rewarm. Within our institution, cooling blankets, intravenous bolus of iced saline, manipulation of the temperature of inspired gases, ice bags to skin, and iced saline lavage are all generally used to either achieve hypothermia or maintain normothermia. It is possible that one of these methods may alter the systemic/brain temperature gradient to a greater degree than the other methods. Moreover, it is possible that other measures of systemic temperature (esophageal, central venous or others) may differ importantly from rectal temperature and be a better approximation of systemic thermoregulatory status. A more detailed analysis of these individual methods would be required to address this possibility.
In summary, we have shown that the RT to BT relationship is temperature but not BSA dependent in children with severe TBI. BT is lower than RT during hypothermia, but higher than RT during hyperthermia after TBI. It is possible that regional variations of temperature within the brain exist that cannot currently be studied clinically and we believe that a greater understanding of the relationship between systemic and brain temperatures could ultimately lead to improved design of clinical trials targeting temperature after brain injuries.
Acknowledgments
Funding for this study provided by NIH T32HD40686.
Footnotes
Dr. Adelson consulted for Traumic and received honoraria/speaking fees from Cyberomics. All other authors have no potential conflicts of interest to disclose.
References
- 1.Thurman DJ, et al. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Rutland-Brown W, et al. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Weisend MP, Feeney DM. The relationship between traumatic brain injury-induced changes in brain temperature and behavioral and anatomical outcome. J Neurosurg. 1994;80(1):120–32. doi: 10.3171/jns.1994.80.1.0120. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita K, et al. The effect of brain temperature on hemoglobin extravasation after traumatic brain injury. J Neurosurg. 2002;97(4):945–53. doi: 10.3171/jns.2002.97.4.0945. [DOI] [PubMed] [Google Scholar]
- 6.Thompson HJ, et al. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12(3):163–73. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 7.Greer DM, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39(11):3029–35. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 8.Stocchetti N, et al. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28(11):1555–62. doi: 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- 9.Marion DW, et al. Treatment of traumatic brain injury with moderate hypothermia. New England J Med. 1997;336:540–6. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 10.Hutchison JS, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 11.Adelson PD, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740–54. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–54. [DOI] [PubMed] [Google Scholar]
- 12.Qiu WS, et al. Therapeutic effect of mild hypothermia on severe traumatic head injury. Chin J Traumatology. 2005;8:27–32. [PubMed] [Google Scholar]
- 13.Latorre JGS, et al. Management of Acute Intracranial Hypertension. The Neurologist. 2009;15(4):193–207. doi: 10.1097/NRL.0b013e31819f956a. [DOI] [PubMed] [Google Scholar]
- 14.Lefrant JY, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003;29(3):414–8. doi: 10.1007/s00134-002-1619-5. [DOI] [PubMed] [Google Scholar]
- 15.Mellergard P, Nordstrom CH. Intracerebral temperature in neurosurgical patients. Neurosurgery. 1991;28(5):709–13. [PubMed] [Google Scholar]
- 16.Schwab S, et al. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48(3):762–7. doi: 10.1212/wnl.48.3.762. [DOI] [PubMed] [Google Scholar]
- 17.Rumana CS, et al. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998;26(3):562–7. doi: 10.1097/00003246-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Stiefel MF, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805–11. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky R, et al. Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas. Neurosurgery. 2004;55(6):1318–23. doi: 10.1227/01.neu.0000143029.42638.2c. discussion 1324. [DOI] [PubMed] [Google Scholar]
- 20.Jaeger M, Soehle M, Meixensberger J. Improvement of brain tissue oxygen and intracranial pressure during and after surgical decompression for diffuse brain oedema and space occupying infarction. Acta Neurochir Suppl. 2005;95:117–8. doi: 10.1007/3-211-32318-x_25. [DOI] [PubMed] [Google Scholar]
- 21.Hemphill JC, 3rd, et al. Brain tissue oxygen monitoring in intracerebral hemorrhage. Neurocrit Care. 2005;3(3):260–70. doi: 10.1385/NCC:3:3:260. [DOI] [PubMed] [Google Scholar]
- 22.Longhi L, et al. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in peri-focal tissue. Intensive Care Med. 2007;33(12):2136–42. doi: 10.1007/s00134-007-0845-2. [DOI] [PubMed] [Google Scholar]
- 23.Jaeger M, et al. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38(3):981–6. doi: 10.1161/01.STR.0000257964.65743.99. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal G, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36(6):1917–24. doi: 10.1097/CCM.0b013e3181743d77. [DOI] [PubMed] [Google Scholar]
- 25.Stiefel MF, et al. Brain tissue oxygen monitoring in pediatric patients with severe traumatic brain injury. J Neurosurg. 2006;105(4 Suppl):281–6. doi: 10.3171/ped.2006.105.4.281. [DOI] [PubMed] [Google Scholar]
- 26.Henker RA, Brown SD, Marion DW. Comparison of brain temperature with bladder and rectal temperatures in adults with severe head injury. Neurosurgery. 1998;42(5):1071–5. doi: 10.1097/00006123-199805000-00071. [DOI] [PubMed] [Google Scholar]
- 27.Childs C, et al. Differences between brain and rectal temperatures during routine critical care of patients with severe traumatic brain injury. Anaesthesia. 2005;60(8):759–65. doi: 10.1111/j.1365-2044.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- 28.Rossi S, et al. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71(4):448–54. doi: 10.1136/jnnp.71.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr Crit Care Med. 2003;4(3 Suppl):S2–4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 30.Dings J, Meixensberger J, Roosen K. Brain tissue pO2-monitoring: catheterstability and complications. Neurol Res. 1997;19(3):241–5. doi: 10.1080/01616412.1997.11740806. [DOI] [PubMed] [Google Scholar]
- 31.Clifton GL, et al. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11(1):114–21. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- 32.Mansfield RT, et al. Effects of hypothermia on traumatic brain injury in immature rats. J Cereb Blood Flow Metab. 1996;16(2):244–52. doi: 10.1097/00004647-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Ao H, et al. Jugular vein temperature reflects brain temperature during hypothermia. Resuscitation. 2000;45(2):111–8. doi: 10.1016/s0300-9572(00)00154-4. [DOI] [PubMed] [Google Scholar]
- 34.Childs C, Jones AK, Tyrrell PJ. Long-term temperature-related morbidity after brain damage: survivor-reported experiences. Brain Inj. 2008;22(7):603–9. doi: 10.1080/02699050802189719. [DOI] [PubMed] [Google Scholar]
- 35.Kilpatrick MM, et al. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47(4):850–5. doi: 10.1097/00006123-200010000-00011. discussion 855–6. [DOI] [PubMed] [Google Scholar]
- 36.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Trauma systems. J Neurotrauma. 2000;17(6–7):457–62. doi: 10.1089/neu.2000.17.457. [DOI] [PubMed] [Google Scholar]
- 37.Soukup J, et al. The importance of brain temperature in patients after severe head injury: relationship to intracranial pressure, cerebral perfusion pressure, cerebral blood flow, and outcome. J Neurotrauma. 2002;19(5):559–71. doi: 10.1089/089771502753754046. [DOI] [PubMed] [Google Scholar]
- 38.Childs C. Human brain temperature: regulation, measurement and relationship with cerebral trauma: part 1. Br J Neurosurg. 2008;22(4):486–96. doi: 10.1080/02688690802245541. [DOI] [PubMed] [Google Scholar]
- 39.Sacho RH, Childs C. The significance of altered temperature after traumatic brain injury: an analysis of investigations in experimental and human studies: part 2. Br J Neurosurg. 2008;22(4):497–507. doi: 10.1080/02688690802245558. [DOI] [PubMed] [Google Scholar]
- 40.Tokutomi T, et al. Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery. 2003;52(1):102–11. discussion 111–2. [PubMed] [Google Scholar]
- 41.Fountas KN, et al. Disassociation between intracranial and systemic temperatures as an early sign of brain death. J Neurosurg Anesthesiol. 2003;15(2):87–9. doi: 10.1097/00008506-200304000-00004. [DOI] [PubMed] [Google Scholar]