Abstract
Findings on the twisting structure and insertional location of the AT on the calcaneal tuberosity are inconsistent. Therefore, to obtain a better understanding of the mechanisms underlying insertional Achilles tendinopathy, clarification of the anatomy of the twisting structure and location of the AT insertion onto the calcaneal tuberosity is important. The purpose of this study was to reveal the twisted structure of the AT and the location of its insertion onto the calcaneal tuberosity using Japanese cadavers. The study was conducted using 132 legs from 74 cadavers (mean age at death, 78.3 ± 11.1 years; 87 sides from men, 45 from women). Only soleus (Sol) attached to the deep layer of the calcaneal tuberosity was classified as least twist (Type I), both the lateral head of the gastrocnemius (LG) and Sol attached to the deep layer of the calcaneal tuberosity were classified as moderate twist (Type II), and only LG attached to the deep layer of the calcaneal tuberosity was classified as extreme twist (Type III). The Achilles tendon insertion onto the calcaneal tuberosity was classified as a superior, middle or inferior facet. Twist structure was Type I (least) in 31 legs (24%), Type II (moderate) in 87 legs (67%), and Type III (extreme) in 12 legs (9%). A comparison between males and females revealed that among men, 20 legs (24%) were Type I, 57 legs (67%) Type II, and eight legs (9%) Type III. Among women, 11 legs (24%) were Type I, 30 legs (67%) Type II, and four legs (9%) Type III. No significant differences were apparent between sexes. The fascicles of the Achilles tendon attach mainly in the middle facet. Anterior fibers of the Achilles tendon, where insertional Achilles tendinopathy is most likely, are Sol in Type I, LG and Sol in Type II, and LG only in Type III. This suggests the possibility that a different strain is produced in the anterior fibers of the Achilles tendon (calcaneal side) where insertional Achilles tendinopathy is most likely to occur in each type. We look forward to elucidating the mechanisms generating insertional Achilles tendinopathy in future biomedical studies based on the present results.
Keywords: Achilles tendon disorders, insertional Achilles tendinopathy, insertional site, Japanese cadavers
Introduction
Achilles tendon (AT) disorders are classified mainly by the positional relationship anatomically and by the site of pain. Noninsertional Achilles tendinopathy is classified as that occurring 2–6 cm proximal to the AT insertion onto the calcaneal tuberosity, and insertional Achilles tendinopathy as that occurring at the AT insertion onto the calcaneal tuberosity (Irwin, 2010; Roche & Calder, 2013).
Since noninsertional Achilles tendinopathy occurs 2–6 cm proximal to the AT insertion onto the calcaneus, where blood supply is poor (Carr & Norris, 1989; Clain & Baxter, 1992; Saltzman & Tearse, 1998) and cross‐sectional area of the AT is small (Magnusson & Kjaer, 2003; Kongsgaard et al. 2005), repetitive stress at this site is not thought to be the principal contributor. In recent years, the unevenness of strain within the AT during overpronation of the calcaneus has come to be viewed as an important factor, and the twisted structure of the AT is regarded as a cause (Lersch et al. 2012; Bojsen‐Moller & Magnusson, 2015; Edama et al. 2015).
In contrast, insertional Achilles tendinopathy is divided into bursitis occurring in the retrocalcaneal bursa and insertional Achilles tendinopathy occurring at the site of AT insertion onto the calcaneal tuberosity. Retrocalcaneal bursitis is thought to result from repeated impact between the bursa and AT. However, few reports have examined the mechanisms leading to insertional Achilles tendinopathy, and findings from those few studies are inconsistent. Since the most commonly involved site on magnetic resonance imaging (MRI) is the anterior fibers of the AT (calcaneal side), inflammation has been speculated to occur with repetitive stress on anterior AT fibers during exercise of the ankle (Irwin, 2010). However, Lyman et al. (2004) reported that strain was greater in posterior fibers of the AT (skin side) than in anterior fibers (calcaneal side) when the ankle was dorsiflexed using fresh cadavers. They also reported that if Achilles insertional tendinopathy is due to mechanical factors, it appears more likely that the tendon breakdown is a result of stress shielding or internal shear forces associated with differential strains. One reason for these differing understandings is thought to be inconsistency of interpretation in anatomical reports regarding the site of AT insertion onto the calcaneal tuberosity.
The only recent report on the twisted structure of the AT examined at its insertion on the calcaneal tuberosity was a study of 12 fresh‐frozen cadavers (Ballal et al. 2014). That report confirmed the twisted structure of the AT at the insertion on the calcaneal tuberosity but found less twisting than in earlier studies investigating the area near the AT insertion on the calcaneal tuberosity (Cummins et al. 1946; Szaro et al. 2009; Edama et al. 2015) and also described a very different structure.
In a study on the site of AT insertion on the calcaneal tuberosity using 12 fresh‐frozen cadavers (Ballal et al. 2014), the insertion was reported to be from the middle facet to the inferior facet of the calcaneal tuberosity. In a study using 40 embalmed legs (Kim et al. 2010), the calcaneal tuberosity was divided into three parts and the AT was reported to insert onto the superior one‐third in 55% of specimens, the middle one‐third in 40% of specimens, and the inferior one‐third in 5% of specimens. In a study using MRI in 69 living subjects (age range, 10–40 years) (Kim et al. 2011), insertional location was seen to differ significantly with age. Findings on the twisting structure and insertional location of the AT on the calcaneal tuberosity are thus inconsistent. Both the small numbers of subjects and methodological differences may be involved in these differences. Therefore, to obtain a better understanding of the mechanisms underlying insertional Achilles tendinopathy, clarification of the anatomy of the twisting structure and location of the AT insertion onto the calcaneal tuberosity is important.
The purpose of this study was to reveal the twisted structure of the AT and the location of its insertion onto the calcaneal tuberosity using Japanese cadavers.
Materials and methods
Cadavers
This investigation examined 132 legs from 74 Japanese cadavers (mean age at death, 78.3 ± 11.1 years; 87 sides from men, 45 from women) that had been switched to alcohol after placement in 10% formalin. Of 132 legs from 74 Japanese cadavers, 110 legs from 60 Japanese cadavers were used in the previous study (Edama et al. 2015). None showed signs of previous major surgery around the foot or ankle or any relevant deformities. There was no prominent degeneration in any specimen. This study was approved by the Ethics Committee at our institution.
Methods
The procedure for dissecting the AT was adopted with reference to previous studies (Ballal et al. 2014; Edama et al. 2015). First, the skin, subcutaneous tissue, and crural fascia were removed from the back of the leg, and the triceps surae was extracted together with the calcaneus. Next, AT fascicles originating in the bellies of the medial head of the gastrocnemius (MG) and lateral head of the gastrocnemius (LG) were separated from AT fascicles originating in the belly of the soleus (Sol). As these fascicles were very strongly fused, the two were carefully teased apart. Afterward, connective tissue around the AT was carefully removed, and the AT fascicles originating in the bellies of the MG and LG were separated. AT fascicles originating in the MG and LG muscle bellies were strongly fused, but the border of each fascicle could be identified and separated by following the course of the relatively thick tendon fibers that represented each tendon fascicle. The twist in the fascicles was classified with reference to a previous study (Edama et al. 2015) into: least twist (Type 1), with only Sol attached to the deep layer of the calcaneal tuberosity (calcaneal side); moderate twist (Type II), with LG and Sol attached to the deep layer of the calcaneal tuberosity; and extreme twist (Type III), with only the LG attached to the deep layer of the calcaneal tuberosity. Next, subcutaneous tissue around the calcaneal tuberosity and retrocalcaneal bursa were carefully removed and the area of AT insertion onto the calcaneal tuberosity was exposed. In line with a previous study (Ballal et al. 2014), the calcaneal tuberosity was divided into three parts: a superior facet; a middle facet; and an inferior facet. The insertional location of each fascicle making up the AT was revealed.
For statistical analysis, a Chi‐square test was used to compare the twisted structure of the AT between right and left sides and between men and women. The level of significance was 5%.
Results
AT fascicles that originated from the bellies of the MG and LG, and AT fascicles that originated from the Sol were difficult to separate in only two legs. The final analysis was thus undertaken using 130 legs from 73 cadavers (85 male legs, 45 female legs).
Classification of twisted structure
AT was Type I (least) in 31 legs (24%), Type II (moderate) in 87 legs (67%), and Type III (extreme) in 12 legs (9%) (Fig. 1).
Figure 1.
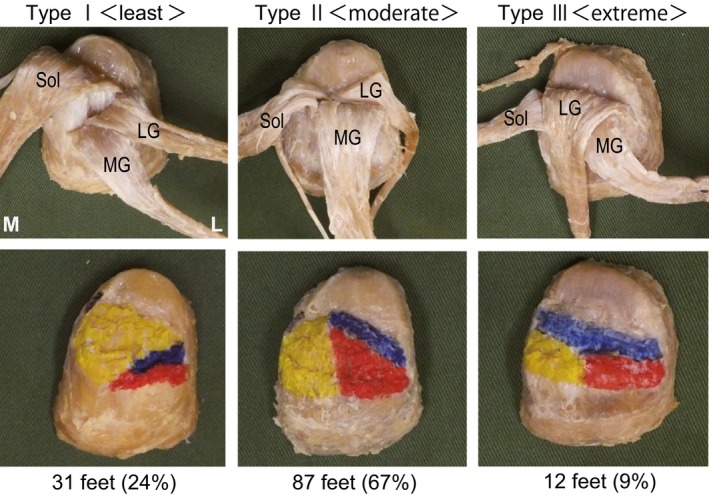
Patterns of ‘twist’ in the right Achilles tendon, posterior view. Type 1 (least twist): with only Sol attached to the deep layer of the calcaneal tuberosity (calcaneal side). Type II (moderate twist): with LG and Sol attached to the deep layer of the calcaneal tuberosity. Type III (extreme twist): with only LG attached to the deep layer of the calcaneal tuberosity LG, fibers from the lateral head of the gastrocnemius; MG, fibers from the medial head of the gastrocnemius; Sol, fibers from the soleus muscle. Blue, fibers from the lateral head of the gastrocnemius; red, fibers from the medial head of the gastrocnemius; yellow, fibers from the soleus muscle. L, lateral; M, medial.
Sex and left–right differences
A comparison between males and females revealed that among men, 20 legs (24%) were Type I, 57 legs (67%) Type II, and eight legs (9%) Type III. Among women, 11 legs (24%) were Type I, 30 legs (67%) Type II, and four legs (9%) Type III. No significant differences were apparent between sexes.
With respect to left–right differences, among the right legs, 16 (28%) were Type I, 35 (61%) Type II, and six (11%) Type III. Among the left legs, 10 (18%) were Type I, 43 (75%) Type II, and four (7%) Type III. Again, no significant differences were apparent between sides. In addition, 34 individual cadavers (57%) showed the same type bilaterally. Five cadavers (15%) showed Type I on both sides, 28 (82%) had Type II bilaterally, and one (3%) had Type III bilaterally.
Classification of insertional location onto the calcaneal tuberosity
The retrocalcaneal bursa exists on the superior facet of the calcaneal tuberosity, and the fascicles of the AT attach mainly in the middle facet (Fig. 2). Some specimens also showed fibers attaching in the transitional area between the middle and inferior facets, but none was connected with the plantar fascia. In Type II, some Sol tendon fibers were seen to attach to the retrocalcaneal bursa (Fig. 3).
Figure 2.
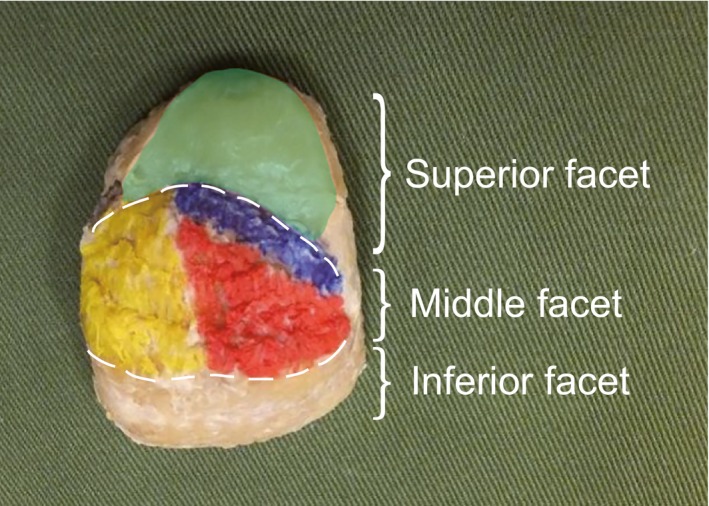
Site of attachment of the Achilles tendon to the calcaneal tuberosity. Right calcaneal tuberosity, posterior view. The calcaneal tuberosity was divided into three parts: superior facet, middle facet, and inferior facet. Blue, fibers from the lateral head of the gastrocnemius; green, retrocalcaneal bursa; red, fibers from the medial head of the gastrocnemius; yellow, fibers from the soleus muscle.
Figure 3.
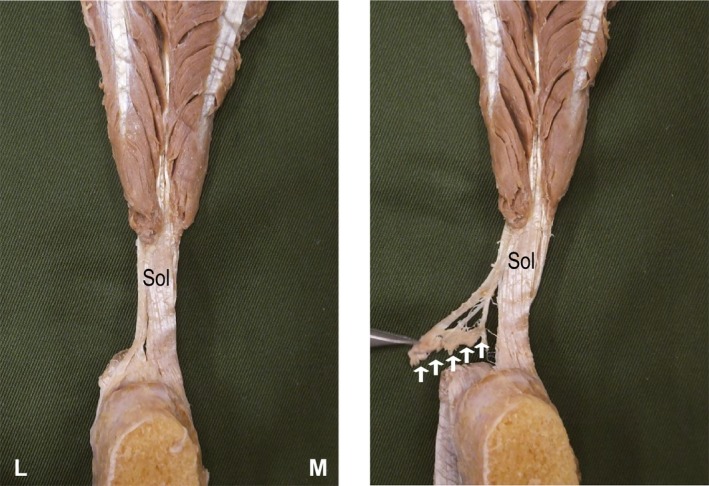
Methods of fine dissection for each fiber bundle. Right Achilles tendon, anterior view. L, lateral; M, medial; Sol, fascicle from the soleus muscle. White arrows: Part of the fascicle from the soleus muscle attached to the retrocalcaneal bursa.
Discussion
In this study, the twisted structure of the AT at the insertion onto the calcaneal tuberosity was classified using Japanese cadavers, and the location of the AT insertion onto the calcaneal tuberosity was revealed. No large‐scale cadaveric studies such as this have been reported previously.
The results identified the same twisted structures as in studies conducted in the vicinity of the calcaneal tuberosity insertion. The twisted structure was Type I in 31 legs (24%), Type II in 87 legs (67%), and Type III in 12 legs (9%). A previous study (Edama et al. 2015) classified tendons 1 cm proximal to the insertion onto the calcaneal tuberosity in 110 legs, finding that 55 legs (50%) were Type I, 47 legs (43%) Type II, and eight legs (7%) Type III. Compared with the present study, a large difference was seen in the proportions of Type I and Type II. The reason for this is thought to be that some Sol fibers that attached to the retrocalcaneal bursa (Fig. 3) were classified as Type I near the insertion onto the calcaneal tuberosity but were classified as Type II at the actual insertion onto the calcaneal tuberosity.
This study showed that anterior fibers of the AT (calcaneal side) where insertional Achilles tendinopathy is most likely to occur are Sol only in Type I, LG and Sol in Type II, and LG only in Type III, with the tissue located at the disease site differing between each type. The mechanism underlying insertional Achilles tendinopathy is not completely understood. One reason for this may be that consistent findings have not been obtained from anatomical studies of the AT insertion onto the calcaneal tuberosity. From studies using ultrasonography or MRI in living subjects (Finni et al. 2003; Bojsen‐Moller et al. 2004; Kinugasa et al. 2008) and studies using strain gauges with cadavers (Wren et al. 2003; Lyman et al. 2004; Defrate et al. 2006), different strains have been shown to be produced in the individual fascia making up the triceps surae during passive movement of the ankle or during contraction of the triceps surae. This suggests the possibility that a different strain is produced in the anterior fibers of the AT (calcaneal side) where insertional Achilles tendinopathy is most likely to occur in each type. Biomechanical studies with the present results as basic data will clearly be necessary in the future.
The present results showed that fascicles of the AT attach mainly to the middle facet of the calcaneal tuberosity, differing from previous findings (Kim et al. 2010, 2011; Ballal et al. 2014). Ballal et al. (2014) reported that the MG extended beyond the calcaneal tuberosity to the plantar fascia in three of 12 legs. In the results of the present study, none of the fascicles that made up the AT extended to the plantar fascia. Factors in these different findings may be the existence of abnormal variations in the shape of the calcaneal tuberosity (Kachlik et al. 2008) and differences in the method of classifying the location of insertion onto the calcaneal tuberosity (Kim et al. 2010, 2011; Ballal et al. 2014). The present results are considered highly reliable compared with previous studies, given the larger‐scale of this cadaveric investigation.
This study was able to reveal the twisted structure of the AT at the insertion onto the calcaneal tuberosity, classified using Japanese cadavers, but there are limitations. This study focused solely on the calcaneal tuberosity insertion in classifying the degree of twist, and did not include quantitative analysis. The longitudinal angle of each fascicle was conjectured to differ with the volumes of gastrocnemius and Sol, and quantification of the angle of twist will be an issue for future studies. Because the triceps surae is a complex three‐dimensional structure (Edama et al. 2015), elucidating the three‐dimensional structure of the AT will also be important.
In conclusion, the results showed that for the anterior fibers of the AT, where insertional Achilles tendinopathy is most likely to occur, the tissue located at the insertion differed in each type. Sol only was seen in Type I, LG and Sol in Type II, and LG only in Type II. This suggests that different strains are produced in the anterior fibers of the AT, where insertional Achilles tendinitis is most likely to occur in each type. We look forward to elucidating the mechanisms generating insertional Achilles tendinopathy in future biomedical studies based on the present results.
Acknowledgements
This study was supported by a Research Activity Young B Grant (20632326) from the Japan Society for the Promotion of Science (JSPS) and a Grant‐in‐Aid program from Niigata University of Health and Welfare (H27B04).
Conflict of interest
None.
References
- Ballal MS, Walker CR, Molloy AP (2014) The anatomical footprint of the Achilles tendon: a cadaveric study. Bone Joint J 96‐b, 1344–1348. [DOI] [PubMed] [Google Scholar]
- Bojsen‐Moller J, Magnusson SP (2015) Heterogeneous loading of the human Achilles tendon in vivo. Exerc Sport Sci Rev 43, 190–197. [DOI] [PubMed] [Google Scholar]
- Bojsen‐Moller J, Hansen P, Aagaard P, et al. (2004) Differential displacement of the human soleus and medial gastrocnemius aponeuroses during isometric plantar flexor contractions in vivo. J Appl Physiol (1985) 97, 1908–1914. [DOI] [PubMed] [Google Scholar]
- Carr AJ, Norris SH (1989) The blood supply of the calcaneal tendon. J Bone Joint Surg Br 71, 100–101. [DOI] [PubMed] [Google Scholar]
- Clain MR, Baxter DE (1992) Achilles tendinitis. Foot Ankle 13, 482–487. [DOI] [PubMed] [Google Scholar]
- Cummins EJ, Anson BJ, et al. (1946) The structure of the calcaneal tendon (of Achilles) in relation to orthopedic surgery, with additional observations on the plantaris muscle. Surg Gynecol Obstet 83, 107–116. [PubMed] [Google Scholar]
- Defrate LE, van der Ven A, Boyer PJ, et al. (2006) The measurement of the variation in the surface strains of Achilles tendon grafts using imaging techniques. J Biomech 39, 399–405. [DOI] [PubMed] [Google Scholar]
- Edama M, Kubo M, Onishi H, et al. (2015) The twisted structure of the human Achilles tendon. Scand J Med Sci Sports 25, e497–e503. [DOI] [PubMed] [Google Scholar]
- Finni T, Hodgson JA, Lai AM, et al. (2003) Nonuniform strain of human soleus aponeurosis‐tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol (1985) 95, 829–837. [DOI] [PubMed] [Google Scholar]
- Irwin TA (2010) Current concepts review: insertional achilles tendinopathy. Foot Ankle Int 31, 933–939. [DOI] [PubMed] [Google Scholar]
- Kachlik D, Baca V, Cepelik M, et al. (2008) Clinical anatomy of the calcaneal tuberosity. Ann Anat 190, 284–291. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Richey JM, Wissman LR, et al. (2010) The variability of the Achilles tendon insertion: a cadaveric examination. J Foot Ankle Surg 49, 417–420. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Martin E, Ballehr L, et al. (2011) Variability of insertion of the Achilles tendon on the calcaneus: an MRI study of younger subjects. J Foot Ankle Surg 50, 41–43. [DOI] [PubMed] [Google Scholar]
- Kinugasa R, Shin D, Yamauchi J, et al. (2008) Phase‐contrast MRI reveals mechanical behavior of superficial and deep aponeuroses in human medial gastrocnemius during isometric contraction. J Appl Physiol (1985) 105, 1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsgaard M, Aagaard P, Kjaer M, et al. (2005) Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol (1985) 99, 1965–1971. [DOI] [PubMed] [Google Scholar]
- Lersch C, Grotsch A, Segesser B, et al. (2012) Influence of calcaneus angle and muscle forces on strain distribution in the human Achilles tendon. Clin Biomech (Bristol, Avon) 27, 955–961. [DOI] [PubMed] [Google Scholar]
- Lyman J, Weinhold PS, Almekinders LC (2004) Strain behavior of the distal achilles tendon: implications for insertional achilles tendinopathy. Am J Sports Med 32, 457–461. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Kjaer M (2003) Region‐specific differences in Achilles tendon cross‐sectional area in runners and non‐runners. Eur J Appl Physiol 90, 549–553. [DOI] [PubMed] [Google Scholar]
- Roche AJ, Calder JD (2013) Achilles tendinopathy: a review of the current concepts of treatment. Bone Joint J 95‐b, 1299–1307. [DOI] [PubMed] [Google Scholar]
- Saltzman CL, Tearse DS (1998) Achilles tendon injuries. J Am Acad Orthop Surg 6, 316–325. [DOI] [PubMed] [Google Scholar]
- Szaro P, Witkowski G, Smigielski R, et al. (2009) Fascicles of the adult human Achilles tendon – an anatomical study. Ann Anat 191, 586–593. [DOI] [PubMed] [Google Scholar]
- Wren TA, Lindsey DP, Beaupre GS, et al. (2003) Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng 31, 710–717. [DOI] [PubMed] [Google Scholar]


