Abstract
A normal feature of the facial anatomy of many species of bat is the presence of bony discontinuities or clefts, which bear a remarkable similarity to orofacial clefts that occur in humans as a congenital pathology. These clefts occur in two forms: a midline cleft between the two premaxillae (analogous to the rare midline craniofacial clefts in humans) and bilateral paramedian clefts between the premaxilla and the maxillae (analogous to the typical cleft lip and palate in humans). Here, we describe the distribution of orofacial clefting across major bat clades, exploring the relationship of the different patterns of clefting to feeding mode, development of the vomeronasal organ, development of the nasolacrimal duct and mode of emission of the echolocation call in different bat groups. We also present the results of detailed radiographic and soft tissue dissections of representative examples of the two types of cleft. The midline cleft has arisen independently multiple times in bat phylogeny, whereas the paramedian cleft has arisen once and is a synapomorphy uniting the Rhinolophidae and Hipposideridae. In all cases examined, the bony cleft is filled in by a robust fibrous membrane, continuous with the periosteum of the margins of the cleft. In the paramedian clefts, this membrane splits to enclose the premaxilla but forms a loose fold laterally between the premaxilla and maxilla, allowing the premaxilla and nose‐leaf to pivot dorsoventrally in the sagittal plane under the action of facial muscles attached to the nasal cartilages. It is possible that this is a specific adaptation for echolocation and/or aerial insectivory. Given the shared embryological location of orofacial clefts in bats and humans, it is likely that aspects of the developmental control networks that produce cleft lip and palate in humans may also be implicated in the formation of these clefts as a normal feature in some bats. A better understanding of craniofacial development in bats with and without clefts may therefore suggest avenues for research into abnormal craniofacial development in humans.
Keywords: cleft palate, bats, premaxilla, comparative anatomy, echolocation
Introduction
Orofacial clefts occur as a craniofacial anomaly in humans in approximately 1 in 700 live births (Mossey et al. 2009; Dixon et al. 2011), with the incidence rate varying among populations (Mossey & Modell, 2012; McDonnell et al. 2014). The two common forms are cleft lip with or without cleft palate (CL/P) and isolated cleft palate (CP), both of which arise as a failure of fusion of neural crest‐derived processes during the development of the face. In normal development, the maxillary processes of the first pharyngeal arch fuse with the medial nasal processes of the frontonasal process of trigeminal neural crest between 5 and 6 weeks of gestation to form the upper lip, the nostril floor, the primary palate and the rostral part of the upper jaw (Cox, 2004; Kuratani, 2005; Depew & Simpson, 2006; Jiang et al. 2006; Dixon et al. 2011). Caudal to this, the palatal shelves of the maxillary processes fuse along the midline between 6 and 12 weeks’ gestation to form the secondary palate (Ferguson, 1988; Bush & Jiang, 2012). Defects in fusion of the palatal shelves result in varying degrees of isolated cleft palate (CP). Defects in fusion of the medial nasal process with the maxillary process produce varying degrees of clefting of the lip and primary palate. This may be associated with defects in fusion of the palatal shelves, producing cleft lip with or without cleft palate (CL/P) (Kernahan & Stark, 1958; Mossey et al. 2009; Dixon et al. 2011). Whereas the cleft of the secondary palate occurs along the midline, the common form of cleft of the lip and primary palate is paramedian and may be either unilateral or bilateral. These three fusion lines or potential clefts trifurcate from the incisive foramen at the junction of the primary and secondary palates (Kernahan & Stark, 1958; Depew & Simpson, 2006; Dixon et al. 2011). A much rarer form of anterior midline cleft of the lip and anterior palate is due to failure of the paired medial nasal processes to fuse at the midline and this type of cleft can continue caudally across the incisive foramen into a cleft of the secondary palate (Tessier, 1976; Allam et al. 2011). Although other types of rare craniofacial clefts have also been described, these do not have a clear origin in failure of fusion of the various facial processes (Tessier, 1976).
The rostral part of the upper jaw thus comes to be formed by paired premaxillary bones (derived from the medial nasal processes), wedged between the maxillae on either side (derived from the maxillary processes). These bones ossify in membrane rather than from cartilage precursors and the pattern of formation of the upper jaw is generally conserved across Amniota (Kuratani, 2005; Richman et al. 2006; Abramyan & Richman, 2015). The molecular signalling pathways underlying development of this region are similarly conserved and best characterised are the Bmp (bone morphogenetic protein), Fgf (fibroblast growth factors), Shh (sonic hedgehog) and Wnt (wingless/integrated) protein pathways (Creuzet et al. 2005; Gritli‐Linde, 2006; Jiang et al. 2006; Richman et al. 2006). Expression of these growth factors is dependent upon positional signals from the developing brain and foregut and nested patterns of expression of homeobox transcription factors such as Dlx and Msx (Creuzet et al. 2005; Depew & Simpson, 2006).
With such shared, conserved mechanisms of facial ontogeny, it is perhaps not surprising that orofacial clefts similar to those in humans have been described in several mammal species, including domestic dogs (Calnan, 1961; Bleicher et al. 1965; Richtsmeier et al. 1994; Martínez‐Sanz et al. 2011), cattle (Shupe et al. 1968), and primates (Kraus & Garrett, 1968; Swindler & Merrill, 1971; Goldschmidt et al. 2010; Krief et al. 2015). However, most non‐human examples are associated with other congenital cranial malformations and have only rarely been observed in the wild (Krief et al. 2015). As an experimental model of craniofacial development, the mouse has been extensively studied in relation to induced clefts and has been invaluable in unravelling the molecular mechanisms controlling facial development (Gritli‐Linde, 2008, 2012; Juriloff & Harris, 2008; Kousa & Schutte, 2016). However, the majority of mouse clefting models are also associated with widespread abnormalities of craniofacial development. In contrast, the majority of children born with CL/P are otherwise normal, healthy individuals (Mossey et al. 2009; Dixon et al. 2011), suggesting that the perturbations of the gene regulatory networks underlying the cleft may be more subtle than those seen in experimental animal models.
Bats (Chiroptera), in addition to their well‐known adaptations for flight and echolocation, display a remarkable variation in craniofacial form and many species have, as a normal feature of their facial anatomy, bony discontinuities or clefts in the rostral part of the upper jaw and midface. The general mammalian condition is for the premaxilla to be firmly attached to the maxilla by a sutural joint, which ossifies to varying degrees across taxa during ontogeny (Ashley‐Montagu, 1935; Barteczko & Jacob, 2004). In contrast, bats exhibit considerable variation in both morphology and degree of attachment of the premaxilla (Giannini & Simmons, 2007). Reduction in size and development of the premaxilla is a consistent feature among bats, and Wible & Novacek (1988) considered this as one of six cranial synapomorphies defining Chiroptera. The degree to which this reduction affects the alveolar, nasal and palatal processes of the premaxilla is characteristic of individual genera and species (Miller, 1907; Wible & Novacek, 1988; Giannini & Simmons, 2007), and the degree of attachment to articulating bones of the face is similarly variable. In some genera there is a tendency for the articulation with the maxilla to be loose, with ligamentous attachments between the bones replacing a sutural joint, whereas other groups show a tendency for fusion (Koopman, 1984; Hutcheon & Kirsch, 2006; Giannini & Simmons, 2007).
The adaptive significance of the morphological variation in the chiropteran premaxilla is currently not understood, with many potential, non‐mutually exclusive, functional and adaptive arguments having been explored. Shortening of the face through reduction of the premaxilla, combined with anterior orientation of the pinnae, could possibly reduce interference with returning echolocation signals. Additionally, a mobile premaxilla could contribute to modulation of nasal acoustic emissions, perhaps related to the function of nose‐leaves, elaborate expansions of the external nasal cartilages that are present in some species of bats and are thought to help focus and direct a nasally emitted echolocation call (Göbbel, 2000, 2002a; Pedersen & Müller, 2013). Alternatively, a mobile premaxilla could allow for increased oral gape, which could then facilitate capture of large prey (Simmons & Geisler, 1998). Reduction of the facial skeleton could reduce overall weight, and therefore the energetic demands of flight (Simmons & Geisler, 1998; Hutcheon & Kirsch, 2006). Reductions in the skeleton of the nasal floor and anterior nasal chamber may also correlate with the variations reported in the nasal part of the lacrimal‐conducting apparatus (Göbbel, 2002b). Finally, because the premaxilla contributes to the anterior floor of the nasal cavity, it is possible that morphological variation is correlated with olfactory adaptation. Specifically, the vomeronasal organ, which forms part of the accessory olfactory system in tetrapods, is highly variable across bats, with widespread loss of expression among groups at both morphological (Wible & Bhatnagar, 1996; Bhatnagar & Meisami, 1998) and genetic levels (Zhao et al. 2011; Hayden et al. 2014). Although olfactory expression has been correlated with feeding mode (Hayden et al. 2014), its relationship to premaxillary anatomy has not been studied.
The variation in the chiropteran premaxilla corresponds to patterns of orofacial clefting along presumed embryological fusion planes (Fig. 1). These occur in two broad patterns. The first is a bilateral paramedian skeletal cleft between the premaxilla and maxilla on either side, analogous to the skeletal component of a bilateral cleft lip and primary palate (Kernahan & Stark, 1958) (Figs 1C and 2B). The second is a midline cleft between the two opposing premaxillary elements, analogous to the skeletal component of the rare midline cleft (Tessier, 1976; Allam et al. 2011) (Figs 1B and 2A). In contrast to what is observed in humans, in bats neither clefting pattern extends further posteriorly than the incisive foramen, and thus is limited to a cleft of the alveolus and anterior (primary) palate.
Figure 1.
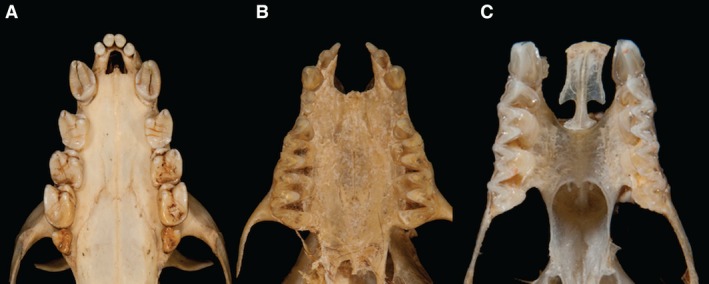
Patterns of variation in the chiropteran premaxilla. (A) Acerodon jubatus (Pteropodidae). The anterior dento‐alveolar arch is intact with no bony cleft. The bony margins of the incisive foramen are reduced, resulting in an enlarged incisive foramen. (B) Myotis myotis (Vespertilionidae). The anterior dento‐alveolar arch is interrupted by a U‐shaped, midline bony cleft between the two premaxillae. Each premaxilla bears incisors and is fused laterally to the maxilla. The cleft extends posteriorly to the anterior margin of the palatal shelves of the maxillae and is continuous with the incisive foramina. (C) Rhinolophus ferrumequinum (Rhinolophidae). The anterior dento‐alveolar arch is interrupted by bilateral paramedian clefts between the premaxillae (which articulate across the midline via a sutural joint and bear diminutive incisors) and the maxillae. The clefts extend posteriorly to the anterior margin of the palatal shelves of the maxillae and are continuous with the incisive foramina. (Photographs: Phil Myers, Museum of Zoology, University of Michigan‐Ann Arbor, www.animaldiversity.org. Creative Commons Attribution‐Noncommercial‐Share Alike 3.0 Unported License).
Figure 2.
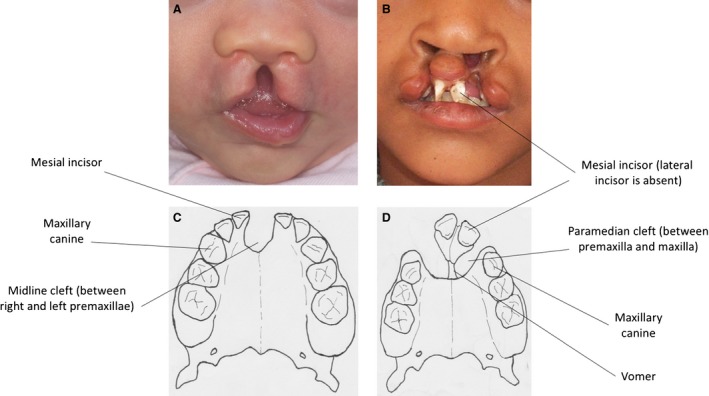
(A,C) Midline cleft lip and alveolus with intact secondary palate in a child. The bony cleft is between the two premaxillae in the midline. This is a very rare form of orofacial clefting in humans. Compare with Fig. 1B. (B,D) Bilateral cleft lip and alveolus with intact secondary palate in a child. The bony cleft in the anterior dento‐alveolar arch is between the premaxilla medially and the maxilla laterally. This is a bilateral form of the most common type of orofacial clefting in humans. Compare with Fig. 1C.
Here, we describe the phylogenetic distribution of anterior orofacial clefting in bats, mapping its occurrence across different species and families of bats. We present the results of detailed radiographic and soft tissue dissections of representative examples of the two types of cleft, and explore the relationship of the different patterns of clefting to feeding mode, development of the vomeronasal organ and lacrimal‐conducting apparatus, and mode of emission of the echolocation call and occurrence of nose‐leaves in different bat groups in an attempt to understand the adaptive significance of these highly unusual morphologies. Further, we suggest that if these bony discontinuities are best understood as a form of clefting, analogous to human congenital pathologies, then investigations into the facial developmental gene regulatory networks that have produced them over evolutionary time may shed light on changes resulting in non‐syndromic clefts in healthy children.
Materials and methods
Survey of the patterns of bony clefting of the mid facial skeleton across Chiroptera
We characterised the bony anatomy of the rostral facial skeleton for 294 species of bat, documenting the morphology of the rostral portions of the oral and nasal cavities with respect to bony clefts (Fig. 3, Supporting Information Table S1). Midline and paramedian clefts were scored in two ways. First, we treated each clefting condition as a distinct character (Characters 1 and 2), allowing for the possibility of both types of cleft to be present in one species. Because there was no observed overlap, we also treated clefting as a single, three‐state unordered character (Character 3). We only considered complete clefts (complete bony discontinuities across the anterior alveolar arch extending into the nasal cavity). We did not score indentations or irregularities in the bony margin as ‘partial’ or ‘incipient’ clefts.
Figure 3.
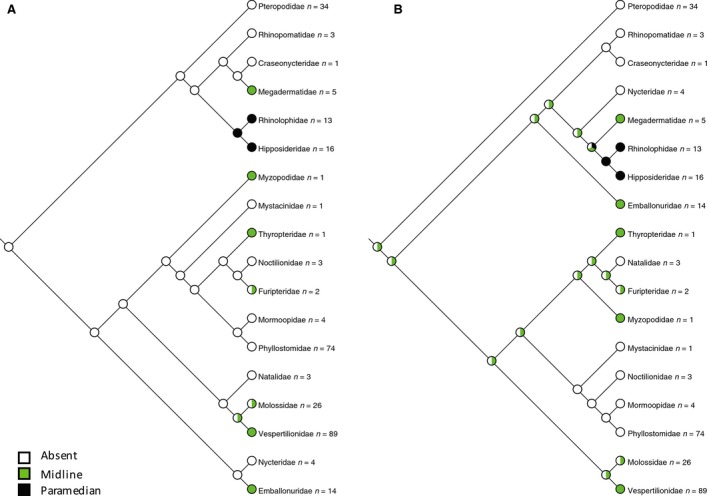
Distribution of orofacial bony clefting mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). Paramedian clefting has arisen once in the clade comprising Rhinolophidae and Hipposideridae. Midline clefting has arisen in seven different families of bats. The ancestral state reconstructs as no clefting in (A) and equivocal for either midline cleft or no cleft in (B).
Midline bony cleft: 0 = absent, 1 = present
Paramedian bony cleft: 0 = absent, 1 = present
-
Oronasal bony cleft: 0 = clefting absent, 1 = midline cleft present, 2 = paramedian cleft present
The 294 species of bats were assembled into two synthetic cladograms. The first was based on the molecular phylogeny of Teeling et al. (2005) with additional phylogenetic detail from recent phylogenetic analyses (Eick et al. 2005; Almeida et al. 2014; Foley et al. 2015). Species not included in molecular phylogenies were incorporated into the cladogram based on family and genus relationships (Jablonski & Finarelli, 2009a,b). The second cladogram was compiled from alternative morphological phylogenies of fossil and extant bats (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). We optimised clefting characters onto both cladograms (Fig. 3) in Mesquite (Maddison & Maddison, 2015).
We coded further characters for taxa where information was available:
-
Mode of emission of laryngeal echolocation signal: 0 = no laryngeal echolocation emission, 1 = oral emission, 2 = nasal emission
Most bat species use echolocation for navigation and foraging, producing an echolocation signal in the larynx, which is emitted through either the mouth or the nose. We used data compiled by Pedersen (1998) and Goudy‐Trainor & Freeman (2002) to classify echolocating species as oral or nasal emitters (Fig. 4). This distinction is not absolute, as some nasal‐emitting bats may emit orally on occasion. Here we use this classification to refer to the usual mode of emission, which correlates with the morphology of the skull base as defined by Pedersen (1998). In oral‐emitting bats the rostrum is rotated dorsally so that the mouth is orientated along the long axis of the head during flight. In nasal‐emitting bats, the skull base retains the ventrally flexed condition of early fetal development so that the nostrils and nose‐leaves are orientated along the long axis of the head in flight (Pedersen, 1998; Pedersen & Müller, 2013). Pteropodidae (Old World fruit bats) do not use laryngeal echolocation, although some species in the genus Rousettus use a form of echolocation based upon orally emitted tongue clicks (Jones & Teeling, 2006). For this analysis we have used only species for which species‐level information was available (Pedersen, 1998; Goudy‐Trainor & Freeman, 2002) but have given Pedersen's family‐level classification in Supporting Information Table S2.
Figure 4.
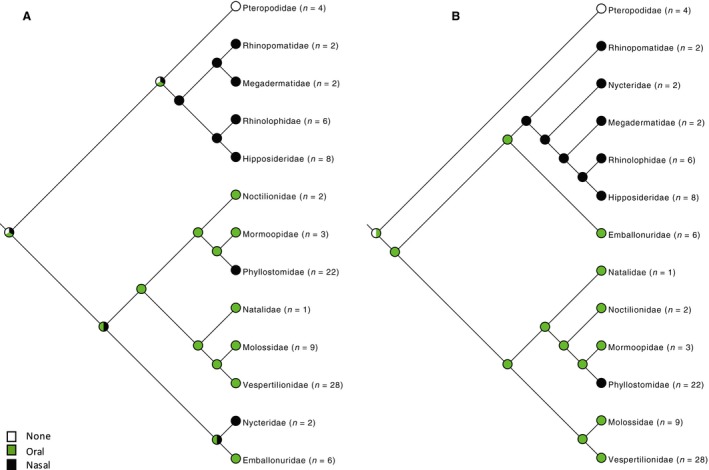 Distribution of mode of emission of laryngeal echolocation signal mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). The ancestral state is equivocal on either tree. All bats with paramedian clefts are nasal emitters, as are two clades with midline cleft and two clades with no cleft. Nasal emission coincides with occurrence of a nose‐leaf (see Fig. 5).
Distribution of mode of emission of laryngeal echolocation signal mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). The ancestral state is equivocal on either tree. All bats with paramedian clefts are nasal emitters, as are two clades with midline cleft and two clades with no cleft. Nasal emission coincides with occurrence of a nose‐leaf (see Fig. 5). -
Presence of nose‐leaf: 0 = no nose‐leaf, 1 = nose‐leaf
The nose‐leaf is considered to be an adaptation to allow focusing of the echolocation call in nasal emitting bats (Pedersen & Müller, 2013). Although there is a wide diversity of nose‐leaf forms, we have simply considered the presence or absence of a nose‐leaf as broadly defined (Göbbel, 2000, 2002a) (Fig. 5).
Figure 5.
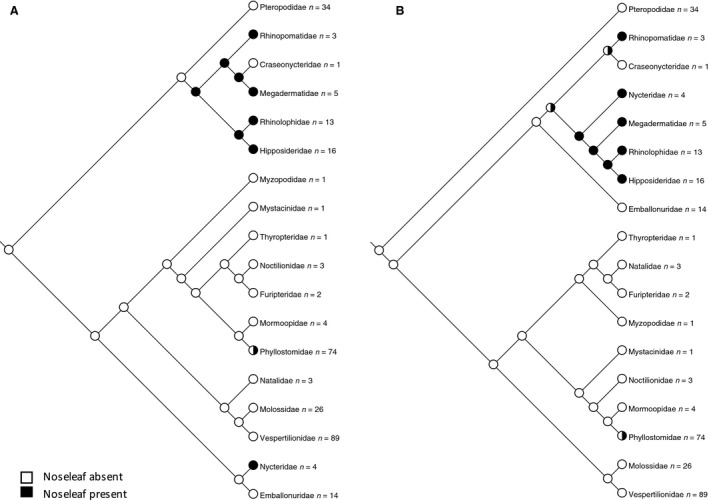
-
Principal feeding mode: 0 = animalivory, 1 = plant visiting, 2 = fruit specialist.
In assigning the principal feeding mode, we followed the classification used by Hayden et al. (2014) (Fig. 6). Animalivory includes insectivory (which constitutes the majority of bats and is most likely the ancestral state (Simmons & Geisler, 1998) as well as carnivory, piscivory, and sanguivory. Plant visiting bats are those that specialise in feeding on pollen or nectar (although they may also take insects present on the visited plant). Fruit specialists are dedicated fruit eaters found among the Pteropodidae and Phyllostomidae (Hayden et al. 2014).
Figure 6.
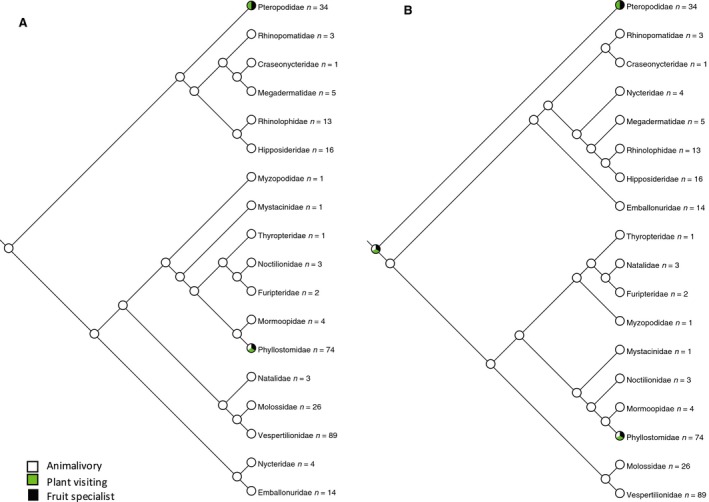 Phylogenetic distribution of principal feeding mode mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). The ancestral state reconstructs as animalivory in (A) with plant visiting and fruit specialism arising independently in Pteropodidae and Phyllostomidae. The ancestral state is equivocal in (B). There is no consistent relationship between orofacial clefting and feeding mode, although no fruit specialists have clefts and all bats with clefts are animalivores.
Phylogenetic distribution of principal feeding mode mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). The ancestral state reconstructs as animalivory in (A) with plant visiting and fruit specialism arising independently in Pteropodidae and Phyllostomidae. The ancestral state is equivocal in (B). There is no consistent relationship between orofacial clefting and feeding mode, although no fruit specialists have clefts and all bats with clefts are animalivores. -
Vomeronasal organ (Wible & Bhatnagar, 1996; Bhatnagar & Meisami, 1998; Hayden et al. 2014): 0 = present, 1 = rudimentary or absent
The vomeronasal organ, an accessory olfactory organ found in the nasal cavity of most tetrapods, is known to be highly variable in its expression in bats (Cooper & Bhatnagar, 1976; Wible & Bhatnagar, 1996; Bhatnagar & Meisami, 1998; Hayden et al. 2014). Wible & Bhatnagar (1996) recognised three character states for the vomeronasal organ: well developed, rudimentary or absent. They also analysed character states for the associated accessory olfactory bulb and the vomeronasal cartilage. However, Hayden et al. (2014) combined absent and rudimentary into a single category, which we follow here (Fig. 7).
Figure 7.
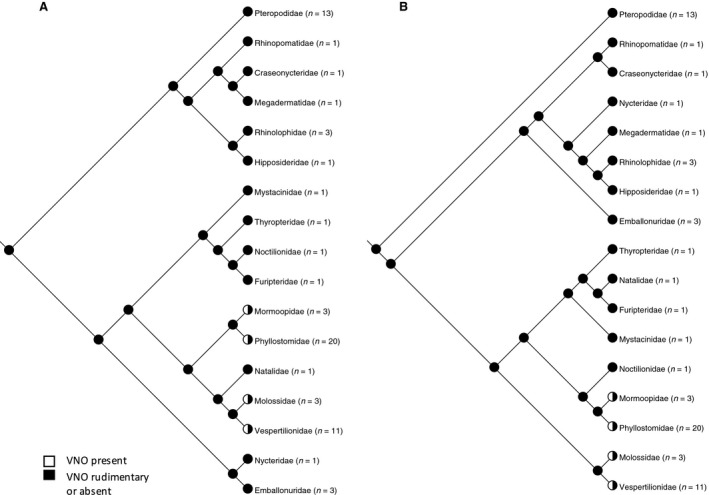 Phylogenetic distribution of development of the vomeronasal organ mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). The ancestral state reconstructs as an absent or rudimentary vomeronasal organ on either tree. A functional vomeronasal organ has evolved in four different families of bats. There is no consistent relationship between orofacial clefting and the degree of development of the vomeronasal organ.
Phylogenetic distribution of development of the vomeronasal organ mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). The ancestral state reconstructs as an absent or rudimentary vomeronasal organ on either tree. A functional vomeronasal organ has evolved in four different families of bats. There is no consistent relationship between orofacial clefting and the degree of development of the vomeronasal organ. -
Lacrimal‐conducting apparatus
The lacrimal‐conducting apparatus consists of the lacrimal canaliculi, the lacrimal sacs and the nasolacrimal ducts (NLD), which drain secretions from the conjunctival space to the nasal cavity. Göbbel (2002b) investigated variations in the arrangement of the terminal intranasal portion of the lacrimal‐conducting apparatus in bats and its relation to the nasopalatine duct and vomeronasal organ complex. She defined five character states, which we follow here:
0 = NLD opens into the floor of the nostrils, 1 = NLD opens into the middle part of the inferior nasal meatus, 2 = NLD opens into a recess of the inferior nasal meatus closely associated with the opening of the nasopalatine duct, 3 = NLD opens into the nasopalatine duct, 4 = Rudimentary lacrimal‐conducting apparatus with cystic or absent NLD (Fig. 8). Complete character matrices are provided in Tables S1 and S2.
Figure 8.
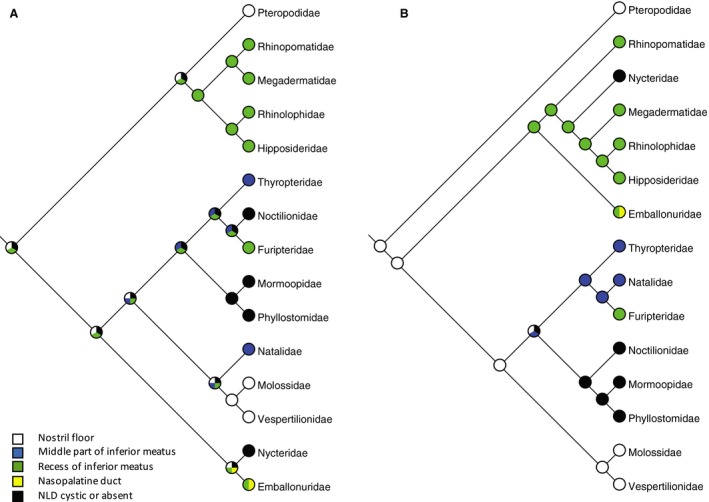 Phylogenetic distribution of development of the lacrimal‐conducting apparatus mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). There is no relationship with oronasal clefting (Fig. 3) or degree of development of the vomeronasal organ (Fig. 7). The ancestral state reconstructs as the duct terminating rostrally in the nostril floor (the general mammalian condition) in (B), but is equivocal in (A).
Phylogenetic distribution of development of the lacrimal‐conducting apparatus mapped to (A) molecular phylogeny (Teeling et al. 2005) and (B) morphological phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005). There is no relationship with oronasal clefting (Fig. 3) or degree of development of the vomeronasal organ (Fig. 7). The ancestral state reconstructs as the duct terminating rostrally in the nostril floor (the general mammalian condition) in (B), but is equivocal in (A).
Three‐dimensional reconstruction of computerised tomographic radiographs of the skull
Complete alcohol‐preserved specimens for two species of bat representing an expected midline cleft (Plecotus auritus) and an expected paramedian cleft (Rhinolophus euryale) were subjected to micro‐computed tomography (μCT) in a small animal μCT scanner (CT120, Trifoil Imaging, Chatsworth, CA, USA) to describe the detailed three‐dimensional bony anatomy of the anterior oral and nasal cavities and associated clefts (see below for details of samples). Micro‐CT image acquisition consisted of 1200 projections taken at 0.3° increments in one full rotation. X‐ray tube settings were 80 kV and 32 μA. The raw data were reconstructed using microview aba software, version 2.4 (General Electric) to create a final image with 25‐μm voxel dimensions. Images were converted to DICOM format before final visualisation with ctvox software, version 2.7.0 (Bruker μCT).
Soft tissue dissections of the palatal regions of three bat species
Although soft tissue clefting can occur in the absence of bony clefts, clefting of the bony palate is invariably associated with soft tissue clefting in humans (Kernahan & Stark, 1958; Malek, 2001). To determine whether the bony clefts observed in bats extended to the associated soft tissues, dissection of the soft tissues in the region of the clefts was performed on three species of bat. Myotis blythii (the Lesser mouse‐eared bat; specimens SP.C.47 and SP.C.48, both juvenile females, Karaftu Cave, Iran [36.3N 46.9E]) and Plecotus auritus (the Brown long‐eared bat, Glendalough, Ireland [53.0N 6.3W]) possess midline clefts, and Rhinolophus euryale (the Mediterranean horseshoe bat; specimen SP.C.57, adult female, Guvenli Water Reservoir, Turkey [37.6N 30.5E]) displays the paramedian cleft condition. Dissections focused on the relationships of the soft tissue anatomy of the hard and soft palate, the roof of the oral cavity and the floor of the anterior nasal cavity with respect to the bony anatomy of the premaxilla. All specimens died of natural causes, and were freshly preserved in ethanol. Dissections were performed under a Nikon SMZ660 dissecting microscope, with photographs taken using a Leica DFC 490 inline digital camera attached to a Leica M165 FC dissecting microscope. See Table S3 for details of specimens dissected.
Results
Anatomy of orofacial clefting in bats
3D μCT scans of bat cranial skeletons
3D reconstructions based on μCT scans permitted detailed visualization of the bony palate. Plecotus auritus (Fig. 9) displays a midline bony cleft. The premaxillae are highly reduced and firmly sutured to the maxillae laterally, with separation along the midline. Two permanent incisors are present in each premaxilla. These are large and pointed, in contrast to the three lower incisors in each half of the mandible, which are spatulate. The bony cleft is bounded posteriorly by the free anterior margin of the palatal processes of the maxilla. The bony nasal septum (vomer) extends anteriorly only to the level of the margin of the maxillary palate.
Figure 9.
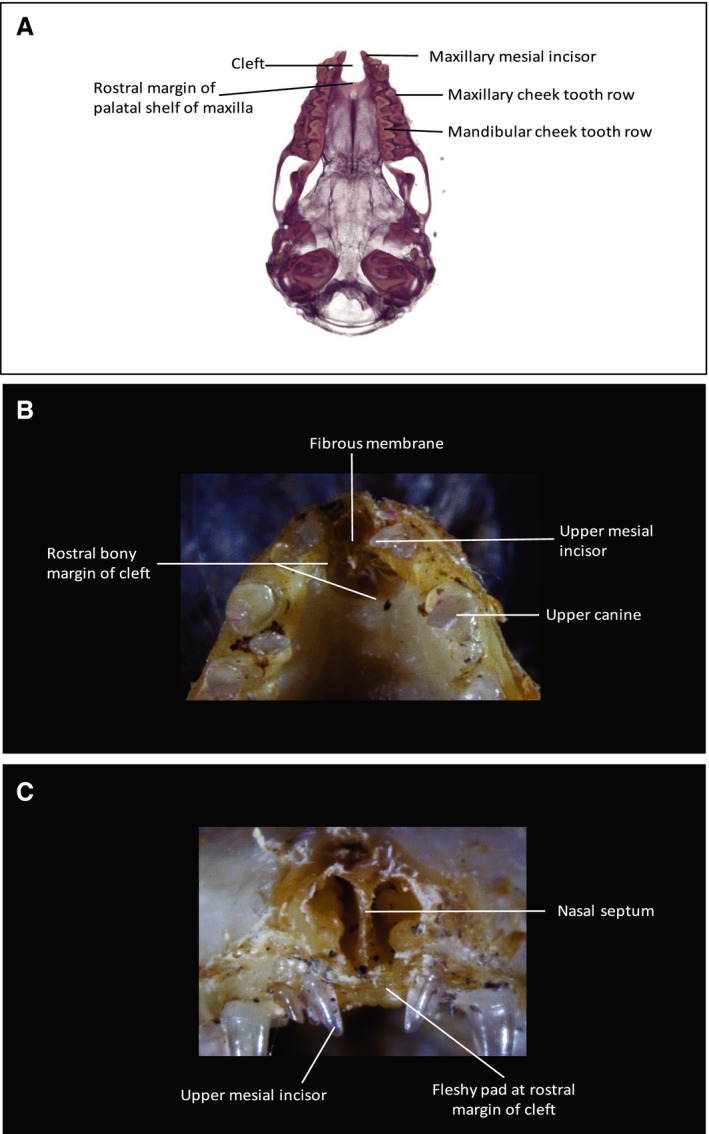
Cranial anatomy of Plecotus auritus. (A) 3D μCT inferior view of skull base cranial skeleton. The body of the mandible has been digitally subtracted to show the palate (the cusps of the mandibular teeth are visible occluding medial to the maxillary dental row). A U‐shaped bony cleft is present between the two premaxillae. Each premaxilla, bearing two conical incisors mesial to the large and pointed maxillary canine, is fused to the maxilla. The cleft is bounded posteriorly by the anterior free bony margin of the palatal shelves. (B) Inferior view of dissection specimen to show anterior palate. (C) Anterior view of dissection specimen to show facial rostrum. A robust fibrous membrane, continuous with the periosteum at the margins, fills in the U‐shaped cleft visible in (A). The anterior free margin of this membrane terminates as a fibrous condensation. The nasal septum (visible in C) attaches to this membrane and the fibrous condensation in the midline.
Rhinolophus euryale (Fig. 10) displays a bilateral paramedian cleft. The premaxillae are separated from the maxillae laterally, and the cleft interrupts the dentoalveolar arch between the diminutive, single premaxillary incisor and the maxillary canine. Right and left premaxillae are not fused along the midline, but rather are joined by a fibrous, interpremaxillary suture. The premaxillae extend posteriorly to the midline junction of the palatal processes of the maxilla with the premaxilla. Anterior to this confluence, the premaxilla is narrowed by a deeply indented notch for the incisive canal. The vomer extends to the anterior margin of the maxillary palate and, in lateral view (Fig. 10a) the premaxillae appear to float freely, at a level dorsal to the plane of dental occlusion. There is a fibrous joint between the posterior margin of the premaxillae and the anterior margin of the maxillae. The entire premaxilla is situated anterior to the bony margin of the nasal cavity.
Figure 10.
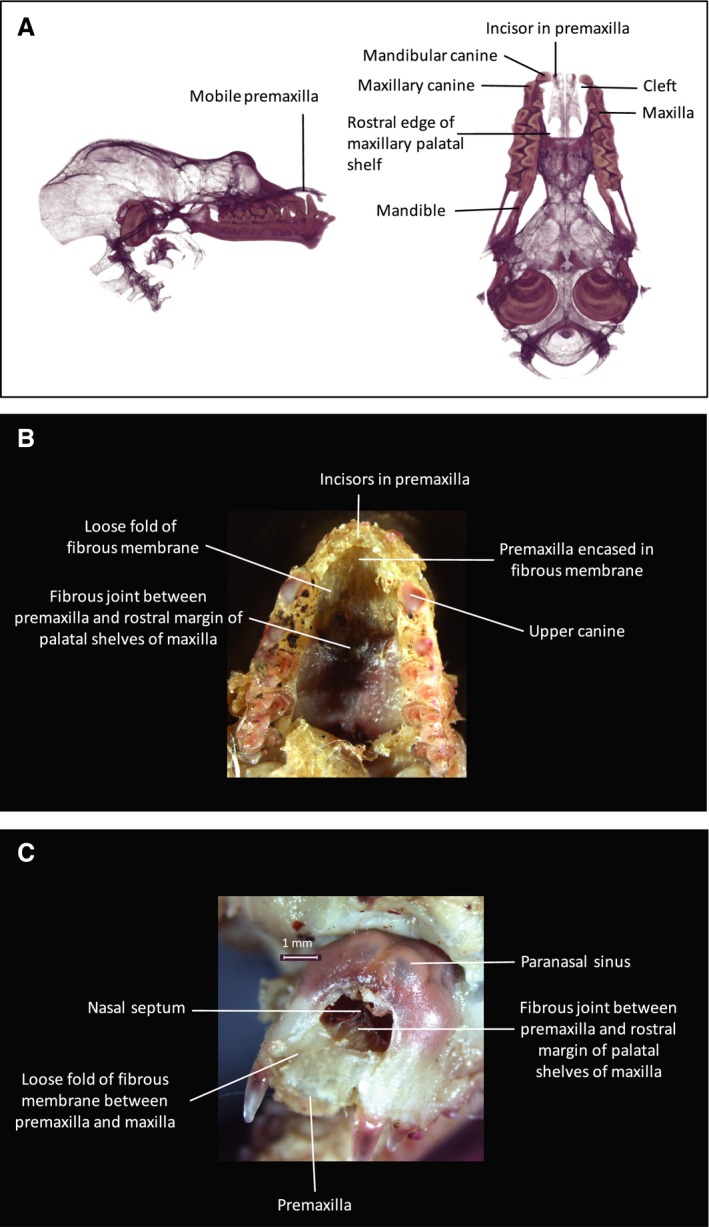
Cranial anatomy of Rhinolophus euryale. (A) 3D μCT inferior and lateral views of cranial skeleton with body of mandible digitally subtracted. Cusps of mandibular teeth are seen occluding medial to the maxillary dental arch. Bilateral paramedian bony clefts are present in the dento‐alveolar arch between the premaxilla and the maxilla. The clefts extend posteriorly to the anterior margin of the maxillary palatal shelves, to which the posterior extremities of the premaxillae are attached via a midline sutural joint. The notched posterolateral margin of the premaxilla represents the site of the incisive foramen on either side. The premaxillae are united in the midline by an unfused sutural joint. The premaxilla is seen to float superior to the occlusal plane, hinging on the midline articulation with the anterior margin of the maxillary palatine shelves. (B) Inferior view of dissection specimen to show anterior palate. (C) Anterior view of dissection specimen to show facial rostrum. The bony cleft is completely filled in by a robust, fibrous membrane that is continuous with the periosteum of the maxillary margins of the cleft and splits to enclose the premaxillae. The membrane is loose in the space between the maxilla and the premaxilla, allowing the premaxilla to move freely in a sagittal plane as it hinges about its posterior sutural joint with the palatal shelf of the maxilla. The nose‐leaf cartilages are attached to the superior surface of the premaxillae and are open superiorly. Thus the mobile premaxilla forms a mobile base to the nose‐leaf, anterior to the nasal cavity. Bullae of the maxillary paranasal sinuses are visible in (C), superior to the anterior bony margin of the nasal cavity.
Soft tissue and bony anatomy of the anterior palate and nasal floor
Two species with midline clefts (Myotis blythii and Plecotus auritus) and one species with a paramedian cleft (Rhinolophus euryale) were dissected. The external appearance of the hard palate is similar for all three species (Supporting Information Figs S1, S2 and S3). The mucoperiosteum adheres to the underlying skeletal structures, presenting bilaterally symmetrical rows of horizontal rugae. This mucosal layer becomes continuous anteriorly with the buccal sulcus of the upper lip. There is no visible clefting in the soft tissues of the roof of the mouth or floor of the nose that corresponds with underlying bony clefts. In all species, the rostral end of the palatal mucosa presents a fleshy pad completing the anterior arch. In Rhinolophus (paramedian condition) this pad overlies the body of the premaxilla and the diminutive incisors erupt anterior to the pad. All species show some degree of dorso‐ventral mobility for this anterior segment when manipulated, although this is notably more pronounced in Rhinolophus.
When the mucosa is removed from the oral surface of the hard palate, the bony palatal shelves of the maxillary bones are visualized. In all three species the palatal bony shelves terminate rostrally in a U‐shaped free margin. In Myotis (Fig. 11) and Plecotus (Fig. 9) this forms a U‐shaped, midline bony cleft. This cleft, which appears empty of a skeletal structure in CT scans or prepared specimens, is filled with a robust, translucent, fibrous membrane, which is continuous with the periosteum of the bony palatal shelves. The rostral free margin of the membrane presents a fibrous condensation, which contributes to the mobile fleshy pad that completes the anterior dental arch. The dorsal surface of the fibrous membrane forms the rostral part of the floor of the nasal cavity. The cartilaginous nasal septum, continuing rostrally from the vomer, attaches along the midline to the fibrous palatal membrane and its rostral fibrous condensation, along with the lateral external nasal cartilages (Figs 9 and 11).
Figure 11.
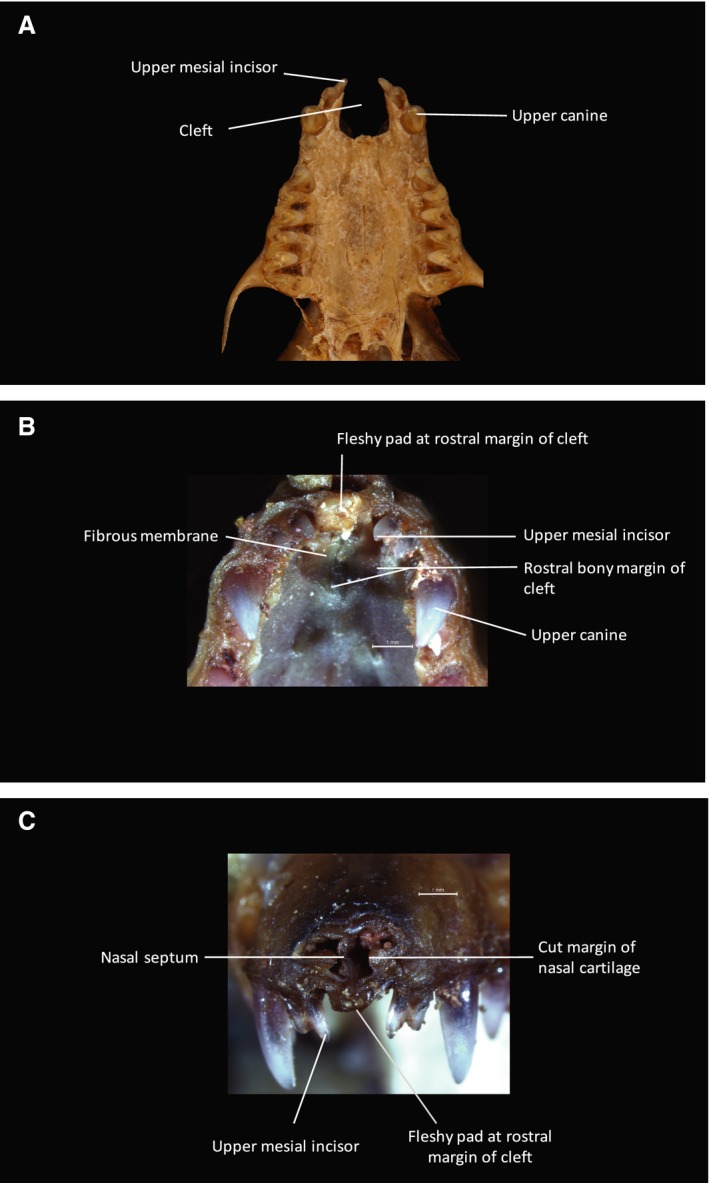
Cranial anatomy of Myotis. (A) Inferior view of cranial skeleton of Myotis myotis to show palate. A U‐shaped bony cleft is present between the two premaxillae. Each premaxilla, bearing two conical incisors mesial to the large and pointed maxillary canine, is fused to the maxilla. The cleft is bounded posteriorly by the anterior free bony margin of the palatal shelves. (B) Inferior view of dissection specimen (Myotis blythii) to show anterior palate. (C) Anterior view of dissection specimen to show facial rostrum. As in Plecotus (Fig. 9) a robust, fibrous membrane, continuous with the periosteum at the margins, fills in the U‐shaped cleft visible in (A). The anterior free margin of this membrane terminates as a fibrous condensation. The nasal septum (visible in C) attaches to this membrane and the fibrous condensation in the midline. (Photograph (A) Phil Myers, Museum of Zoology, University of Michigan‐Ann Arbor, www.animaldiversity.org. Creative Commons Attribution‐Noncommercial‐Share Alike 3.0 Unported License).
In Rhinolophus (Fig. 10) the same fibrous membrane is observed, although it encloses the premaxilla in the midline. In addition, the membrane forms a loose fold in the bony cleft between the premaxillae and the maxillae. This laxity allows the premaxilla to pivot in the sagittal plane about the mobile, midline fibrous joint formed between the caudal margin of the premaxillae and the rostral margin of the palatal shelves. Thus, despite the fragile appearance of the premaxilla in the μCT scan and in the prepared crania, it is enclosed in a tough fibrous membrane and is quite robust. Unlike the condition in Myotis and Plecotus, the dorsal surface of the fibrous membrane (and enclosed premaxillae) in Rhinolophus is rostral to the nasal cavity and forms a mobile floor for the elaborate nose‐leaf, formed by an expansion of the superior and lateral nasal cartilages and the expanded rostral margin of the nasal septal cartilage. Thus, the nose‐leaf, premaxilla and fibrous rostral palatal membrane form an anatomical unit that is mobile in a sagittal plane, hinging on the premaxillary‐maxillary fibrous joint. We observed no muscles attaching directly to the premaxilla, but the facial muscles do attach indirectly via the nasal and nose‐leaf cartilages and presumably provide the active movement seen in living specimens.
Assignment of oronasal bony cleft character state
Assignment of cleft character states was generally unambiguous, although there were a few cases where prepared specimens retained remnants of soft tissues obscuring underlying bony morphology. These were resolved by cross‐referencing with 3D CT images and reference to congeners. Character optimisation of clefting onto the molecular phylogeny of Teeling et al. (2005), treating the two types of cleft as either separate binary characters or a single multistate character resulted in an ancestral reconstruction of no clefting (Fig. 3A). Paramedian clefting arose once, in the Rhinolophoidea. In contrast, median clefting evolved in seven different bat families. No clefting is observed in any member of the Pteropodidae (Old World fruit bats). Mapping cleft characters to the morphology‐based phylogeny (Simmons & Geisler, 1998; Gunnell & Simmons, 2005) similarly shows a single origin for paramedian clefting with multiple occurrences of midline clefting, although the ancestral state is equivocal (either midline cleft or absence of cleft) (Fig. 3B).
Relationship of oronasal bony clefting to echolocation mode and nose‐leaf occurrence
In this dataset, the ancestral state for echolocation is equivocal on either tree (Fig. 4). There is no clear association between cleft state and emission of echolocation signal: although all bats with paramedian clefts are nasal emitters, nasal emitters with midline clefts (Megadermatidae and Rhinopomatidae) and no cleft (Nycteridae and Phyllostomidae) also exist. The six families in which nasal emission arose occurred on either three occasions (molecular tree) or two occasions (morphology‐based tree) independently. Nasal emission coincides more or less exactly with the presence of a nose‐leaf, as broadly defined, supporting the hypothesis that the nose‐leaf is a specific adaptation related to nasal emission of the echolocation call.
Relationship of oronasal bony clefting to principal feeding mode
Principal feeding mode was mapped onto the phylogenies to test for association with oronasal clefting (Fig. 6). The majority of bats are animalivorous (primarily insectivores) and this is reconstructed as the ancestral feeding mode. Plant visiting (feeding on nectar and pollen) and specialised fruit eating has arisen only in those clades lacking bony clefts (Pteropodidae and Phyllostomidae). All bats possessing oronasal bony clefts are animalivores, although there are several animalivorous families that lack bony clefts.
Relationship of oronasal bony clefting to development of the vomeronasal organ
For taxa in which information was available on the degree of development of the vomeronasal organ, Fig. 7 shows vomeronasal organ character state mapped onto the phylogeny. In this dataset, the ancestral state for bats reconstructs as an absent or rudimentary vomeronasal organ with evolution of a functional vomeronasal organ in four different families (Phyllostomidae, Mormoopidae, Miniopteridae and Molossidae). In most species in which either a median or paramedian cleft is present, the vomeronasal organ is absent or rudimentary. The exception is the two species of Miniopterus in which there is both a midline cleft and a functioning vomeronasal organ. However, absent or rudimentary vomeronasal organ is associated with both cleft types and no cleft.
Relationship of oronasal bony clefting to variations in the terminal portion of the nasolacrimal duct
Anatomical variations of the nasolacrimal duct as described by Göbbel (2002b) show no relationship with oronasal clefting or degree of development of the vomeronasal organ (Fig. 8). The ancestral state reconstructs as the duct terminating rostrally in the nostril floor (the general mammalian condition) on the morphology‐based tree but is equivocal on the molecular tree.
Discussion
Here, we draw an analogy between the conditions of the chiropteran premaxilla (Fig. 1) and the bony component of cleft lip and palate observed as a pathology in humans (Fig. 2). Paramedian clefts separate the premaxilla from the maxilla laterally, whereas midline clefts separate the right and left premaxillae along the midline. Clefting in the Chiroptera is confined to skeletal structures, with no evidence of a cleft in the overlying soft tissues. However, the clefts are easily characterised in prepared crania and μCT scans. Dissections reveal that bony clefts in the facial skeleton are filled in by a robust, translucent fibrous membrane that appears continuous with the periosteum of the maxillae (Figs 9, 10, 11). The oral surface of this membrane is covered by an otherwise typical horizontally rugose mucosa in continuity with the mucosa of the rest of the hard palate Figs S1, S2, and S3. In the two examined species with midline clefts, the free rostral border of the membrane presents a fibrous condensation contributing to a mobile fleshy pad that completes the edentulous portion of the anterior dental arch (Figs 9 and 11). In Rhinolophus (paramedian cleft) the fibrous membrane encloses the premaxilla and forms a loose fold laterally, providing a skeletal base for the nose‐leaf that is considerably mobile, pivoting in a sagittal plane about the midline maxillary‐premaxillary fibrous joint (Fig. 10). Movement of this complex is enacted by facial muscles inserting into the nose‐leaf.
Previous authors have described the marked variation of the premaxilla in Chiroptera (Miller, 1907; Koopman, 1984; Wible & Novacek, 1988; Simmons, 1994; Simmons & Geisler, 1998; Gunnell & Simmons, 2005; Giannini et al. 2006b; Hutcheon & Kirsch, 2006; Giannini & Simmons, 2007) and numerous morphological characters of potential phylogenetic importance have been drawn from this variation (Miller, 1907; Koopman, 1984; Wible & Novacek, 1988; Simmons & Geisler, 1998; Giannini & Simmons, 2007). However, these bony discontinuities have not been recognised as potentially analogous to pathological orofacial clefts in humans. Miller (1907) in discussing ‘Microchiroptera’ referred to the premaxilla as being present or absent and described reductions in the nasal or palatal processes and whether there is fusion and/or contact between the premaxillae and maxilla. However, he did not describe clefts or major bony discontinuities. Koopman (1984) divided microbats into ‘Yinochiroptera’ and ‘Yangochiroptera’ on the basis of premaxillary mobility (Hutcheon & Kirsch, 2006), although he was not specific about the anatomical basis behind this mobility. (In fact, Koopman referred to a ‘moveable maxilla’ (1984), although it is generally accepted that this was intended to describe the condition of the premaxilla). The most comprehensive account of the chiropteran premaxilla and its morphological variation was presented by Giannini & Simmons (2007), in which they describe 16 phylogenetic characters of the premaxilla and its various processes, foramina and articulations with adjacent bones. The midline cleft of this study coincides with states 0, 1, and 2 of Character 1 in Giannini & Simmons (2007), although they did not explicitly describe this in terms of clefting. Paramedian clefting, as considered here, could potentially be interpreted as state 2 of Character 9 in Simmons & Geisler (1998).
The distribution of clefting across bat phylogeny
Mapping the two different cleft characters onto either the molecular or morphological cladogram reveals two very different patterns (Fig. 3). The paramedian cleft is an unambiguous synapomorphy for a clade that includes Rhinolophidae and Hipposideridae, arising only once in the evolution of bats. Molecular dating estimates this branch split from the remainder of the Rhinolophoidea approximately 51 mya (Foley et al. 2015). In contrast, midline clefts have evolved convergently in several clades of bats. Some caution is required, as it may be argued that the midline clefts represent a heterogeneous complex of anatomies if details of the premaxillary anatomy are considered (Miller, 1907; Giannini & Simmons, 2007). In most cases of midline clefting the premaxillae are reduced, but in three genera of Megadermatidae (Lavia, Macroderma, and Megaderma), Giannini & Simmons (2007) consider the premaxilla to be entirely absent. However, a common feature across all of these taxa is the suppression bone and tooth formation along the midline of the rostral portion of the upper jaw.
Adaptive significance
The functional adaptive significance of these clefts is not clear, but a number of non‐mutually exclusive possibilities exist. It is possible that midline or paramedian clefting is an adaptation to aerial insectivory (that is, taking prey in flight), given that the forelimbs are constrained to be involved in flight and are therefore unavailable for food manipulation (Hutcheon & Kirsch, 2006). Moving the incisors to a lateral position or suppressing them entirely (as in a midline cleft) could lead to a wider effective gape, thus allowing easier capture of large insects. A similar advantage might be achieved by the sagittal mobility of the anterior palate and dental arch produced by the paramedian cleft – perhaps an instance of cranial kinesis in mammals (Frazzetta, 1966). All bats with either cleft type are animalivorous (Figs 3 and 6), although there are many species of animalivorous bats lacking clefts entirely. In addition, absence of a cleft is shared by both frugivorous clades. It is likely that a complete anterior dental arch supported in a solid bony substructure is important for biting into fruit and was therefore retained. Olfaction is also potentially important in frugivorous foraging and it is possible that optimal olfactory function is favoured in some way by a complete bony nasal cavity (although we find no clear relationship to the degree of development of the vomeronasal organ – see Figs 3 and 7). However, almost half of all insectivorous families lack clefts and it is therefore not essential to aerial insectivory.
The paramedian cleft only occurs in association with an elaborate nose‐leaf and potentially allows active movement/orientation of the nose‐leaf in the sagittal plane. It is possible that this is an adaptation to allow directional focussing of the echolocation call for nasal‐emitting species. Although all bats with a paramedian cleft are nasal emitters (Figs 3 and 4), such a cleft is clearly not a required adaptation for nasal emission, as three families of nasal emitting bats possess either no cleft or midline clefts. The clade of bats characterised by a paramedian cleft is principally insectivorous and nasal emitting (Figs 3, 4, and 6). In their review of the bat ‘guild’ concept, integrating foraging and echolocation modes, Denzinger & Schnitzler (2013) describe rhinolophids and hipposiderids as ‘narrow space, flutter‐detecting foragers,’ noting that this mode of foraging has only evolved one other time, in the mormoopid, Pteronotus parnellii (an oral emitting insectivore lacking a cleft). It seems likely that the paramedian cleft is a particular adaptation for this mode of echolocation and foraging ecology in Rhinolophidae and Hipposideridae. A relationship between midline clefting and echolocation is far less straightforward: bats with midline clefts may be oral or nasal emitters and nasal emission may be carried out by bats with no cleft, a midline cleft or a paramedian cleft (Figs 3 and 4).
Bats show considerable variation among taxa in the degree of development of both the vomeronasal organ (Wible & Bhatnagar, 1996; Bhatnagar & Meisami, 1998; Hayden et al. 2014) and the terminal part of the lacrimal‐conducting apparatus (Göbbel, 2002b). Because the anterior facial clefts occur in anatomical proximity to these structures, we considered the possibility that suppression of bony structures in this region might be related in either direction to suppression of the vomeronasal organ or the lacrimal‐conducting apparatus. However, the occurrence of anterior orofacial clefting appears quite independent of the degree of suppression of either structure (Figs 3, 7, and 8). On the other hand, the rostrum of rhinolophid and hipposiderid bats is a highly derived structure with large resonance chambers within the expanded nasal cavity (Pedersen, 1993, 1995, 1996, 1998). It has been argued (Pedersen, 1995, 1998; Santana & Lofgren, 2013) that these nasal domes constitute a novel mammalian cranial module (Goswami, 2006), whose evolution has been driven by the demands of nasal echolocation. As such, suppression of skeletal, vomeronasal, and lacrimal‐conducting structures in the nasal cavity may be a by‐product of this process (Pedersen & Müller, 2013), although Hayden et al. (2014) found no evidence of a trade‐off between olfaction and echolocation in their study of olfactory genes in bats.
It is also possible that the reduction of the bony portion of the premaxilla is part of a general strategy of reducing non‐essential bone weight as an adaptation to flight. However, the bone in this region is extremely thin and the amount of weight saved is likely minimal.
Ontogeny
Although the bony anatomy is similar, complete absence of any soft tissue evidence of cleft makes the conditions of the premaxilla observed in bats quite different to pathological cleft lip and palate. In human clefts there is always at least a minor degree of soft tissue cleft (lip or soft palate) if a bony cleft exists (Kernahan & Stark, 1958). Paramedian clefting in humans arises from failure of fusion of the embryological medial nasal process of the frontonasal process with the maxillary process, whereas midline clefting is due to failure of fusion of the paired medial nasal processes (Jiang et al. 2006). Published embryological series for Chiroptera have not concentrated in detail on the anatomy of the face, although bats show a typical mammalian pattern of craniofacial development, with no obvious failures of fusion of the medial nasal or maxillary processes (Cretekos et al. 2005; Giannini et al. 2006a; Tokita, 2006; Hockman et al. 2009; Nolte et al. 2009). Nevertheless, the bony discontinuities in bats bearing these clefts do occur at the junction of the medial nasal processes with each other or with the maxillary processes and they give rise to bony clefts that show striking similarity to the skeletal pathologies in failure of fusion in humans. It seems likely that at least some aspects of the developmental control networks behind bony clefts in bats and pathological clefts in humans are shared between the taxa.
The bones of the facial skeleton begin as sheets, or membranes, of undifferentiated mesenchyme derived from the neural crest and ossify directly in membrane (Richman et al. 2006). Migration and differentiation of cranial neural crest cells is under complex controls involving Shh, Wnt proteins, BMPs and FGFs, among others (Creuzet et al. 2005; Gritli‐Linde, 2006; Jiang et al. 2006). High levels of BMPs induce neural crest to differentiate into cartilage, but lower levels cause differentiation to osteoblast precursors expressing Runx2 transcription factor. Indian hedgehog induces these cells to mature as osteoblasts, secreting bone matrix within a periosteal membrane (Richman et al. 2006; Abzhanov et al. 2007). There is also evidence that differentiation of primordial skeletal tissues may be influenced by mechanical stimuli in the developing embryo, with shearing forces activating pathways that lead to bone formation and compressive forces leading to cartilage formation (Radlanski & Renz, 2006). In the dissected bat specimens, although bone formation in the cleft has been suppressed, there remains a tough, fibrous membrane topologically consistent with the position of the absent ossification of the premaxilla that we speculate is derived from the primordial osteogenic membrane. In the midline cleft this coincides with an arrest of medial migration of the incisor tooth buds, which instead form laterally. The presence of fibrous membranes that are continuous across the clefts would seem to indicate that the mechanism of formation in bats is not a failure of fusion of the medial nasal and maxillary processes, but a suppression of bone along the lines of fusion.
Conclusions
Orofacial bony discontinuities in bats represent a normally occurring morphology that is analogous to the pathological cleft condition in humans. Investigating these characters through detailed anatomical dissection and 3D μCT scanning, the anatomy has been clarified as a basis for better understanding the function and evolutionary significance of these unusual morphologies. The present work could lay the foundation for a naturally occurring model of orofacial clefting, serving to better illuminate our understanding of the causal basis and development of the condition in humans.
Although the mouse has proven to be a highly useful experimental model for understanding mammalian craniofacial development, models of orofacial clefting in the mouse generally involve mutations in genes coding for growth factors, receptors, transcription factors, and other molecules with large numbers of pleiotropic effects, the results of which are complex, and often lethal, accompanying abnormalities (Gritli‐Linde, 2008). The same appears to be true of the majority of reported instances of orofacial clefting in non‐human primates (Kraus & Garrett, 1968; Swindler & Merrill, 1971; Goldschmidt et al. 2010; Krief et al. 2015). In contrast, clefts in bats occur as a part of normal anatomy and are thus more likely to be determined by subtle changes in the timing and pattern of gene expression than by large‐scale mutations radically impacting important genes. An investigation into why some species of bats produce clefts and others do not may elucidate the causation of cleft lip and palate in otherwise normal and healthy children (Mossey et al. 2009; Dixon et al. 2011). If the bony discontinuities in bats serve as model of clefting in humans, understanding their developmental biology will be crucial. Some embryological series of bat species have been published (Pedersen, 1995, 1996; Cretekos et al. 2005; Giannini et al. 2006a; Tokita, 2006; Hockman et al. 2009; Nolte et al. 2009; Wang et al. 2010) but detailed research covering the critical stages of formation of the anterior face in bats with different cleft patterns has not been carried out.
Bioinformatic analysis may shed light on the molecular developmental controls of these cleft states by identifying variations between midline, paramedian, and non‐clefting bats in genes or gene regulatory regions (Uslu et al. 2014) known to be important in craniofacial development. A useful place to start might be with homologues of regions of the human genome that have been identified as quantitative trait loci for cleft lip and palate (Zucchero et al. 2004; Mangold et al. 2010; Dixon et al. 2011). Paramedian clefts are by far the more common variety in humans (Allam et al. 2011; Dixon et al. 2011). Their counterpart in bats has a single evolutionary origin, and likely a common developmental basis, implying that a rhinolophid or hipposiderid bat may be a suitable model organism (Wang et al. 2010).
Supporting information
Figure S1. Myotis blythii. View of inferior aspect of hard palate. Note horizontally rugose mucosa with no soft tissue cleft.
Figure S2. Plecotus auritus. View of inferior aspect of hard palate. Note horizontally rugose mucosa with no soft tissue cleft.
Figure S3. Rhinolophus euryale. View of inferior aspect of hard palate. Note horizontally rugose mucosa with no soft tissue cleft.
Table S1. Character matrix for 294 species included in this analysis.
Table S2. Character matrix for families of bats.
Table S3. Bat specimens examined by dissection.
Acknowledgements
The authors would like to thank E. Conroy (Conway Institute and School of Biomolecular and Biomedical Science at University College, Dublin) for carrying out the μCT scans and assisting with reconstruction of the images; W. Gallagher for providing access to the imaging facility; and, M. Kelly‐Quinn (School of Biology and Environmental Science, University College, Dublin) for the specimen of Plecotus auritus. E. C. Teeling is funded by a European Research Grant (ERC‐2012‐StG311000).
References
- Abramyan J, Richman JM (2015) Recent insights into the morphological diversity in the amniote primary and secondary palates. Dev Dyn 244, 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abzhanov A, Rodda SJ, McMahon AP, et al. (2007) Regulation of skeletogenic differentiation in cranial dermal bone. Development 134, 3133–3144. [DOI] [PubMed] [Google Scholar]
- Allam KA, Wan DC, Kawamoto HK, et al. (2011) The spectrum of median craniofacial dysplasia. Plast Reconstr Surg 127, 812–821. [DOI] [PubMed] [Google Scholar]
- Almeida FC, Giannini NP, Simmons NB, et al. (2014) Each flying fox on its own branch: a phylogenetic tree for Pteropus and related genera (Chiroptera: Pteropodidae). Mol Phylogenet Evol 77, 83–95. [DOI] [PubMed] [Google Scholar]
- Ashley‐Montagu MF (1935) The Premaxilla in the primates. Q Rev Biol 10, 32–59. [Google Scholar]
- Barteczko K, Jacob M (2004) A re‐evaluation of the premaxillary bone in humans. Anat Embryol (Berl) 207, 417–437. [DOI] [PubMed] [Google Scholar]
- Bhatnagar KP, Meisami E (1998) Vomeronasal organ in bats and primates: extremes of structural variability and its phylogenetic implications. Microsc Res Tech 43, 465–475. [DOI] [PubMed] [Google Scholar]
- Bleicher N, Sloan RF, Gault IG, et al. (1965) Cleft palate in a dog. Cleft Palate J 45, 56–61. [PubMed] [Google Scholar]
- Bush JO, Jiang R (2012) Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development 139, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan J (1961) The comparative anatomy of cleft lip and palate. Part I. Classification of cleft lip and palate in dogs. Br J Plast Surg 14, 180–184. [DOI] [PubMed] [Google Scholar]
- Cooper JG, Bhatnagar KP (1976) Comparative anatomy of the vomeronasal organ complex in bats. J Anat 122, 571–601. [PMC free article] [PubMed] [Google Scholar]
- Cox TC (2004) Taking it to the max: the genetic and developmental mechanisms coordinating midfacial morphogenesis and dysmorphology. Clin Genet 65, 163–176. [DOI] [PubMed] [Google Scholar]
- Cretekos CJ, Weatherbee SD, Chen CH, et al. (2005) Embryonic staging system for the short‐tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive‐bred animals. Dev Dyn 233, 721–738. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Douarin NM (2005) Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat 207, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzinger A, Schnitzler HU (2013) Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front Physiol 4, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA (2006) 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn 235, 1256–1291. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, et al. (2011) Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet 12, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick GN, Jacobs DS, Matthee CA (2005) A nuclear DNA phylogenetic perspective on the evolution of echolocation and historical biogeography of extant bats (chiroptera). Mol Biol Evol 22, 1869–1886. [DOI] [PubMed] [Google Scholar]
- Ferguson MW (1988) Palate development. Development 103(Suppl), 41–60. [DOI] [PubMed] [Google Scholar]
- Foley NM, Thong VD, Soisook P, et al. (2015) How and why overcome the impediments to resolution: lessons from rhinolophid and hipposiderid bats. Mol Biol Evol 32, 313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazzetta TH (1966) Studies on the morphology and function of the skull in the boidae (Serpentes). Part II. Morphology and function of the jaw apparatus in Python sebae and Python molurus . J Morphol 118, 217–295. [DOI] [PubMed] [Google Scholar]
- Giannini NP, Simmons NB (2007) The Chiropteran premaxilla: a reanalysis of morphological variation and its phylogenetic interpretation. Am Mus Novit, 3585, 1–44. [Google Scholar]
- Giannini N, Goswami A, Sanchez‐Villagra MR (2006a) Development of integumentary structures in Rousettus amplexicaudatus (Mammalia: Chiroptera: Pteropodidae) during late‐embryonic and fetal stages. J Mammal 87, 993–1001. [Google Scholar]
- Giannini NP, Wible JR, Simmons NB (2006b) On the cranial osteology of Chiroptera. I. Pteropus (Megachiroptera: Pteropodidae). Bull Am Mus Nat Hist, 295, 1–134. [Google Scholar]
- Göbbel L (2000) The external nasal cartilages in Chiroptera: significance for intraordinal relationships. J Mamm Evol 7, 167–201. [Google Scholar]
- Göbbel L (2002a) Morphology of the external nose in Hipposideros diadema and Lavia frons with comments on its diversity and evolution among leaf‐nosed microchiroptera. Cells Tissues Organs 170, 39–60. [DOI] [PubMed] [Google Scholar]
- Göbbel L (2002b) Ontogenetic and phylogenetic transformations of the lacrimal‐conducting apparatus among Microchiroptera. Mamm Biol 67, 338–357. [Google Scholar]
- Goldschmidt B, Lopes CAA, Moura M, et al. (2010) Cleft lip and palate associated with other malformations in a neotropical primate (Saimiri ustus). J Am Assoc Lab Anim Sci 49, 357–360. [PMC free article] [PubMed] [Google Scholar]
- Goswami A (2006) Cranial modularity shifts during mammalian evolution. Am Nat 168, 270–280. [DOI] [PubMed] [Google Scholar]
- Goudy‐Trainor A, Freeman PW (2002) Call parameters and facial features in bats: a surprising failure of form following function. Acta Chiropt 4, 1–16. [Google Scholar]
- Gritli‐Linde A (2006) Molecular control of secondary palate development. Dev Biol 301, 309–326. [DOI] [PubMed] [Google Scholar]
- Gritli‐Linde A (2008) The etiopathogenesis of cleft lip and cleft palate: usefulness and caveats of mouse models. Curr Top Dev Biol 84, 37–138. [DOI] [PubMed] [Google Scholar]
- Gritli‐Linde A (2012) The Mouse as a developmental model for cleft lip and palate In: Cleft Lip and Palate Epidemiology, Aetiology and Treatment (ed. Cobourne MT.), pp. 32–51. Basel: Karger. [DOI] [PubMed] [Google Scholar]
- Gunnell GF, Simmons NB (2005) Fossil evidence and the origin of bats. J Mamm Evol 12, 209–246. [Google Scholar]
- Hayden S, Bekaert M, Goodbla A, et al. (2014) A cluster of olfactory receptor genes linked to frugivory in bats. Mol Biol Evol 31, 917–927. [DOI] [PubMed] [Google Scholar]
- Hockman D, Mason MK, Jacobs DS, et al. (2009) The role of early development in mammalian limb diversification: a descriptive comparison of early limb development between the Natal long‐fingered bat (Miniopterus natalensis) and the mouse (Mus musculus). Dev Dyn 238, 965–979. [DOI] [PubMed] [Google Scholar]
- Hutcheon JM, Kirsch JAW (2006) A moveable face: deconstructing the Microchiroptera and a new classification of extant bats. Acta Chiropt 8, 1–10. [Google Scholar]
- Jablonski D, Finarelli JA (2009a) Congruence of morphologically‐defined genera with molecular phylogenies. Proc Natl Acad Sci U S A 106, 8262–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski D, Finarelli JA (2009b) Reply to Smith and O'Meara: the utility of morphogenera. Proc Natl Acad Sci U S A 106, E99–E100. [Google Scholar]
- Jiang R, Bush JO, Lidral AC (2006) Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn 235, 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Teeling EC (2006) The evolution of echolocation in bats. Trends Ecol Evol 21, 149–156. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ (2008) Mouse genetic models of cleft lip with or without cleft palate. Birth Defects Res A 82, 63–77. [DOI] [PubMed] [Google Scholar]
- Kernahan DA, Stark RB (1958) A new classification for cleft lip and cleft palate. Plast Reconstr Surg Transplant Bull 22, 435–441. [DOI] [PubMed] [Google Scholar]
- Koopman KF (1984) A synopsis of the families of bats, part VII. Bat Res News 25, 25–29. [Google Scholar]
- Kousa YA, Schutte BC (2016) Toward an orofacial gene regulatory network. Dev Dyn 245, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BS, Garrett WS (1968) Cleft palate in a marmoset: report of a case. Cleft Palate J 5, 340–343. [PubMed] [Google Scholar]
- Krief S, Watts DP, Mitani JC, et al. (2015) Two cases of cleft lip and other congenital anomalies in wild chimpanzees living in Kibale National Park, Uganda. Cleft Palate Craniofac J 52, 743–750. [DOI] [PubMed] [Google Scholar]
- Kuratani S (2005) Cephalic neural crest cells and the evolution of craniofacial structures in vertebrates: morphological and embryological significance of the premandibular‐mandibular boundary. Zoology (Jena) 108, 13–25. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2015) Mesquite: a modular system for evolutionary analysis. Version 3.04 http://mesquiteproject.org/.
- Malek R (2001) Classification and anatomo‐clinical forms In: Cleft Lip and Palate: Lesions, Pathophysiology and Primary Treatment, pp. 17–25. London: Martin Dunitz. [Google Scholar]
- Mangold E, Ludwig KU, Birnbaum S, et al. (2010) Genome‐wide association study identifies two susceptibility loci for nonsyndromic cleft lip with or without cleft palate. Nat Genet 42, 24–26. [DOI] [PubMed] [Google Scholar]
- Martínez‐Sanz E, Casado‐Gómez I, Martín C, et al. (2011) A new technique for feeding dogs with a congenital cleft palate for surgical research. Lab Anim 45, 70–80. [DOI] [PubMed] [Google Scholar]
- McDonnell R, Owens M, Delany C, et al. (2014) Epidemiology of orofacial clefts in the East of Ireland in the 25‐year period 1984–2008. Cleft Palate Craniofac J 51, e63–e69. [DOI] [PubMed] [Google Scholar]
- Miller GS (1907) The families and genera of bats. Bull US Nat Mus 57, 1–282. [Google Scholar]
- Mossey PA, Modell B (2012) Epidemiology of oral clefts 2012: an international perspective. Front Oral Biol 16, 1–18. [DOI] [PubMed] [Google Scholar]
- Mossey PA, Little J, Munger RG, et al. (2009) Cleft lip and palate. Lancet 374, 1773–1785. [DOI] [PubMed] [Google Scholar]
- Nolte MJ, Hockman D, Cretekos CJ, et al. (2009) Embryonic staging system for the Black mastiff bat, Molossus rufus (Molossidae), correlated with structure‐function relationships in the adult. Anat Rec 292, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SC (1993) Cephalometric correlates of echolocation in the chiroptera. J Morphol 218, 85–98. [DOI] [PubMed] [Google Scholar]
- Pedersen SC (1995) Cephalometric correlates of echolocation in the chiroptera: II. Fetal development. J Morphol 225, 107–123. [DOI] [PubMed] [Google Scholar]
- Pedersen SC (1996) Skull growth and the presence of auxiliary fontanels in rhinolophoid bats (Microchiroptera). Zoomorphology 116, 205–212. [Google Scholar]
- Pedersen SC (1998) Morphometric analysis of the chiropteran skull with regard to mode of echolocation. J Mammal 79, 91–103. [Google Scholar]
- Pedersen SC, Müller R (2013) Nasal‐emission and Nose leaves In: Bat Evolution, Ecology and Conservation (eds. Adams RA, Pedersen SC.), pp. 71–91. New York: Springer. [Google Scholar]
- Radlanski RJ, Renz H (2006) Genes, forces, and forms: mechanical aspects of prenatal craniofacial development. Dev Dyn 235, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Richman JM, Buchtova M, Boughner JC (2006) Comparative ontogeny and phylogeny of the upper jaw skeleton in amniotes. Dev Dyn 235, 1230–1243. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Sack GH, Grausz HM, et al. (1994) Cleft palate with autosomal recessive transmission in Brittany spaniels. Cleft Palate Craniofac J 31, 364–371. [DOI] [PubMed] [Google Scholar]
- Santana SE, Lofgren SE (2013) Does nasal echolocation influence the modularity of the mammal skull? J Evol Biol 26, 2520–2526. [DOI] [PubMed] [Google Scholar]
- Shupe JL, James LF, Binns W, et al. (1968) Cleft palate in cattle. Cleft Palate J 5, 346–355. [PubMed] [Google Scholar]
- Simmons NB (1994) The case for chiropteran monophyly. Am Mus Novit 3103, 1–54. [Google Scholar]
- Simmons NB, Geisler JH (1998) Phylogenetic relationships of Icaronycteris, Archaeonycteris, Hassianycteris, and Palaeochiropteryx to extant bat lineages, with comments on the evolution of echolocation and foraging strategies in Microchiroptera. Bull Am Mus Nat Hist 235, 1–182. [Google Scholar]
- Swindler DR, Merrill OM (1971) Spontaneous cleft lip and palate in a living nonhuman primate, Macaca mulatta . Am J Phys Anthropol 34, 435–439. [DOI] [PubMed] [Google Scholar]
- Teeling EC, Springer MS, Madsen O, et al. (2005) A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307, 580–584. [DOI] [PubMed] [Google Scholar]
- Tessier P (1976) Anatomical classification of facial, cranio‐facial and latero‐facial clefts. J Maxillofac Surg 4, 69–92. [DOI] [PubMed] [Google Scholar]
- Tokita M (2006) Normal embryonic development of the Japanese pipistrelle, Pipistrellus abramus . Zoology (Jena) 109, 137–147. [DOI] [PubMed] [Google Scholar]
- Uslu VV, Petretich M, Ruf S, et al. (2014) Long‐range enhancers regulating Myc expression are required for normal facial morphogenesis. Nat Genet 46, 753–758. [DOI] [PubMed] [Google Scholar]
- Wang Z, Han N, Racey PA, et al. (2010) A comparative study of prenatal development in Miniopterus schreibersii fuliginosus, Hipposideros armiger and H. pratti . BMC Dev Biol 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible JR, Bhatnagar KP (1996) Chiropteran vomeronasal complex and the interfamilial relationships of bats. J Mamm Evol 3, 285–314. [Google Scholar]
- Wible JR, Novacek MJ (1988) Cranial evidence for the monophyletic origin of bats. Am Mus Novit, 2911, 1–19. [Google Scholar]
- Zhao H, Xu D, Zhang S, et al. (2011) Widespread losses of vomeronasal signal transduction in bats. Mol Biol Evol 28, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchero TM, Cooper ME, Maher BS, et al. (2004) Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med 351, 769–780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Myotis blythii. View of inferior aspect of hard palate. Note horizontally rugose mucosa with no soft tissue cleft.
Figure S2. Plecotus auritus. View of inferior aspect of hard palate. Note horizontally rugose mucosa with no soft tissue cleft.
Figure S3. Rhinolophus euryale. View of inferior aspect of hard palate. Note horizontally rugose mucosa with no soft tissue cleft.
Table S1. Character matrix for 294 species included in this analysis.
Table S2. Character matrix for families of bats.
Table S3. Bat specimens examined by dissection.


