Abstract
A previous manuscript [Fernández B, et al. (2008) J Anat 212, 12] reported on the unusual coronary artery patterns in mice belonging to the C57BL/6 strain. The aim here was to elucidate whether this pattern is unique to C57BL/6 mice or appears in other laboratory mouse strains and in wild‐living mice. Stereomicroscopy, scanning electron microscopy, light microscopy and a corrosion cast technique were used to examine 597 adult mice belonging to three inbred strains (C57BL/6, Balb/c, DBA/2), three outbred stocks (CD1, OF1, NMRI) two hybrid lines (129sv × BL/6, CD2F1) and wild mice. It was shown that lock‐like ostium is an exclusive trait of C57BL/6 mice, whereas left septal artery, accessory ostium, high take‐off, intramural course and solitary ostium in aorta are all present in the different laboratory strains and wild mice included in the present study. However, each mouse population shows a specific incidence of these coronary conditions. Several clinically relevant human coronary artery anomalies are present in healthy mice from different strains that may serve as animal models for humans. These results should be taken into consideration in research concerning the murine coronary system, especially in coronary artery occlusion experiments and in studies on cardiovascular developmental biology using murine mutant lines.
Keywords: coronary arteries, genetic background, mouse, strain
Introduction
A previous report described unusual arrangements in the origin of the coronary arteries in C57BL/6 mice (Fernández et al. 2008). This strain is extensively used in the generation of mutant mouse models and in studies dealing with the cardiovascular system. The coronary artery pattern of C57BL/6 mice shows several unusual anatomical conditions, some of which are clinically relevant in humans. The C57BL/6 coronary artery pattern includes solitary ostium in aorta, accessory ostium, high take‐off, aortic intramural course, slit‐like ostium, lock‐like ostium and origin of a septal artery from the left coronary artery (Fernández et al. 2008). In the C57BL/6 strain, these conditions show a distinct incidence, appearing in different combinations.
It is clear that these findings must be taken into consideration in studies dealing with the anatomy and embryology of the murine coronary arteries, as well as in studies in which myocardial ischaemia is induced by surgical occlusion of the coronary arteries. This may be particularly relevant when the studies are comparative and include mutant mouse strains. As demonstrated in the previous report, an inadequate choice of the control mouse strain, particularly when generating mutant lines with mixed genetic backgrounds, may cause misinterpretation of the results obtained (Fernández et al. 2008). In order to take full advantage of these findings and to avoid erroneous deductions derived from the study of strains with different genetic backgrounds, it is necessary to know whether the C57BL/6‐associated coronary phenotypes are exclusive to this strain or also appear in other mouse populations. In this regard, it must be mentioned that, besides the extensive use of laboratory mice in studies dealing with the coronary system, little attention has been paid on the precise anatomy of the murine coronary arteries. In addition to the preceding paper (Fernández et al. 2008), two other studies have described with certain degree of detail the normal coronary anatomy in Swiss albino mice (Icardo & Colvee, 2001) and in wild‐living mice (Mus musculus; Durán et al. 1992).
The present report aimed to elucidate whether the distinct coronary artery anatomical conditions detected in C57BL/6 mice are also present in seven frequently used mouse strains, i.e. Balb/c, DBA/2, CD1, OF1, NMRI, 129sv × BL/6, CD2F1, and in wild mice, as a representative cohort of the species. Given that the general anatomy of the coronary arteries in C57BL/6 mice was reported in the preceding paper, the whole coronary pattern of each mouse population examined is not described here, but only the origin and course of the coronary arteries that were identified as unusual in the previous study.
Materials and methods
The data included in the present research were obtained retrospectively from the authors' database. A total of 597 mice belonging to nine different populations were used. C57BL/6 (n = 108), Balb/c (n = 33), DBA/2 (n = 84), CD1 (n = 112), OF1 (n = 85), NMRI (n = 88), CD2F1 (n = 18) and 129sv × BL/6 (n = 10) mice were purchased from Charles River Laboratories. Wild mice (n = 59) were obtained from archives in the authors' laboratory (see Durán et al. 1992 for material collection). The animals were handled in accordance with the European and Spanish guidelines for animal welfare. They were killed by anaesthetic overdose (Ketamin/Xylazine), CO2 inhalation or cervical dislocation.
The origin of the coronary artery trunks and the course of the main branches were assessed by means of stereomicroscopy, scanning electron microscopy, histological methods for light microscopy or a corrosion‐cast technique. These methods have been described elsewhere (Durán et al. 1992; Sans‐Coma et al. 1993, 1996) and are summarized in the previous study (Fernández et al. 2008).
Results
Table 1 shows the variation in the origin of the septal artery, as well as in the number and position of the coronary ostia in the different groups of mice examined. According to the number and origin of the septal arteries, three types could be distinguished: (i) one septal artery originating from the right coronary artery trunk or sinus (Fig. 1A); (ii) one septal artery originating from the left coronary artery trunk or sinus (Fig. 1B); and (iii) two septal arteries, each originating from one of the coronary artery trunks or sinuses (Fig. 1C). In the C57BL/6 strain, each of these types was present in about one‐third of the specimens examined. In the other groups, a single right septal artery (type 1) was the most common anatomical type (67–87%). In Balb/c and CD2F1 mice, a single left septal artery (type 2) was never observed. The type of septal artery could not be assessed in DBA/2, CD1, OF1 and NMRI mice because their hearts were examined by stereomicroscopy.
Table 1.
Number and proportion of mice of the nine groups studied, with the different coronary artery conditions examined. See the text for further explanation
| Mouse group | Septal artery | Accessory ostium | High take‐off | Single coronary artery | ||
|---|---|---|---|---|---|---|
| Right | Left | Both | ||||
|
n = 108 C57BL/6 |
n = 49 (C+H) | n = 107 (C+H+S) | n = 108 (C+H+S) | n = 108 (C+H+S) | ||
| 16 (32%) | 13 (27%) | 20 (41%) | 33 (31%) | 63 (58%) | 6 (6%) | |
|
n = 33 Balb/c |
n = 9 (C) | n = 33 (C+S) | n = 33 (C+S) | n = 33 (C+S) | ||
| 7 (78%) | 0 | 2 (22%) | 13 (39%) | 0 | 0 | |
|
n = 84 DBA/2 |
– | – | – | n = 84 (S) | n = 84 (S) | n = 84 (S) |
| 19 (23%) | 4 (5%) | 1 (1%) | ||||
|
n = 112 CD1 |
– | – | – | n = 112 (S) | n = 112 (S) | n = 112 (S) |
| 34 (30%) | 18 (16%) | 0 | ||||
|
n = 85 OF1 |
– | – | – | n = 85 (S) | n = 85 (S) | n = 85 (S) |
| 9 (11%) | 5 (6%) | 1 (1%) | ||||
|
n = 88 NMRI |
– | – | – | n = 88 (S) | n = 88 (S) | n = 88 (S) |
| 21 (24%) | 10 (11%) | 3 (3%) | ||||
|
n = 18 CD2F1 |
n = 17 (H) | n = 18 (H) | n = 17 (H) | n = 18 (H) | ||
| 11 (67%) | 0 | 6 (33%) | 4 (22%) | 0 | 0 | |
|
n = 10 129sv × BL/6 |
n = 10 (H) | n = 10 (H) | n = 10 (H) | n = 10 (H) | ||
| 8 (80%) | 1 (10%) | 1 (10%) | 3 (30%) | 4 (40%) | 0 | |
|
n = 59 Wild mouse |
n = 57 (C) | n = 53 (C) | n = 58 | n = 57 (C) | ||
| 50 (87%) | 1 (2%) | 6 (11%) | 16 (30%) | 3 (5%) | 0 | |
C, corrosion‐cast technique; H, light microscopy; n, number of specimens examined; S, scanning electron microscopy.
Figure 1.
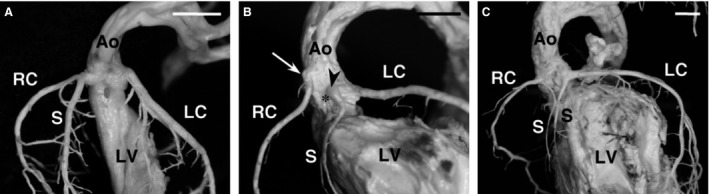
The septal artery. Internal casts of the left ventricle and the coronary arteries of C57BL/6J mice. Frontal views. (A) The septal artery and the right coronary artery arise from a common ostium in the right aortic sinus. (B) The septal artery originates from a separate ostium (arrowhead) in the left aortic sinus, close to the ventral commissure (asterisk), whereas the right coronary ostium (arrow) displays a high take‐off. (C) A septal artery originates from the right coronary artery and another from the left coronary artery. In both cases, the septal artery and the right or left coronary artery arise from a common ostium in the right and left aortic sinuses. Ao, aorta; LC, left coronary artery; LV, left ventricle; RC, right coronary artery; S, septal artery. Scale bars: 100 μm. (Reprinted from Fernández et al. 2008.)
In all the animals examined, the coronary arteries arose from coronary ostia located in the right and left aortic sinuses, adjacent to or facing the pulmonary root. No coronary ostia arising from the non‐adjacent aortic sinus were observed. In most cases, each coronary artery arose from a coronary ostium located in one of the aortic sinuses. However, between about a quarter and a third of the animals of each group (Table 1) exhibited more than one coronary ostium in one of the aortic sinuses (Fig. 2A). The only exception was the OF1 strain, with only 11% of animals showing one accessory ostium. Usually, one of the ostia corresponded to the main coronary trunk, whereas the other, accessory ostium corresponded to the septal or the conal artery (Fig. 1B). There was a high variability between individuals in the number of ostia: from animals with just one ostium per sinus to animals with two or three ostia in each sinus.
Figure 2.
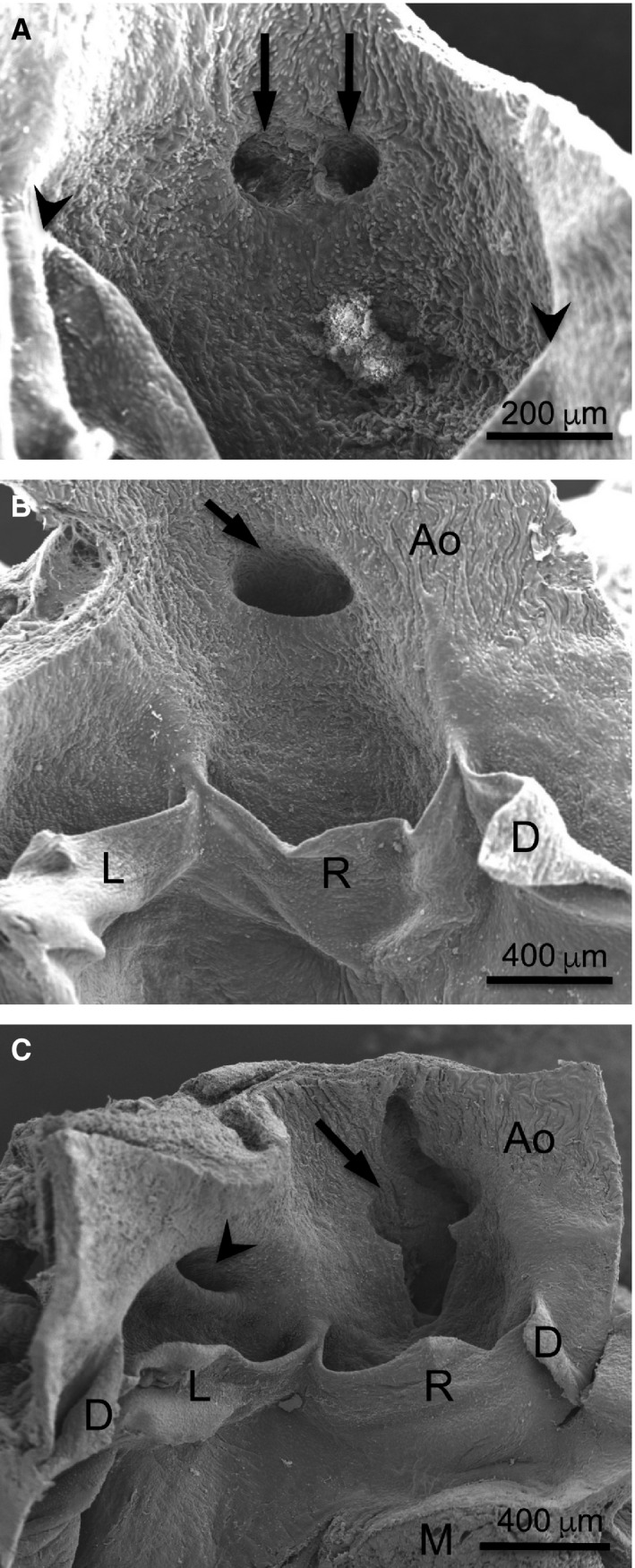
Number, position and shape of the coronary ostia. Scanning electron micrographs of the aortic valve from the luminal side. (A) In a Balb/c mouse, two coronary ostia with rounded openings (arrows) are located in the centre of the right sinus. Arrowheads = commissures. (B) In a C57BL/6 mouse, the right coronary ostium (arrow) shows a high take‐off. (C) In another C57BL/6 mouse, the right coronary ostium, which shows a high take‐off, is large and lock‐like (arrow), whereas the left ostium is slit‐like (arrowhead). Ao, aorta; D, L, R, dorsal, left and right leaflets; M, myocardium.
In 45 out of 108 (42%) C57BL/6 mice, the coronary ostia were located in the aortic sinuses. In the remaining 63 (58%), one or more ostia showed a high take‐off, i.e. they were located in the ascending aorta, above the sinutubular junction (Figs 1B, 2B,C). In addition to the C57BL/6 strain, a high incidence of high take‐off was only found in the 129sv × BL/6 hybrid line (40%). In the remaining groups, the incidence of high take‐off ranged from 0% in Balb/c and CD2F1 to 16% in CD1 (Table 1).
As a rule, the coronary ostia located in the aortic sinuses showed rounded openings (Fig. 2A). However, the ostia corresponding to high take‐off usually displayed a slit‐ (Figs 2C, 3B) or a lock‐like (Fig. 2C) shape. While slit‐like ostia were present in specimens from the seven populations showing high take‐off, lock‐like ostia were only detected in C57BL/6 mice (Table 1). This bizarre ostium morphology was characterized by a large opening, sometimes as wide as halve the size of the whole valve sinus, with a contour similar to a keyhole (Fig. 2C).
Figure 3.
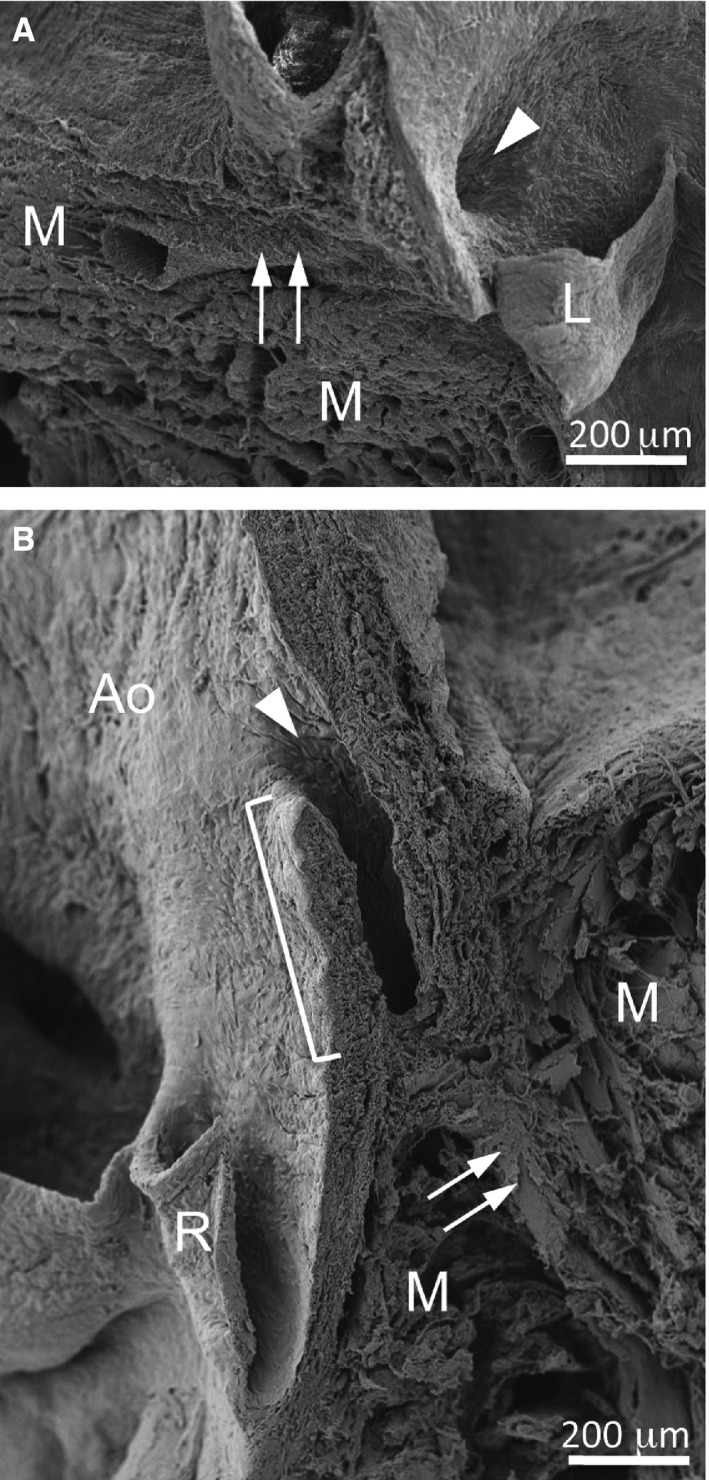
The normal and intramural courses of the coronary artery stems. Scanning electron micrographs of the left (A) and right (B) aortic sinus and adjacent myocardium of a Balb/c (A) and a C57BL/6 mouse (B). (A) The left coronary ostium (arrowhead) is located at the level where the myocardium contacted the aortic root, and the coronary artery stem (arrows) runs intramyocardially from its origin. (B) The right coronary ostium (arrowhead) shows a slit‐like shape. The bracket indicates the portion of the coronary artery stem with an intramural course. The arrows point to the intramyocardial portion of the coronary artery. Ao, aorta; L, left leaflet; M, myocardium; R, right leaflet.
In most cases in which the coronary artery originated from the aortic sinuses, the ostium was located in the middle of the sinus, just at the level where the myocardium contacted the aortic root (Fig. 3A). Therefore, the proximal segment (stem) of the coronary artery became immediately intramyocardial. However, in coronary arteries with a high take‐off, the ostium was separated from the myocardium by the aortic root and a segment of the tubular aorta, which were crossed by the coronary artery stem before becoming intramyocardial (Fig. 3B). In these cases, the stem showed an intramural course, sharing part of the media with the aortic wall. This condition was present in all specimens with a high take‐off, being more frequent but not exclusive to the C57BL/6 strain.
A solitary ostium in aorta was found in six specimens (6%) belonging to the C57BL/6 strain. The single coronary ostium was located in the right aortic sinus (Fig. 4). In these cases, the left ventricle was irrigated by an anatomically normal left coronary artery, which originated from the right coronary artery, crossed the crest of the muscular interventricular septum towards the left ventricle, and gave off the left coronary arterial components. In two out of six cases, the septal artery arose from the left coronary artery as it crossed the septum, whereas in the remaining four cases it originated from the right coronary artery. A solitary ostium in aorta was additionally found in an animal of the DBA/2 strain (1%), another of the OF1 strain (1%), and in a further three of the NMRI strain (3%; Table 1), although in these cases the course of the main coronary arteries and the origin of the septal artery could not be assessed because the animals were examined by scanning electron microscopy.
Figure 4.
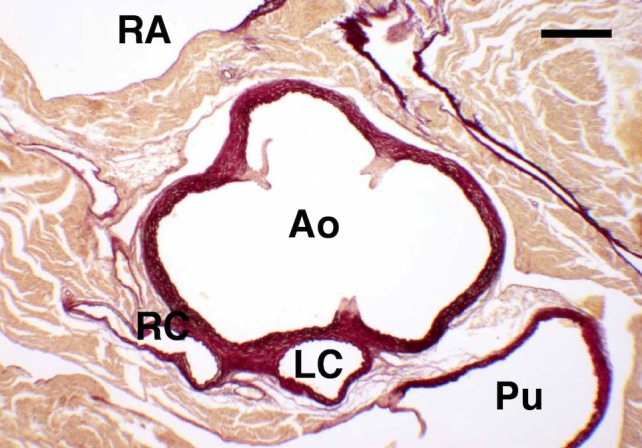
Solitary ostium in aorta. Transversal histological section of the aortic valve of a C57BL/6 mouse stained with resorcin‐fuchsin. This specimen has a solitary, high take‐off, coronary ostium in aorta. The section corresponds to the level where the single coronary trunk divides into a right (RC) and a left (LC) coronary artery. The right coronary artery courses the wall of the right ventricle. The left coronary artery runs towards the muscular outlet ventricular septum. Ao, aortic root; Pu, pulmonary root; RA, right atrium. Scale bar: 200 μm. (Reprinted from Fernández et al. 2008.)
Discussion
The present results were obtained from the study of overall 597 hearts from adult mice belonging to three inbred strains, three outbred stocks, two hybrid lines and wild‐living mice. It was considered that this sample was sufficiently representative of the species Mus musculus.
As in other rodent species, the murine coronary arteries are intramyocardial. In mice, three main coronary arteries can be usually recognized (Durán et al. 1992; Fernández et al. 2008): (i) the right coronary artery, which originates from the right aortic sinus, runs parallel to the right atrioventricular sulcus and crosses obliquely the right ventricle dorsal wall, turning towards the apex as a dorsal interventricular artery; (ii) the left coronary artery, which arises from the left aortic sinus, divides into the left circumflex artery and the obtuse marginal artery that runs along the left margin of the ventricle until the apex; and (iii) the septal artery originates from the main right coronary artery trunk or directly from the right aortic sinus in wild (Durán et al. 1992) and in Swiss albino mice (Icardo & Colvee, 2001). It irrigates the interventricular septum and frequently reaches the apex of the heart. In many cases, the apical portion of the septum is supplied by branches from the dorsal interventricular artery. When compared with humans, although the septal artery takes over, to a certain extent, the role of the anterior interventricular artery, it should not be taken as an equivalent, nor should it be the inferior interventricular artery. In the murine heart, the equivalents of the anterior and inferior interventricular arteries are the obtuse marginal and the dorsal interventricular arteries, respectively, whereas the septal artery can be considered the ‘third’ coronary artery. Therefore, we propose that in the mouse, the origin of the septal artery (right or left) establishes the right or left coronary arterial dominance.
The present findings agree with the previous report (Fernández et al. 2008) and with Salto‐Tellez et al. (2004) that a significant proportion of C57BL/6 mice shows only one septal artery originating from the left coronary artery trunk or from the left aortic sinus (27%), or two septal arteries (41%), each originating from a coronary artery trunk or sinus. In addition, we report here that in other mouse strains, most (67–87%) animals show only one right septal artery, while others (10–33%) show double septal arteries. In contrast, the single left septal artery was the less represented condition (0–10%). Thus, although the presence of a right septal artery is the most common condition in mice, the origin of this important murine coronary artery seems to be quite variable, as it occurs in other rodent species (Durán et al. 1992; Sans‐Coma et al. 1993). These results are particularly important in the context of experimental research on cardiac ischaemia using the mouse model of experimental coronary artery occlusion, which is conventionally performed by surgical ligation of the proximal segment of the left coronary artery trunk (Michael et al. 1995; Guo et al. 1998; Buehler et al. 2002; Jones et al. 2015). Obviously, the existence of a septal artery branching from a more or less distal portion of the left coronary artery trunk in a proportion of animals may certainly lead to a high variability in the extension of the infarct area.
Although the origin of the coronary arteries in mammals is normally located in the aortic sinuses, this is not the situation in C57BL/6 mice. Overall, 58% of the present animals belonging to this strain showed one or more coronary arteries with a high take‐off, i.e. the coronary ostia were located above the sinutubular junction. This anatomical condition was not exclusive to the C57BL/6 strain, but appeared in other hybrid (129sv × BL/6), inbred (DBA/2), outbred (CD1, OF1 and NMRI) and wild populations. Thus, although not exclusive to C57BL/6 mice, a high incidence of high take‐off can be considered a specific characteristic of this strain. Interestingly, while most mice populations showed a relatively low incidence of high take‐off (0–16% in Balb/c, DBA/2, CD1, OF1, NMRI, CD2F1 and wild mice), 129sv × BL/6 hybrid mice showed an intermediate incidence (40%). This can be explained by the hybrid nature of this line, in which the C57BL/6 genetic background accounts for 50% of the genotype.
In humans, high take‐off does not cause any physiological constraint per se, but it is usually associated with a slit‐like opening of the coronary artery, what confers a high risk to severe myocardial ischaemia and even sudden death (Mahowald et al. 1986; García Rinaldi et al. 1994; Basso et al. 2002). In the current mouse population, slit‐like ostia were found in most cases with high take‐off. In addition, some C57BL/6 mice showed lock‐like ostia associated with high take‐off. This bizarre ostium morphology was first described in iv/iv mice by Icardo & Colvee (2001). Later, it was found not to be associated with the iv mutation but with the C57BL/6 background (Fernández et al. 2008). The results of the present report indicate that lock‐like ostia are exclusive to the C57BL/6 strain, as they were absent in other mouse strains.
In addition to slit‐ and lock‐like ostia, high take‐off was strictly associated with an intramural course of the coronary artery stem, as described previously (Fernández et al. 2008). In humans, this anatomical condition is associated with severe myocardial ischaemia due to compression of the coronary artery wall (Sacks et al. 1977; Mahowald et al. 1986). In mice, however, these anomalies (slit‐like ostia, lock‐like ostia and intramural course) cause no apparent disadvantage, as affected animals have a normal life span and no sign of myocardial ischaemia.
In humans (Becker, 1981) and other mammals (Fernández et al. 2007, 2008), the presence of supernumerary coronary ostia (more than one ostium in at least one aortic sinus) is not regarded as an anomaly, because this condition is present in a significant number of individuals and does not entail the risk of clinical complications. In the mouse populations studied, the incidence of supernumerary ostia ranged between a quarter and a third, with the only exception of the OF1 strain, with only 11% of animals presenting this condition. Thus, supernumerary or accessory coronary ostia can be considered a normal trait in mice.
Human patients with solitary ostium in aorta are at risk of ischaemic heart disease and sudden death (Cheitlin et al. 1974; Liberthson et al. 1979; Barth & Roberts, 1986; Basso et al. 2000). Ischaemia is caused by compression of the coronary artery trunk, what may occur when the proximal portion of one coronary artery passes between the aortic and pulmonary roots. This anatomical condition is quite rare (0.04–0.66%) in humans (Desmet et al. 1992; Petit & Reig, 1993). However, the results of the present report and those from the previous study (Fernández et al. 2008) indicate that the incidence of this anomalous condition is notably higher (6%) in C57BL/6 mice compared with humans. Moreover, we report here that three additional mouse strains show a relatively high incidence of solitary ostium in aorta (1% in DBA/2 and OF1 and 3% in NMRI). In previous reports, this coronary artery pattern was detected in wild specimens of different rodent species with intramyocardial coronary arteries, including the house mouse (Arqué et al. 1986; Durán et al. 2005). Taking these data together, the presence of a solitary ostium in aorta, as opposed to humans, seems not to produce cardiac ischaemia in the mouse. This may be due to the fact that in rodents, the coronary arteries have an intramyocardial course (Durán et al. 1992). In mice and hamsters with a solitary ostium in aorta, the stem of the coronary artery with an abnormal origin does not usually pass between the aortic and pulmonary roots, but becomes intramyocardial and either crosses the muscular interventricular septum or runs ventrally within the infundibular wall of the right ventricle (Durán et al. 2005; Fernández et al. 2008).
In summary, except for the lock‐like ostia, the other unusual anatomical conditions of the coronary arteries previously detected in the C57BL/6 mouse strain (accessory ostium, left septal artery, high take‐off, slit‐like ostium, intramural course and solitary ostium) are not exclusive to this particular strain, but are found in a variety of conventional inbred and outbred mouse strains (Balb/c, DBA/2, CD1, OF1, NMRI, 129sv × BL/6 and CD2F1) and in wild mice. The occurrence and incidence of these anatomical conditions varies according to each strain, where their combination and high incidence are characteristic of the C57BL/6 strain.
Finally, two conclusions derived from the current results should be highlighted: (i) different common strains of laboratory mouse may serve as animal models to investigate relevant aspects of congenital anomalies of the coronary arteries, some of them causing sudden death in humans; and (ii) different mouse strains may show dissimilar responses to experimental occlusion of the coronary arteries. Care should be taken in the selection of appropriate experimental animals in research on myocardial ischaemia employing the mouse model.
Author contributions
Alejandro López‐García: data acquisition, analysis and interpretation; study design. María Teresa Soto‐Navarrete: data acquisition, analysis and interpretation. María Carmen Fernández: data acquisition, analysis and interpretation; study design. Javier Moncayo‐Arlandi: data acquisition, analysis and interpretation. Ana Carmen Durán: data interpretation; manuscript revision and approval. Juan Horacio Alonso‐Briales: manuscript revision and approval. Miguel Ángel López‐Unzu: data acquisition, analysis and interpretation. Borja Fernández: data interpretation; manuscript drafting; study design.
Acknowledgements
This work was supported by Grants PI‐0888/2012 and PI‐0591/2010 from Junta de Andalucía (Sevilla, Spain); and Red de Investigación Cardiovascular (RIC; RETICs) (Madrid, Spain). The authors would like to thank Prof. Dr Valentín Sans‐Coma, Málaga, for his valuable suggestions, Mr Luis Vida, Málaga, for his technical assistance and Mr Gregorio Martín, Málaga, for assistance in operating the scanning electron microscope.
The authors declare that no competing interests exist.
References
- Arqué JM, Cruz V, Rosado LM, et al. (1986) Congenital anomalies of coronary arteries in rodents. Am J Cardiol 57, 498–499. [DOI] [PubMed] [Google Scholar]
- Barth CW III, Roberts WC (1986) Left main coronary artery originating from the right sinus of Valsalva and coursing between the aorta and the pulmonary trunk. J Am Coll Cardiol 7, 366–373. [DOI] [PubMed] [Google Scholar]
- Basso C, Maron B, Corrado D, et al. (2000) Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 35, 1493–1501. [DOI] [PubMed] [Google Scholar]
- Basso C, Corrado D, Thiene G (2002) Coronary artery anomalies and sudden death. Cardiac Electrophysiol Rev 6, 107–111. [DOI] [PubMed] [Google Scholar]
- Becker AE (1981) Variations of the main coronary arteries In: Paediatric Cardiology. (eds Becker AE, Losekoot G, Marcelleti C, Anderson RH.), pp. 263–277. Edinburgh: Churchill Livingstone. [Google Scholar]
- Buehler A, Martire A, Strohm C, et al. (2002) Angiogenesis‐independent cardioprotection in FGF‐1 transgenic mice. Cardiovasc Res 55, 768–777. [DOI] [PubMed] [Google Scholar]
- Cheitlin MD, De Castro CM, Mcallister HA (1974) Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva. Circulation 50, 780–787. [DOI] [PubMed] [Google Scholar]
- Desmet W, Vanhaecke J, Vrolix M, et al. (1992) Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J 13, 1637–1640. [DOI] [PubMed] [Google Scholar]
- Durán AC, Sans‐Coma V, Arqué JM, et al. (1992) Blood supply to the interventricular septum of the heart of rodents with intramyocardial coronary arteries. Acta Zool 73, 223–229. [Google Scholar]
- Durán AC, Fernández‐Gallego T, Fernández B, et al. (2005) Solitary coronary ostium in the aorta of Syrian hamsters. A morphological study of 130 cases. Cardiovasc Pathol 14, 303–311. [DOI] [PubMed] [Google Scholar]
- Fernández B, Durán AC, Palomo LJ, et al. (2007) How many coronary arteries are there in mammals? J Morphol 268, 1072. [Google Scholar]
- Fernández B, Durán AC, Fernández MC, et al. (2008) The coronary arteries of the C57BL/6 mouse strains: implications for comparison with mutant models. J Anat 212, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Rinaldi R, Cabardillo J, Giles R, et al. (1994) Right coronary artery with anomalous origin and slit ostium. Ann Thorac Surg 58, 828–832. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wu WJ, Qiu Y, et al. (1998) Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol 275, H1375–H1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardo JM, Colvee E (2001) Origin and course of the coronary arteries in normal mice and iv/iv mice. J Anat 199, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SP, Tang XL, Guo Y, et al. (2015) The NHLBI‐sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct‐sparing interventions in mice, rabbits, and pigs. Circ Res 116, 572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberthson RR, Dinsmore RE, Fallon JT (1979) Aberrant coronary artery origin from the aorta. Report of 18 patients, review of the literature and management. Circulation 59, 748–754. [DOI] [PubMed] [Google Scholar]
- Mahowald JM, Blieden LC, Coe JI, et al. (1986) Ectopic origin of a coronary artery from the aorta. Sudden death in 3 of 23 patients. Chest 89, 669–672. [DOI] [PubMed] [Google Scholar]
- Michael LH, Entman ML, Hartley CJ, et al. (1995) Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269, H2147–H2154. [DOI] [PubMed] [Google Scholar]
- Petit M, Reig J (1993) Arterias Coronarias. Aspectos Anatomo‐Clínicos. Barcelona: Masson‐Salvat, pp258 [Google Scholar]
- Sacks JH, Londe SP, Rosenbluth A, et al. (1977) Left main coronary bypass for aberrant (aortic) intramural left coronary artery. J Thorac Cardiovasc Surg 73, 733–737. [PubMed] [Google Scholar]
- Salto‐Tellez M, Lim SY, El Oakley RM, et al. (2004) Myocardial infarction in the C57BL/6J mouse. A quantifiable and highly reproducible experimental model. Cardiovasc Pathol 13, 91–97. [DOI] [PubMed] [Google Scholar]
- Sans‐Coma V, Arqué JM, Durán AC, et al. (1993) The coronary arteries of the Syrian hamster, Mesocricetus auratus (Waterhouse, 1839). Ann Anat 175, 53–57. [DOI] [PubMed] [Google Scholar]
- Sans‐Coma V, Fernández B, Durán AC, et al. (1996) Fusion of valve cushions as a key factor in the formation of congenital bicuspid aortic valves in Syrian hamsters. Anat Rec 244, 490–498. [DOI] [PubMed] [Google Scholar]


