Abstract
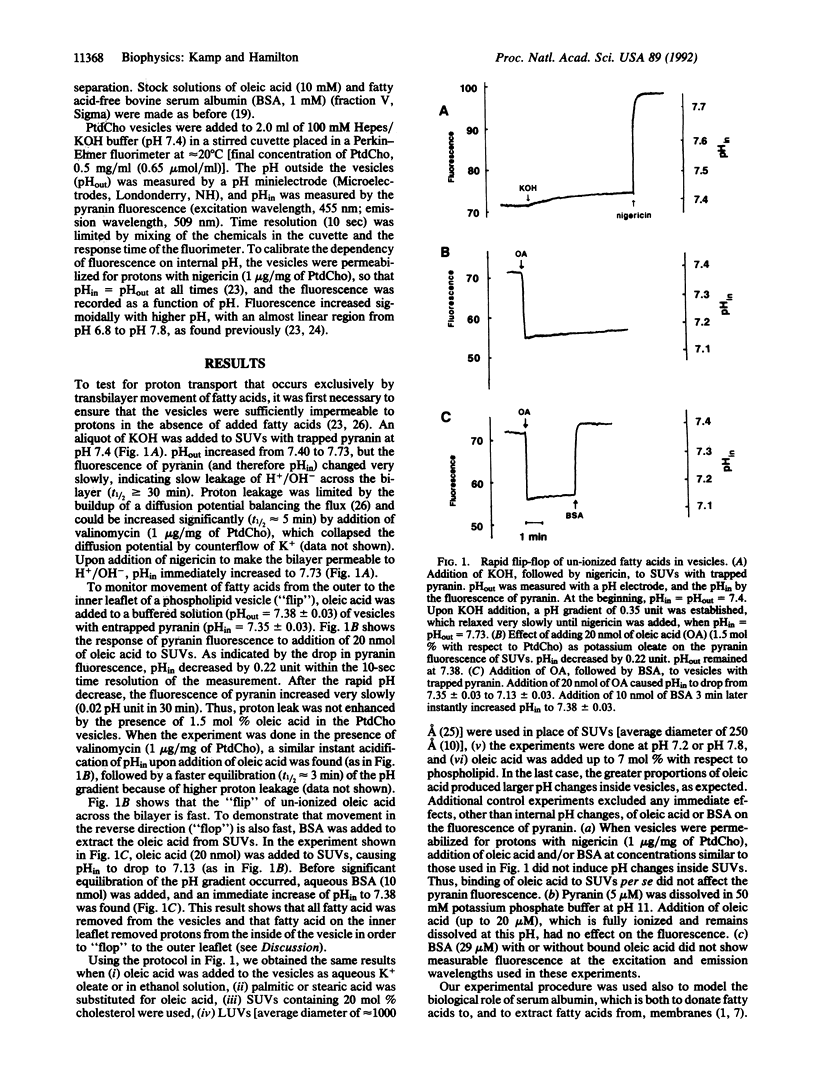
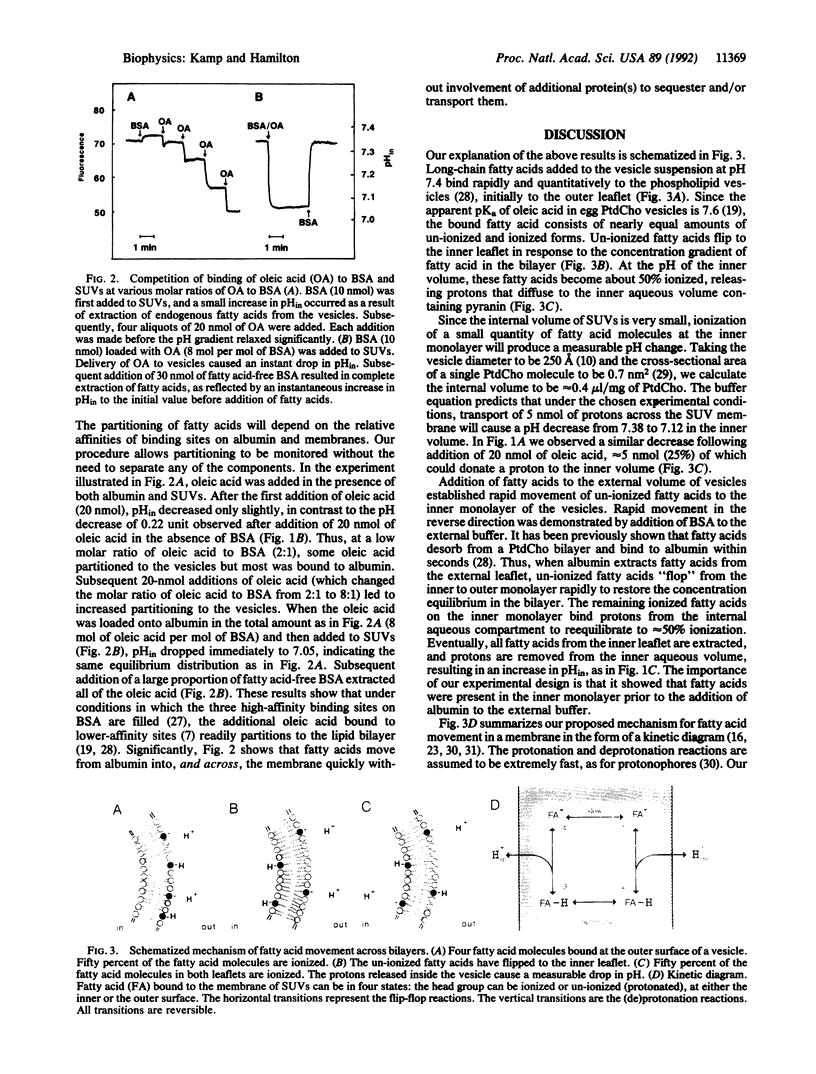
A central, unresolved question in cell physiology is how fatty acids move across cell membranes and whether protein(s) are required to facilitate transbilayer movement. We have developed a method for monitoring movement of fatty acids across protein-free model membranes (phospholipid bilayers). Pyranin, a water-soluble, pH-sensitive fluorescent molecule, was trapped inside well-sealed phosphatidylcholine vesicles (with or without cholesterol) in Hepes buffer (pH 7.4). Upon addition of a long-chain fatty acid (e.g., oleic acid) to the external buffer (also Hepes, pH 7.4), a decrease in fluorescence of pyranin was observed immediately (within 10 sec). This acidification of the internal volume was the result of the "flip" of un-ionized fatty acids to the inner leaflet, followed by a release of protons from approximately 50% of these fatty acid molecules (apparent pKa in the bilayer = 7.6). The proton gradient thus generated dissipated slowly because of slow cyclic proton transfer by fatty acids. Addition of bovine serum albumin to vesicles with fatty acids instantly removed the pH gradient, indicating complete removal of fatty acids, which requires rapid "flop" of fatty acids from the inner to the outer monolayer layer. Using a four-state kinetic diagram of fatty acids in membranes, we conclude that un-ionized fatty acid flip-flops rapidly (t1/2 < or = 2 sec) whereas ionized fatty acid flip-flops slowly (t1/2 of minutes). Since fatty acids move across phosphatidylcholine bilayers spontaneously and rapidly, complex mechanisms (e.g., transport proteins) may not be required for translocation of fatty acids in biological membranes. The proton movement accompanying fatty acid flip-flop is an important consideration for fatty acid metabolism in normal physiology and in disease states such as cardiac ischemia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Bihain B. E., Deckelbaum R. J., Yen F. T., Gleeson A. M., Carpentier Y. A., Witte L. D. Unesterified fatty acids inhibit the binding of low density lipoproteins to the human fibroblast low density lipoprotein receptor. J Biol Chem. 1989 Oct 15;264(29):17316–17321. [PubMed] [Google Scholar]
- Bröring K., Haest C. W., Deuticke B. Translocation of oleic acid across the erythrocyte membrane. Evidence for a fast process. Biochim Biophys Acta. 1989 Nov 27;986(2):321–331. doi: 10.1016/0005-2736(89)90484-7. [DOI] [PubMed] [Google Scholar]
- Cabral D. J., Small D. M., Lilly H. S., Hamilton J. A. Transbilayer movement of bile acids in model membranes. Biochemistry. 1987 Apr 7;26(7):1801–1804. doi: 10.1021/bi00381a002. [DOI] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- Cooper R. B., Noy N., Zakim D. Mechanism for binding of fatty acids to hepatocyte plasma membranes. J Lipid Res. 1989 Nov;30(11):1719–1726. [PubMed] [Google Scholar]
- Daniels C., Noy N., Zakim D. Rates of hydration of fatty acids bound to unilamellar vesicles of phosphatidylcholine or to albumin. Biochemistry. 1985 Jun 18;24(13):3286–3292. doi: 10.1021/bi00334a032. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Nichols J. W. Proton flux mechanisms in model and biological membranes. J Membr Biol. 1989 Feb;107(2):91–103. doi: 10.1007/BF01871715. [DOI] [PubMed] [Google Scholar]
- Doody M. C., Pownall H. J., Kao Y. J., Smith L. C. Mechanism and kinetics of transfer of a fluorescent fatty acid between single-walled phosphatidylcholine vesicles. Biochemistry. 1980 Jan 8;19(1):108–116. doi: 10.1021/bi00542a017. [DOI] [PubMed] [Google Scholar]
- Eastman S. J., Hope M. J., Cullis P. R. Transbilayer transport of phosphatidic acid in response to transmembrane pH gradients. Biochemistry. 1991 Feb 19;30(7):1740–1745. doi: 10.1021/bi00221a002. [DOI] [PubMed] [Google Scholar]
- Gutknecht J. Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J Membr Biol. 1988 Nov;106(1):83–93. doi: 10.1007/BF01871769. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Cistola D. P. Transfer of oleic acid between albumin and phospholipid vesicles. Proc Natl Acad Sci U S A. 1986 Jan;83(1):82–86. doi: 10.1073/pnas.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Era S., Bhamidipati S. P., Reed R. G. Locations of the three primary binding sites for long-chain fatty acids on bovine serum albumin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2051–2054. doi: 10.1073/pnas.88.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G. E., Williams B. R. Signal transduction by interferon-alpha through arachidonic acid metabolism. Science. 1991 Jan 11;251(4990):204–207. doi: 10.1126/science.1898993. [DOI] [PubMed] [Google Scholar]
- Kasianowicz J., Benz R., McLaughlin S. How do protons cross the membrane-solution interface? Kinetic studies on bilayer membranes exposed to the protonophore S-13 (5-chloro-3-tert-butyl-2'-chloro-4' nitrosalicylanilide). J Membr Biol. 1987;95(1):73–89. doi: 10.1007/BF01869632. [DOI] [PubMed] [Google Scholar]
- Katiyar S. S., Shrago E. Differential interaction of fatty acids and fatty acyl CoA esters with the purified/reconstituted brown adipose tissue mitochondrial uncoupling protein. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1104–1111. doi: 10.1016/0006-291x(91)91679-7. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991 Jan 30;1061(2):297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Noy N., Donnelly T. M., Zakim D. Physical-chemical model for the entry of water-insoluble compounds into cells. Studies of fatty acid uptake by the liver. Biochemistry. 1986 Apr 22;25(8):2013–2021. doi: 10.1021/bi00356a027. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Prestegard J. H., Cramer J. A., Viscio D. B. Nuclear magnetic resonance determinations of permeation coefficients for maleic acid in phospholipid vesicles. Biophys J. 1979 Jun;26(3):575–584. doi: 10.1016/S0006-3495(79)85272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991 Dec 9;294(3):158–162. doi: 10.1016/0014-5793(91)80658-p. [DOI] [PubMed] [Google Scholar]
- Small D. M. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res. 1967 Nov;8(6):551–557. [PubMed] [Google Scholar]
- Storch J., Kleinfeld A. M. Transfer of long-chain fluorescent free fatty acids between unilamellar vesicles. Biochemistry. 1986 Apr 8;25(7):1717–1726. doi: 10.1021/bi00355a041. [DOI] [PubMed] [Google Scholar]
- Storch J., Lechene C., Kleinfeld A. M. Direct determination of free fatty acid transport across the adipocyte plasma membrane using quantitative fluorescence microscopy. J Biol Chem. 1991 Jul 25;266(21):13473–13476. [PubMed] [Google Scholar]
- Storch J. Mechanism for binding of fatty acids to hepatocyte plasma membranes: different interpretation. Hepatology. 1990 Dec;12(6):1447–1449. doi: 10.1002/hep.1840120632. [DOI] [PubMed] [Google Scholar]
- Upreti G. C., Jain M. K. Effect of the state of phosphatidylcholine on the rate of its hydrolysis by phospholipase A2 (bee venom). Arch Biochem Biophys. 1978 Jun;188(2):364–375. doi: 10.1016/s0003-9861(78)80021-6. [DOI] [PubMed] [Google Scholar]