Abstract
In our previous work we identified the presence of meso-zeaxanthin [(3R,3′S)-zeaxanthin] in trout flesh and skin (Nolan et al., 2014), but were not able to quantify this carotenoid with the method used at that time. In the present study, we developed a protocol that allows for the quantification of lutein and the three stereoisomers of zeaxanthin [(3R,3′R)-zeaxanthin, meso-zeaxanthin and (3S,3′S)-zeaxanthin] in fish flesh. We tested this protocol in two species of farmed trout (Oncorhynchus mykiss and Salmo Trutta), and we detected and quantified these carotenoids. The concentrations of each carotenoid detected (ranging from 1.18 ± 0.68 ng g−1 flesh for meso-zeaxanthin to 38.72 ± 15.87 ng g−1 flesh for lutein) were highly comparable for the two fish species tested. In conclusion, we report, for the first time, the concentrations of zeaxanthin stereoisomers (including meso-zeaxanthin) and lutein in trout flesh. This work adds further to the knowledge on the presence of these carotenoids in the human food chain.
Keywords: Meso-zeaxanthin; Zeaxanthin; (3S,3′S)-zeaxanthin; Lutein; Trout flesh; Carotenoids; Chiral HPLC
1. Introduction
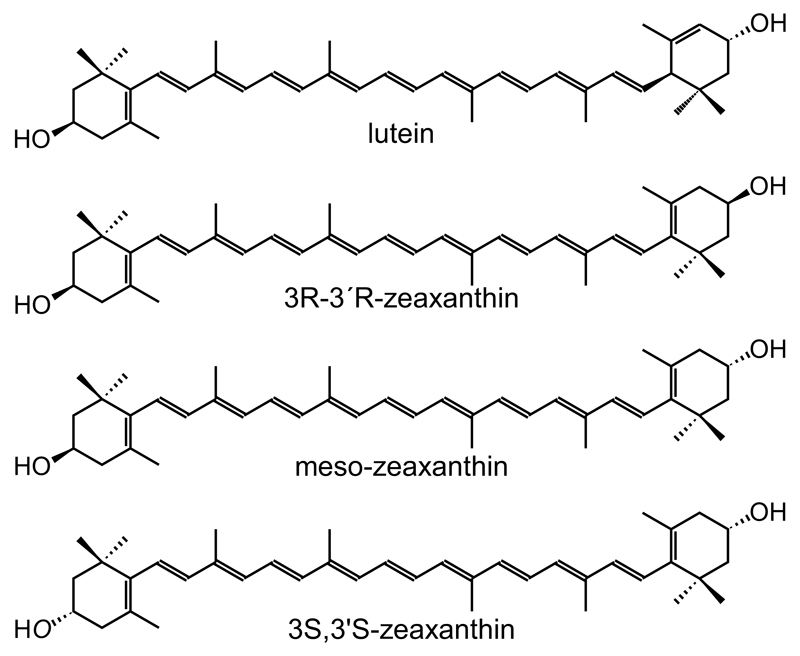
The macula is the region of the retina that mediates sharp central and color vision (Hammond et al., 1998; Howarth and Bradley, 1986), and its yellow color is due to the presence of the macular carotenoids lutein [(3R,3′R,6′R)-β,ε-carotene-3,3′-diol], (3R,3′R)-zeaxanthin, and (3R,3′S)-zeaxanthin (meso-zeaxanthin) at this tissue, where they are collectively referred to as macular pigment (MP) or the macular carotenoids. In addition to the major carotenoids at the macula (i.e. lutein, (3R,3′R)-zeaxanthin and meso-zeaxanthin), another stereoisomer of zeaxanthin [(3S,3′S)-zeaxanthin] has been previously identified, but is present in minuscule concentrations compared to lutein, (3R,3′R)-zeaxanthin and meso-zeaxanthin (Bone et al., 1997; Bone et al., 1993) (Fig. 1). These carotenoids are believed to support macular health and function via their powerful antioxidant and short-wavelength (blue) light-filtering properties. Importantly, however, the macular carotenoids cannot be synthesized by mammals, and therefore their presence in the macula relies entirely on obtaining them from diet.
Fig. 1.
Structure of the carotenoids quantified in this study, lutein and the three isomers of zeaxanthin. Stucture of lutein [(3R,3′R,6′R)-β,ε-carotene-3,3′-diol], (3R,3′R)-zeaxanthin [(3R,3′R)-β,β-carotene-3,3′-diol], meso-zeaxanthin [(3R,3′ S)-β,β-carotene-3,3′-diol] and (3S,3′S)-zeaxanthin [(3S,3′S)-β,β-carotene-3,3′-diol].
It is estimated that the average intake of lutein in a western diet is between 1.0 and 3.6 mg/day, mainly from leafy green vegetables and fruits (Nebeling et al., 1997). The intake of (3R,3′R)-zeaxanthin is much lower, typically from eggs, corn, and paprika, with the average intake of this carotenoid reported to be 0.1 mg/day (Johnson et al., 2010; Nebeling et al., 1997). Meso-zeaxanthin and (3S,3′S)-zeaxanthin are traditionally classified as non-dietary, because they have not been consistently detected in the human diet to date. However, up until recently, most studies reporting on dietary sources of these carotenoids did not use methods capable of separating or quantifying the zeaxanthin stereoisomers.
Meso-zeaxanthin and (3S,3′S)-zeaxanthin were detected for the first time in nature in fish integuments, turtle fat depot and shrimp carapace (Maoka et al., 1986). Shortly after this publication, two further studies detected zeaxanthin in the flesh of Rainbow trout (Salmo gairdneri), but the stereoisomeric composition of this carotenoid was only determined in the integuments (Katsuyama et al., 1987; Schiedt et al., 1986). Only recently, the topic of the presence of zeaxanthin stereoisomers in fish was revisited (Rasmussen et al., 2012), and the authors of this work reported that the fish species analyzed either did not contain any of the zeaxanthin stereoisomers or lutein, or that these carotenoids were present in concentrations below their detection level. Furthermore, the authors hypothesized that in view of their results, the zeaxanthin stereoisomers reported by Maoka et al. (1986) in fish were due to an artifact from saponification. Recently, however, our group published a study that confirmed the presence of meso-zeaxanthin in Rainbow trout flesh (Oncorhynchus mykiss), but meso-zeaxanthin was not quantified in that study due to the difficulties of carotenoid purification from fish tissue (Nolan et al., 2014).
With this work, we challenge the results reported by Rasmussen et al. (2012) and, to our knowledge, quantify meso-zeaxanthin, 3S,3′S-zeaxanthin, 3R,3′R-zeaxanthin and lutein in fish flesh for the first time.
2. Material and methods
2.1. Carotenoid standards and solvents
Lutein standard [(3R,3′R,6′R)-β,ε-carotene-3,3′-diol] and zeaxanthin standard (racemic mixture of the three zeaxanthin stereoisomers [(3R,3′R)-β,β-carotene-3,3′-diol, (3S,3′S)-β,β-carotene-3,3′-diol and (3R,3′S)-β,β-carotene-3,3′-diol)] were supplied by CaroteNature GmbH (Ostermundigen, Switzerland). The Standard Reference Material (SRM) 968e (Fat-Soluble Vitamins, Carotenoids, and Cholesterol in Human Serum) was obtained from NIST (National Institute for Standards and Technology, Gaithersburg, MD, USA). Hexane and isopropanol, HPLC grade, were purchased from Sigma-Aldrich (Vale Road, Arklow, Wicklow, Ireland) or Thermo Fisher Scientific (Blanchardstown Corp Pk 2, Ballycoolin, Dublin, Ireland). BHT (butylated hydroxytoluene) was purchased from Sigma-Aldrich.
2.2. HPLC analysis
Lutein and zeaxanthin stereoisomers were separated and quantified on an Agilent Technologies (Palo Alto, CA) 1260 Series HPLC system equipped with a Diode Array Detector (DAD, G1315C), binary pump, degasser, thermostatically-controlled column compartment, thermostatically-controlled High Performance auto-sampler (G1367E) and thermostatically-controlled analytical fraction collector.
System 1 (for lutein and zeaxanthin isomers purification) was performed using a PVA-Sil semipreparative column (100 mm × 10 mm; 5 μm YMC, Maynooth, Kildare, Ireland) with a guard column. Isocratic elution was performed with hexane:isopropanol (90:10, v/v) and a flow rate of 2 mL min−1. The column temperature was set at 25 °C.
System 2 (for lutein and zeaxanthin isomers analysis) was performed using a Daicel Chiralpak AD-3 column (Amylose derivative coated on silica-gel, 250 × 4.6 mm i.d., 3 μm; Chiral Technologies Europe, Cedex, France) with a guard column. A gradient was performed from 100% solvent A (hexane:isopropanol (95:5, v/v)) to 20% solvent B (hexane:isopropanol (90:10, v/v)) in 15 min at a flow rate of 0.5 mL min−1, and these conditions were maintained up to minute 40, when the system was returned to the starting conditions within one minute. The column temperature was set at 25 °C.
2.3. Calibration
We confirmed the accuracy of our quantification method using the Standard Reference Material 968e in HPLC system 2. Lutein measured was 0.157 μmol L−1, which is within the limits of the certified value reported by NIST for this carotenoid (0.170 ± 0.013 μmol L−1).
Quantification was achieved by constructing two standard curves, one for lutein and one for (3R,3′R)-zeaxanthin, (3S,3′S)-zeaxanthin and meso-zeaxanthin, using a UV–vis spectrophotometer UVmini-1240 (Shimadsu) and HPLC system 2, with the DAD detector set to 444 nm for lutein and 450 nm for zeaxanthin stereoisomers. For the lutein standardcurve, lutein was previously purified in our laboratory up to 96% purity (based on peak area) as previously described (Prado-Cabrero et al., 2016). Six concentrations were measured in triplicate within the linear range 0.3–3.1 mg L−1 and the molar extinction coefficient applied was 147.3 × 103 L moL−1 cm−1 in hexane. The resulting regression line was given by the formula y = 0.0425x + 0.216 (r2 = 0.997), where y is lutein concentration and x is the peak area. For the (3R,3′R)-zeaxanthin, (3S,3′S)-zeaxanthin and meso-zeaxanthin standard curve, the zeaxanthin stereoisomeric mix from Carotenature was used (94% purity based on peak area). Identical spectral characteristics were assumed for the three stereoisomers present in the standard, therefore a zeaxanthin molar extinction coefficient was applied, 141.1 × 103 L moL−1 cm−1 in hexane. Five concentrations within the linear range 0.04–0.8 mg L−1 were used and the resulting regression line was given by the formula y = 0.0549x + 0.0719 (r2 = 0.996), where y is zeaxanthin stereoisomer concentration and x is the peak area.
We established the Limit of Quantification (LOQ) of our HPLC system for lutein and zeaxanthin assessing the lowest concentration of each carotenoid quantifiable with a Relative Standard Deviation (RSD) lower than 5%. LOQ was 57.4 pmol for lutein (RSD = 1.2%, n = 9) and 3.2 pmol for zeaxanthin (RSD = 4.2%, n = 9).
2.4. Sample sourcing
Farmed Brown trout (Salmo Trutta) fillets were purchased from Lidl stores (Waterford, Ireland), which sourced the trout from the fish farm Keohane Seafoods Ltd. (Cork, Ireland). Farmed Rainbow trout (Oncorhynchus mykiss) fillets were purchased from the local fish monger M.J. Flanagan (Waterford, Ireland), who sourced them from Goatsbridge Trout Farm (Thomastown, Co. Kilkenny, Ireland). In both cases, trout was presented for sale as half fish fillets without bones and with skin.
2.5. Carotenoid analysis
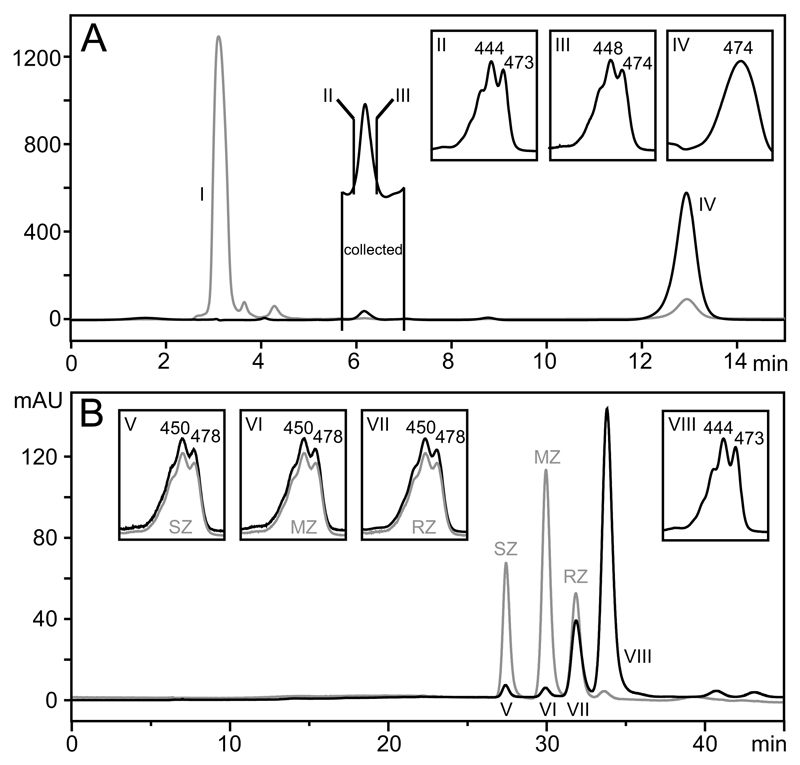
A raw half trout fillet, as is usually presented in the market, was analyzed. Five separate trout samples were analyzed for each trout species. The skin was manually separated from the flesh and discarded. The flesh was diced and weighted (each trout fillet weighted between 60 and 120 g). The diced flesh was placed in a 500 mL beaker and covered with 300 mL of Ethanol 96% with 0.1% BHT per 100 g of sample. The sample was then homogenized in an Ultra-Turrax T50 basic homogenizer with a S 50 N–W 65 SK cutting head (IKA-Werke GmbH & Co. KG, Stafen, Germany). The resulting slurry was distributed in four 250 mL polypropylene centrifuge bottles and the bottles were incubated under agitation at 250 rpm at room temperature for 30 min. Then the bottles were centrifuged at 4248g in a Sigma 3–18 K centrifuge for 10 min at 15 °C to remove debris and denatured proteins. The supernatants were transferred to an evaporating flask and dried in a rotavapor RII (Buchi, Mason Technology Ltd., Dublin, Ireland) at 30 °C. The final aqueous slurry was extracted with hexane twice, filtered with nylon filters of 0.45 μm pore diameter (Chromafil, Apex Scientific Ltd., Kildare, Ireland) and dried again in rotavapor. The dry sample containing the carotenoids was re-suspended in 5 mL of hexane:isopropanol (90:10) and two injections of 50 μL were performed using HPLC system 1. The peak containing lutein and total zeaxanthin was collected (Fig. 2A, peak including spectra II and III, selected to note the portion of the peak enriched in lutein and zeaxanthin respectively). In this way, we eliminated phospholipids (Fig. 2A, Peak I) and astaxanthin (Fig. 2A, Peak IV), as these compounds would overload the chiral column when present in high concentrations. The collections were dried in rotavapor and re-suspended in 100 μL of hexane:isopropanol (95:5) and analyzed using HPLC system 2. Each sample was injected in duplicate, whereas the first injection was typically of 50 μl in order to obtain a clear spectrum of meso-zeaxanthin and (3S,3′S)-zeaxanthin. The second injection, of 10 μl, was performed to quantify the carotenoids. Using this analysis method we were able to quantify carotenoid concentrations as low as 0.9 ng of meso-zeaxanthin per gram of trout flesh.
Fig. 2.
HPLC chromatograms exemplifying the carotenoid extraction protocol performed. A. HPLC chromatogram of a carotenoid extract run in a PVA-Sil semipreparative column (HPLC system 1). In grey, chromatogram at 250 nm, where peak I contains phospholipids of the sample; In black, chromatogram at 450 nm, where between bars and labeled as “collected” is the peak of interest, containing lutein (spectrum shown in inset II) and zeaxanthin stereoisomers (spectrum inset III); peak IV contains astaxanthin. B. HPLC chromatogram representing the chromatogram of the peak collected in A and run in a chiral column (HPLC system 2). In grey and labeled as SZ, MZ and RZ [(3S,3′S)-zeaxanthin, (3R,3′S)-zeaxanthin and (3R,3′R)-zeaxanthin respectively) are shown the peaks of the authentic standard containing the three stereoisomers of zeaxanthin. The chromatogram in black shows peak V of the sample exhibiting the same retention time and spectrum of authentic (3S,3′S)-zeaxanthin (see inset including the spectrum of authentic (3S,3′S)-zeaxanthin in grey). Peak VI is a peak of the sample exhibiting the same retention time and spectrum of authentic meso-zeaxanthin (see inset including the spectrum of authentic meso-zeaxanthin in grey). Peak VII is a peak of the sample exhibiting the same retention time and spectrum of (3R,3′R)-zeaxanthin (see inset including the spectrum of authentic (3R,3′R)-zeaxanthin in grey). Peak VIII is a peak of the sample exhibiting the same spectrum of lutein (see inset VIII). The authentic standard for lutein is not shown in this chromatogram.
2.6. Statistical methods
The statistical software package SPSS 20 was used for analysis. Inter-species similarity for each carotenoid quantified was analyzed using Mann-Whitney U Test, considering samples as independent and taking the significance level 0.05. Correlation between the carotenoids quantified was performed using two-tailed Spearman’s rho correlation test.
3. Results
3.1. Carotenoids in trout flesh
Lutein, (3R,3′R)-zeaxanthin, meso-zeaxanthin and (3S,3′S)-zeaxanthin were detected and quantified in the fresh flesh of two farmed species of trout (See Fig. 2B for an example chromatogram). The concentrations (mean ± SD) of these carotenoids are presented in Table 1. The concentrations ranged from 1.18 ± 0.68 ng g−1 flesh for meso-zeaxanthin to 38.72 ± 15.87 ng g−1 flesh for lutein, and the concentrations of each carotenoid detected were highly comparable across the two species tested. Furthermore, we found a strong correlation between the concentrations of meso-zeaxanthin and (3S,3′S)-zeaxanthin detected in the fish (r2 = 0.927, P < 0.01, n = 10).
Table 1.
Concentration of zeaxanthin stereoisomers and lutein in trout flesh.
| Trout species | Sample |
Carotenoid ng/g flesh (mean ± SD) |
||||
|---|---|---|---|---|---|---|
| number | weight (g) | 3S,3′S-zeaxanthin | 3R,3′S (meso)-zeaxanthin | 3R,3′R-zeaxanthin | lutein | |
| Rainbow trout | 1 | 85,82 | 3,11 | 2,34 | 19,80 | 32,27 |
| 2 | 111,05 | 1,25 | 0,84 | 6,77 | 27,84 | |
| 3 | 68,52 | 0,95 | 0,58 | 12,47 | 56,94 | |
| 4 | 59,33 | 1,49 | 1,02 | 8,53 | 26,37 | |
| 5 | 104,47 | 1,40 | 1,12 | 4,75 | 20,21 | |
| mean ± SD | 1,64 ± 0.85 | 1,18 ± 0.68 | 10,46 ± 5.94 | 32,73 ± 14.21 | ||
| Brown trout | 1 | 112,80 | 2,18 | 1,17 | 9,64 | 43,24 |
| 2 | 121,21 | 1,43 | 0,86 | 12,94 | 32,32 | |
| 3 | 93,40 | 2,28 | 1,72 | 14,88 | 61,07 | |
| 4 | 104,88 | 1,50 | 1,34 | 12,78 | 39,35 | |
| 5 | 110,26 | 1,76 | 1,49 | 4,24 | 17,61 | |
| mean ± SD | 1,83 ± 0.39 | 1,31 ± 0.33 | 10,89 ± 4.17 | 38,72 ± 15.87 | ||
4. Discussion
This work follows on from our previous publication that confirmed the presence of meso-zeaxanthin in fish (Nolan et al., 2014). To our knowledge, this is the first time that zeaxanthin stereoisomers have been quantified in fish flesh. To achieve this, we designed a protocol that allowed for the analysis and quantification of low concentrations of carotenoids by extracting large weight representative samples of an animal (i.e. by extracting carotenoids from half a trout). By doing this, however, the extract contained high concentrations of undesirable molecules such as proteins, lipids, and other major carotenoids not the subject of this investigation (e.g. astaxanthin), and these molecules needed to be removed before analysis. In order to remove lipids, we decided to use a method not requiring saponification, because our experience is that saponification does not remove lipids to an extent that allows us to quantify low concentrated carotenoids, whilst preserving carotenoid stability (Nolan et al., 2014). Therefore, we purified our sample from lipids using a semipreparative PVA-Sil column. The use of this column also allowed us to discard astaxanthin from our sample. Subsequently, we proceeded to analyse zeaxanthin stereoisomers using a chiral column, using a previously described method (Khachik et al., 2002).
In our experiment, we found that the concentration of (3R,3′R)-zeaxanthin, meso-zeaxanthin, (3S,3′S)-zeaxanthin and lutein were comparable between the two trout species analyzed (see Table 1), which is a very interesting finding given that the two trout species analyzed belong to different genus (Salmo for Brown trout and Oncorhynchus for Rainbow trout) (Murata et al., 1993), and also given that the species were cultured in different fish farms, and presumably under differing habitat conditions (e.g. feed, temperature, light, etc.). To explain this interesting finding we propose the following possibilities: a. it is possible that carotenoid metabolism in the genera Salmo and Onchorhyncus is very similar and yields these carotenoids in comparable concentrations in these species, with possible functional roles for the carotenoids at these ratios and concentrations; b. the feed provided in the fish farms was comparable and already contain the carotenoids assessed. Either way, this experiment confirms the presence of meso-zeaxanthin in the human food chain.
5. Conclusion
We have developed a method that allows for the quantification of zeaxanthin stereoisomers and lutein in fish flesh. Using this method we have been able to detect and quantify, for the first time, meso-zeaxanthin and (3S,3′S)-zeaxanthin in fish flesh. This work adds further to the knowledge on the presence of these carotenoids in the human food chain. Further work is needed to understand the exact origins of these carotenoids, and study into their specific functions in fish is warranted.
Acknowledgement
We would like to thank Prof. David Thurnham, from the University of Ulster, for his valuable comments on this study.
Footnotes
Conflict of interests
APC is a Howard Fellow and his research program is supported by the Howard Foundation (English Charity reg. number 285822) via a commercial grant from Iosa (Industrial Orgánica S.A., Monterrey, Nuevo León, Mexico), MacuHealth LLC™ (Birmingham, MI, USA) and Alliance Pharma plc (Chippenham, Wiltshire, UK); all of the above organisations have an interest in commercially available supplements containing the macular carotenoids. JMN and SB do consultancy work for nutraceutrical companies as directors of Nutrasight Consultancy Ltd. AH is an ‘honorary director’ of Howard Foundation Holdings Limited and Nutri-products Ltd., which licence and supply nutraceutical ingredients. The sponsors had no involvement in study design, collection, analysis or interpretation of data, in the writing of the manuscript or in the decision to submit the article for publication.
References
- Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human mascular carotenoids. Invest Ophthalmol Vis Sci. 1993;34(6):2033–2040. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang WL. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64(2):211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Jr, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Invest Ophthalmol Vis Sci. 1998;39(2):397–406. [PubMed] [Google Scholar]
- Howarth PA, Bradley A. The longitudinal chromatic aberration of the human eye, and its correction. Vis Res. 1986;26(2):361–366. doi: 10.1016/0042-6989(86)90034-9. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Maras JE, Rasmussen HM, Tucker KL. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J Am Diet Assoc. 2010;110(9):1357–1362. doi: 10.1016/j.jada.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Katsuyama M, Komori T, Matsuno T. Metabolism of three stereoisomers of astaxanthin in the fish, rainbow trout and tilapia. Comp Biochem Physiol – Part B Biochem Mol Biol. 1987;86(1):1–5. doi: 10.1016/0305-0491(87)90165-9. [DOI] [PubMed] [Google Scholar]
- Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Invest Ophthalmol Vis Sci. 2002;43(11):3383–3392. [PubMed] [Google Scholar]
- Maoka T, Arai A, Shimizu M, Matsuno T. The 1st isolation of enantiomeric and meso-zeaxanthin in nature. Comp Biochem Physiol – Part B Biochem Mol Biol. 1986;83(1):121–124. doi: 10.1016/0305-0491(86)90341-x. [DOI] [PubMed] [Google Scholar]
- Murata S, Takasaki N, Saitoh M, Okada N. Determination of the phylogenetic relationships among Pacific salmonids by using short interspersed elements (SINEs) as temporal landmarks of evolution. Proc Natl Acad Sci U S A. 1993;90(15):6995–6999. doi: 10.1073/pnas.90.15.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebeling LC, Forman MR, Graubard BI, Snyder RA. Changes in carotenoid intake in the United States: the 1987 and 1992 national health interview surveys. J Am Diet Assoc. 1997;97(9):991–996. doi: 10.1016/S0002-8223(97)00239-3. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Beatty S, Meagher KA, Howard AN, Kelly D, Thurnham DI. Verification of meso-zeaxanthin in fish. J Food Process Technol. 2014;5(6):335. doi: 10.4172/2157-7110.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Cabrero A, Beatty S, Howard A, Stack J, Bettin P, Nolan JM. Assessment of lutein, zeaxanthin and meso-zeaxanthin concentrations in dietary supplements by chiral high-performance liquid chromatography. Eur Food Res Technol. 2016;242(4):599–608. doi: 10.1007/s00217-015-2569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HM, Muzhingi T, Eggert EMR, Johnson EJ. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J Food Compos Anal. 2012;27(2):139–144. [Google Scholar]
- Schiedt K, Vecchi M, Glinz E. Astaxanthin and its metabolites in wild rainbow trout (Salmo gairdneri R.) Comp Biochem Physiol – Part B Comp Bioch. 1986;83(1):9–12. [Google Scholar]