Abstract
Purpose
We compared the clinical characteristics and treatment outcomes of patients with eosinophilic and neutrophilic COPD exacerbations requiring hospital admission.
Patients and methods
This was a retrospective multicenter study performed between January 2010 and December 2014. In all, 1,688 COPD patients admitted via the outpatient clinics or emergency departments of six university hospitals were enrolled. The patients were grouped by complete blood counts: eosinophilic group, >2% peripheral blood eosinophils, and neutrophilic group, >65% peripheral blood neutrophils or >11,000 leukocytes/mL. The patients with radiographic evidence of pneumonia at the time of admission, those with lung cancer, those admitted for treatment of other medical problems, and those who chronically used steroids were excluded.
Results
A total of 605 patients hospitalized with COPD exacerbations (177 eosinophilic and 380 neutrophilic) were included. Pulmonary functions, including the forced expiratory volume in 1 second and forced vital capacity, were better in patients with eosinophilic exacerbations. Treatment outcomes, including the rate of admission to the intensive care unit and mortality, were poorer in patients with neutrophilic exacerbations (4.5% vs 12.4%, P=0.004; 1.1% vs 4.5%, P=0.043, respectively). Congestive heart failure (odds ratio [OR] =3.40, 95% confidence interval [CI]: 1.28–9.01) and neutrophilic exacerbation (OR = 2.81, 95% CI: 1.21–6.52) were independent risk factors for intensive care unit admission.
Conclusion
COPD patients with neutrophilic exacerbations experienced worse clinical outcomes than did those with eosinophilic exacerbations. The peripheral blood eosinophil count may be a useful predictor of clinical progress during hospitalization of COPD patients with acute exacerbations.
Keywords: eosinophilia, neutrophilia, pulmonary disease, chronic obstructive, exacerbations, intensive care unit
Introduction
Acute exacerbation of COPD is associated with substantial morbidity and mortality. It is known that such exacerbation is typically associated with an increase in neutrophilic (and, to a lesser extent, eosinophilic) airway inflammation.1,2 However, COPD exacerbations are heterogeneous in terms of both airway inflammation and etiology. Bafadhel et al classified patients with COPD exacerbations into four distinct biological clusters. As expected, the bacterial cluster was the largest, but the eosinophilia-predominant cluster constituted 28% of all exacerbations.3
Inhaled or systemic steroids are used to minimize the symptoms of eosinophilic airway inflammation in patients with severe COPD exacerbations.4 However, treatment failure is more common in noneosinophilic (compared to eosinophilic) COPD patients receiving systemic steroids.5 Ultimately, eosinopenia is associated with acute infection and inflammation; these conditions, combined with leukocytosis, are predictive of further bacterial infection.6 Eosinopenia is known to be an independent predictor of in-hospital mortality in patients with COPD exacerbations.7,8 Treatment outcomes differ by the cause of exacerbation. Thus, phenotyping of COPD exacerbations is clinically important.
Several biomarkers of eosinophilic COPD exacerbations have been developed.3,9–11 Of these, the peripheral blood eosinophil percentage is a simple and sensitive biomarker of sputum production and bronchial eosinophilia.3,12 A cutoff of 2% peripheral blood eosinophilia accurately identifies a sputum eosinophilia of >3% upon exacerbation.3
In the present study, we classified COPD patients into eosinophilic and neutrophilic exacerbation (at the time of hospital admission) groups, using data from complete blood cell counts. We compared the clinical characteristics and treatment outcomes of the two groups.
Patients and methods
This was a multicenter retrospective study conducted in six university hospitals in the Republic of Korea from 2010 to 2014. The study was approved by the institutional review boards of all participating centers (The Catholic University of Korea Bucheon St Mary’s Hospital, The Catholic University of Korea Seoul St Mary’s Hospital, The Catholic University of Korea Yeouido St Mary’s Hospital, The Catholic University of Korea St Paul’s Hospital, The Catholic University of Korea Incheon St Mary’s Hospital, The Catholic University of Korea St Vincent’s Hospital; IRB No XC16RIMI0030). All data were collected from hospital databases. The requirement for informed consent was waived by the institutional review boards because the study was based on retrospective chart reviews.
Patients
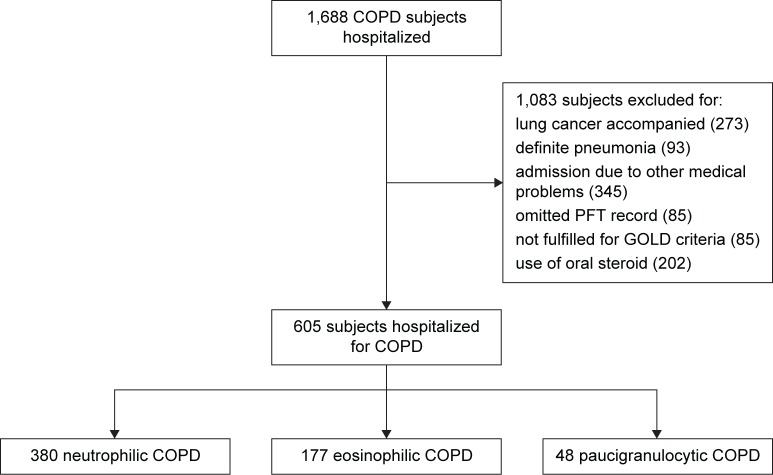
Patients previously diagnosed with COPD using the International Classification of Diseases Version 10 codes J440, J441, J448, and J449 and who were hospitalized with exacerbations were included. Patients with underlying lung cancer, who chronically used steroids, who were admitted because of other medical problems, who did not fulfill the Global Initiative for Chronic Obstructive Lung Disease criteria (not having results of spirometry without bronchodilator or forced expiratory volume in 1 second [FEV1]/forced vital capacity ≥0.70), who lacked pulmonary function test (PFT) data, and who exhibited definite pneumonic infiltrations on chest X-ray at the time of admission were excluded. Only the most recent hospitalization event was considered. The study flow is summarized in Figure 1.
Figure 1.
Study flowchart.
Abbreviations: PFT, pulmonary function test; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Definition
A COPD exacerbation was defined as an event developing during the natural progress of disease featuring aggravation of symptoms such as dyspnea and increased purulence of respiratory secretions, requiring a change in regular treatment.13 An eosinophilic exacerbation was defined as a serum eosinophil count >2%.5 A neutrophilic exacerbation was defined as a leukocyte count >11,000 leukocytes/mL or a neutrophil proportion >65%. Cases that met both eosinophilia (serum eosinophil count >2%) and neutrophilia (neutrophil proportion >65% or leukocyte count >11,000 leukocytes/mL) were categorized as eosinophilic exacerbations.
Any case that did not belong to these groups was classified into paucigranulocytic exacerbations.
Data
We extracted the following data from medical records: patient demographics; any history of comorbid disease such as hypertension, diabetes mellitus, myocardial infarction, congestive heart failure (CHF), or cerebrovascular accident; smoking history; the number of hospital or emergency room (ER) admissions in the previous year; the types of regular COPD medications taken; laboratory data (including those pertaining to arterial blood gas analysis and C-reactive protein [CRP] level); PFT results; hospital days; admission to the intensive care unit (ICU); length of ICU stay; any need for mechanical ventilation (MV); the duration of MV; any need for noninvasive ventilation; and treatment results.
COPD exacerbations were treated with nebulizers, antibiotics, and systemic steroids. Nebular forms of salbutamol (2.5 mg/2.5 mL nebules) and budesonide (500 µg/2 mL nebules) were given every 6 hours and 12 hours, respectively. Prescription of antibiotics and systemic steroids was at the discretion of attending clinicians.
Based on laboratory data, COPD patients were classified into two groups: eosinophilic and neutrophilic exacerbation groups. Clinical parameters and treatment outcomes, including ICU admission and in-hospital mortality, were compared between the two groups. Severe COPD exacerbations requiring ICU admission have a major impact on mortality.14,15 In view of the differences in disease severity between the two groups, COPD patients were further classified into ICU and non-ICU admission groups.
Statistical analysis
Baseline demographics and clinical outcomes were compared between patients with eosinophilic and neutrophilic exacerbations. We used Pearson’s chi-squared test to compare discrete variables and Student’s t-test to compare continuous variables. Multiple logistic regression analysis was used to identify significant independent factors associated with ICU admission. A two-sided P-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows software (Version 20.0; IBM Corporation, Armonk, NY, USA).
Results
Overall, 1,688 COPD patients with severe exacerbations were admitted to the hospital over the study period, of whom 1,083 met the exclusion criteria of underlying lung cancer, definite pneumonia at the time of admission, admission because of other medical problems, absence of PFT data, and/or chronic use of steroids. Thus, 605 patients were finally included. Of these, 177, 380, and 48 patients were classified into the eosinophilic, neutrophilic, and paucigranulocytic COPD exacerbation groups, respectively. We compared patients with eosinophilic and neutrophilic COPD exacerbations.
The clinical characteristics of patients are summarized in Table 1. The proportion of males was lower, and the mean age higher, in the neutrophilic group (P<0.001). The mean body mass index was higher in the eosinophilic group (P=0.019). The frequencies of comorbidities and self-reported history of allergy or asthma were similar in the two groups. The rate of never-smokers was higher in the neutrophilic group. The proportions of patients who had experienced one or more hospital or ER admissions in the previous year did not differ significantly between the two groups. In terms of regular medications prescribed at the outpatient clinics, patients with neutrophilic exacerbations used significantly more phosphodiesterase 4 inhibitors than did those with eosinophilic exacerbations (P=0.026).
Table 1.
Comparison of clinical characteristics between COPD patients with eosinophilic and neutrophilic exacerbations
| Eosinophilic | Neutrophilic | P-value | |
|---|---|---|---|
| Subjects, n | 177 | 380 | |
| Male, n (%) | 151 (85.3) | 262 (68.9) | <0.001 |
| Age (years), mean ± SD | 69.89±11.25 | 73.06±9.34 | <0.001 |
| BMI, mean ± SD | 22.61±3.49 | 21.76±3.86 | 0.019 |
| Comorbidities, n (%) | |||
| Hypertension | 61 (34.5) | 145 (38.2) | 0.400 |
| DM | 35 (19.8) | 75 (19.7) | 0.992 |
| MI history | 8 (4.5) | 16 (4.2) | 0.867 |
| CHF | 10 (5.6) | 21 (5.5) | 0.953 |
| CVA history | 8 (4.5) | 21 (5.5) | 0.619 |
| Allergy history, n (%) | 9 (5.1) | 11 (2.9) | 0.270 |
| Asthma history, n (%) | 30 (16.9) | 73 (19.2) | 0.522 |
| Smoking history, n (%) | |||
| Never | 25 (14.1) | 98 (25.8) | 0.002 |
| Ex-smoker | 92 (52.0) | 168 (44.2) | 0.087 |
| Current smoker | 53 (29.9) | 100 (26.3) | 0.372 |
| Smoking (PY), mean ± SD | 41.39±26.59 | 36.25±28.76 | 0.069 |
| ≥1 hospital or ER admission in the previous year, n (%) | 46 (26.0) | 111 (29.2) | 0.431 |
| COPD medication, n (%) | |||
| ICS | 1 (0.6) | 8 (2.1) | 0.179 |
| LAMA | 77 (43.55) | 170 (44.7) | 0.785 |
| LABA | 11 (6.2) | 18 (4.7) | 0.465 |
| ICS + LABA | 71 (40.1) | 181 (47.6) | 0.097 |
| PDE4 inhibitor | 2 (1.1) | 19 (5.0) | 0.026 |
Abbreviations: SD, standard deviation; BMI, body mass index; DM, diabetes mellitus; MI, myocardial infarction; CHF, congestive heart failure; CVA, cerebrovascular accident; PY, pack-year; ER, emergency room; ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta agonist; PDE4, phosphodiesterase 4.
The laboratory data and PFT results are shown in Table 2. The parameters of arterial blood gas analysis did not differ between the two groups. The CRP level was higher in the neutrophilic group. On the PFT, FEV1 and forced vital capacity were higher in the eosinophilic group (P<0.001 and P=0.009, respectively), but the severity of airway obstruction (based on the Global Initiative for Chronic Obstructive Lung Disease criteria) did not differ between the two groups. The treatment strategies, ICU admission data, approaches toward MV, and mortality are summarized in Table 3. In terms of treatment strategies, steroid-only prescription was more common in the eosinophilic group (P<0.001), and steroids combined with antibiotics were prescribed more often in the neutrophilic group (P<0.001). The proportion of patients who used nebulizers only (ie, who did not take steroids or antibiotics) was higher among those with eosinophilic exacerbations (P=0.028). The length of hospital stay did not differ between the two groups, but the rates of ICU admission and MV were higher in the neutrophilic group (4.5% vs 12.4%, P=0.004; 2.8% vs 9.5%, P=0.005, respectively). Both total and early mortality were higher in the neutrophilic group (1.1% vs 4.5%, P=0.043; 0.0% vs 2.9%, P=0.022, respectively).
Table 2.
The laboratory findings on admission and baseline PFT
| Eosinophilic | Neutrophilic | P-value | |
|---|---|---|---|
| ABGA on admission, mean ± SD | |||
| pH | 7.41±0.78 | 7.42±0.83 | 0.403 |
| PaCO2 | 42.73±15.39 | 43.42±15.90 | 0.649 |
| PaO2 | 75.30±25.97 | 71.15±35.44 | 0.189 |
| HCO3 | 26.16±7.22 | 26.69±5.70 | 0.378 |
| CRP | 13.89±29.06 | 38.69±64.89 | ,0.001 |
| PFT (post BD), mean ± SD | |||
| FEV1/FVC | 46.20±10.98 | 46.20±11.40 | 0.995 |
| FEV1 (L) | 1.22±0.52 | 1.04±0.42 | <0.001 |
| FEV1, % | 51.71±20.69 | 49.46±18.42 | 0.198 |
| FVC (L) | 2.65±0.87 | 2.37±1.33 | 0.009 |
| FVC, % | 78.25±23.08 | 74.32±22.49 | 0.057 |
| RV, % | 140.75±75.06 | 146.84±73.58 | 0.485 |
| BDR, % | 7.65±11.04 | 5.74±8.53 | 0.026 |
| DLco, % | 62.71±28.81 | 59.953±27.06 | 0.322 |
| GOLD classification, n (%) | |||
| 1 | 17 (9.6) | 28 (7.4) | 0.367 |
| 2 | 69 (39.0) | 134 (35.3) | 0.396 |
| 3 | 75 (42.4) | 176 (46.3) | 0.384 |
| 4 | 16 (9.0) | 42 (11.1) | 0.469 |
Abbreviations: PFT, pulmonary function test; ABGA, arterial blood gas analysis; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; HCO3, bicarbonate; CRP, C-reactive protein; BD, bronchodilator; SD, standard deviation; FEV1, forced expiratory volume in 1 second; VC, forced vital capacity; RV, residual volume; BDR, bronchodilator response; DLco, diffusing capacity of the lung for carbon monoxide; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 3.
The strategies of treatment, ICU admission, MV approach, and mortality
| Eosinophilic | Neutrophilic | P-value | |
|---|---|---|---|
| Treatment, n (%) | |||
| Steroid only | 32 (18.1) | 19 (5.0) | <0.001 |
| Steroid + antibiotics | 119 (67.2) | 314 (82.6) | <0.001 |
| Antibiotics mono | 14 (7.9) | 38 (10.0) | 0.430 |
| Nebulizer only | 10 (5.6) | 8 (2.1) | 0.028 |
| Length of hospital stay (days), mean ± SD | 9.51±27.76 | 9.87±8.01 | |
| ICU admission, n (%) | 8 (4.5) | 47 (12.4) | 0.004 |
| Length of ICU stay (days), mean ± SD | 11.38±21.61 | 9.51±12.63 | 0.732 |
| MV, n (%) | 5 (2.8) | 36 (9.5) | 0.005 |
| Duration of MV (days), mean ± SD | 15.60±27.33 | 8.86±9.08 | 0.237 |
| Noninvasive ventilation, n (%) | 0 (0.0) | 6 (1.6) | 0.093 |
| Treatment results, n (%) | |||
| Resolve | 175 (98.9) | 363 (95.5) | 0.043 |
| Mortality | 2 (1.1) | 17 (4.5) | 0.043 |
| Death within 28 days | 0 (0.0) | 11 (2.9) | 0.022 |
| Death after 28 days | 2 (1.1) | 6 (1.6) | 0.678 |
Abbreviations: ICU, intensive care unit; MV, mechanical ventilation; SD, standard deviation.
The study groups were subgrouped in terms of ICU admission. Upon univariate analysis, age, body mass index, CHF, and neutrophilic exacerbation were associated with ICU admission (Table 4). Multiple logistic regression was performed to identify independent risk factors for ICU admission. CHF (odds ratio [OR] =3.40, 95% CI 1.28–9.01, P=0.014) and neutrophilic exacerbation (OR =2.81, 95% CI 1.21–6.52, P=0.016) were independent risk factors for ICU admission (Table 5).
Table 4.
Comparison of clinical characteristics between non-ICU and ICU admission
| Non-ICU admission | ICU admission | P-value | |
|---|---|---|---|
| Subjects, n | 502 | 55 | |
| Male, n (%) | 376 (74.9) | 37 (67.3) | 0.220 |
| Age, years, mean ± SD | 69.89±11.25 | 73.06±9.34 | ,0.001 |
| BMI, mean ± SD | 22.61±3.49 | 21.76±3.86 | 0.019 |
| Comorbidities, n (%) | |||
| Hypertension | 182 (36.3) | 24 (43.6) | 0.282 |
| DM | 94 (18.7) | 16 (29.1) | 0.067 |
| MI history | 20 (4.0) | 4 (7.3) | 0.254 |
| CHF | 24 (4.8) | 7 (12.7) | 0.015 |
| CVA history | 26 (5.2) | 3 (5.5) | 0.930 |
| Allergy history, n (%) | 20 (4.0) | 0 (0.0) | 0.301 |
| Asthma history, n (%) | 92 (18.3) | 11 (20.0) | 0.762 |
| Smoking history, n (%) | |||
| Never | 111 (22.1) | 12 (21.8) | 0.960 |
| Ex-smoker | 231 (46.0) | 29 (52.7) | 0.344 |
| Current smoker | 141 (28.1) | 12 (21.8) | 0.323 |
| Smoking (PY), mean ± SD | 37.93±28.18 | 38.48±27.91 | 0.907 |
| Neutrophilic exacerbation, n (%) | 333 (66.3) | 47 (85.5) | 0.004 |
| ≥1 hospital or ER admission in the previous year, n (%) | 137 (27.3) | 20 (36.4) | 0.156 |
| GOLD classification, n (%) | |||
| 1 | 42 (8.4) | 3 (5.5) | 0.452 |
| 2 | 188 (37.5) | 15 (27.3) | 0.137 |
| 3 | 220 (43.8) | 31 (56.4) | 0.076 |
| 4 | 52 (10.4) | 6 (10.9) | 0.899 |
Abbreviations: ICU, intensive care unit; SD, standard deviation; BMI, body mass index; DM, diabetes mellitus; MI, myocardial infarction; CHF, congestive heart failure; CVA, cerebrovascular accident; PY, pack-year; ER, emergency room; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 5.
Results of multiple logistic regression analysis for ICU admission
| Factor | OR (95% CI) | P-value |
|---|---|---|
| CHF | 3.40 (1.28–9.01) | 0.014 |
| BMI | 0.97 (0.89–1.06) | 0.482 |
| Age | 1.02 (0.99–1.06) | 0.178 |
| Neutrophilic AE | 2.81 (1.21–6.52) | 0.016 |
Abbreviations: ICU, intensive care unit; OR, odds ratio; CI, confidence interval; CHF, congestive heart failure; BMI, body mass index; AE, acute exacerbations.
Discussion
We compared the clinical characteristics and treatment outcomes of COPD patients with neutrophilic and eosinophilic exacerbations based on complete blood count. We found that 29.3% of hospitalized COPD patients with acute exacerbations had peripheral eosinophilia exceeding 2%. Patients with eosinophilic COPD exacerbations had better clinical outcomes than did those with neutrophilic exacerbations. Significantly more neutrophilic than eosinophilic COPD patients required MV and ICU admission. Patients with eosinophilic exacerbations had a lower early mortality rate than did those with neutrophilic exacerbations.
One possible explanation for the association between poor prognosis and neutrophilic exacerbation is that neutrophilia is known to be a marker of bacterial infection.16 Most COPD exacerbations are associated with such infections, and COPD exacerbations caused by bacteria are associated with longer hospital stays and more frequent exacerbations.3,8,17 Also, neutrophilia was associated with a greater probability of hospital admission in patients visiting ERs with COPD exacerbations.16 In patients with neutrophilia, the CRP level is a useful serum biomarker of bacteria-associated exacerbation.3,18 A higher CRP level at admission increases the risk of treatment failure.19 In our present study, the CRP level was significantly higher in patients with neutrophilic than eosinophilic exacerbations.
Traditionally, COPD exacerbation has been associated with neutrophilic airway inflammation. However, one study found that eosinophilic airway inflammation accounted for a considerable proportion (nearly 30%) of COPD exacerbations,3 which is consistent with our data. In patients with COPD exacerbations, airway eosinophilia is evident upon bronchial biopsy.20 Sputum eosinophil levels increase during COPD exacerbations.4,11 Furthermore, bronchial hyperresponsiveness and reversibility may be evident in certain subgroups of COPD patients; these characteristics are associated with airway eosinophilia.21
Bronchoalveolar lavage and endobronchial biopsies have been performed and sputum collected for decades to examine the nature of airway inflammation in COPD patients.20,22 However, these methods are difficult to apply to COPD patients exhibiting acute exacerbations. Sputum induction can trigger additional bronchoconstriction, and invasive techniques such as bronchoscopy are difficult in patients in poor condition.23 Measurements of interleukin-15 and fractional exhaled nitric oxide levels can identify eosinophilic airway inflammation. However, these methods are difficult to apply in routine clinical practice.9–11 Recently, a peripheral blood eosinophil proportion >2% has been suggested to be a simple and useful marker of sputum eosinophilia.3 Blood eosinophilia is associated with increased all-cause mortality in general populations with asthma and COPD.24,25 Eosinophilia is associated with frequent exacerbations only among COPD patients who are not currently smoking.26 In COPD patients with eosinophilic exacerbations, as revealed by peripheral blood eosinophil levels, the length of hospital stay and readmission rate were shorter and lower, respectively, than in those with noneosinophilic exacerbations.27 Patients with COPD exacerbations featuring acute respiratory failure (and who thus required ICU admission) had better outcomes in terms of a lower median length of ICU stay and lower ICU mortality than did patients with peripheral eosinophilia.28
High doses of bronchodilators and systemic steroids are the mainstay of management of COPD exacerbations.29,30 Antibiotics are beneficial if the sputum is purulent, but routine antibiotics are only marginally efficacious.30,31 In clinical practice, most COPD patients with acute exacerbations receive antibiotics.32 However, only 20%–30% of COPD patients with exacerbations exhibited positive sputum bacterial cultures; indiscriminate prescription of antibiotics triggers antibiotic resistance.30 Although systemic corticosteroids are well known to be effective in COPD patients with acute exacerbations, corticosteroids can influence bacterial clearance, rendering pathogen eradication incomplete, thereby triggering relapses and clinical failure.33 Thus, COPD phenotypes must be characterized and treatment individualized. As mentioned earlier, neutrophilic and eosinophilic COPD exacerbations exhibit different characteristics and treatment outcomes, indicating that different treatment strategies are required. However, few comparisons have been made between the two groups.
Our study had certain limitations. First, the work was retrospective in nature. Nonetheless, this was a multicenter study, with a large sample size, which yielded valuable clinical information on eosinophilic and neutrophilic exacerbations. Second, COPD management was not identical in all patients. The rates of use of steroids only and steroids combined with antibiotics were higher in patients with eosinophilic and neutrophilic exacerbations, respectively. Prescription of steroids and/or antibiotics was at the discretion of pulmonologists treating exacerbations in each hospital. A prospective study is thus needed to clarify appropriate phenotype-specific therapies for subgroups of COPD patients with peripheral eosinophilia or neutrophilia.
Despite these limitations, our research had several strengths. First, we included a large number of patients from six teaching hospitals. Second, although several comparative studies on eosinophilic and non-eosinophilic COPD exacerbations requiring hospital admission have appeared, few studies have directly compared eosinophilic and neutrophilic exacerbations in COPD patients.27,28,34,35 In addition, the cited studies included COPD patients taking steroids prior to enrollment, and those with histories of steroid use were unknown. Corticosteroids affect eosinophils and induce eosinopenia; we thus excluded patients who had taken steroids prior to possible enrollment.36 Finally, our work suggests that peripheral neutrophilia evident on admission is a risk factor for ICU admission. This information will aid clinicians who must evaluate, and predict the clinical course of patients hospitalized with COPD exacerbations.
Conclusion
COPD patients with neutrophilic exacerbations experienced poorer clinical outcomes than did those with eosinophilic exacerbations. The peripheral blood eosinophil count may be a useful marker for predicting clinical progress in COPD patients exhibiting acute exacerbations during hospitalization. Prospective studies are needed to clarify the utility of peripheral eosinophilia in terms of the classification of COPD phenotypes and individualization of treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proc Am Thorac Soc. 2004;1(2):109–114. doi: 10.1513/pats.2306029. [DOI] [PubMed] [Google Scholar]
- 3.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 4.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 5.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil H, Magy N, Mauny F, Dupond JL. Value of eosinopenia in inflammatory disorders: an “old” marker revisited. Rev Med Interne. 2003;24(7):431–435. doi: 10.1016/s0248-8663(03)00138-3. French. [DOI] [PubMed] [Google Scholar]
- 7.Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–976. doi: 10.1136/thoraxjnl-2012-202103. [DOI] [PubMed] [Google Scholar]
- 8.Holland M, Alkhalil M, Chandromouli S, Janjua A, Babores M. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15(1):165–167. doi: 10.1111/j.1440-1843.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao P, Zhang J, He X, Hao Y, Wang K, Gibson PG. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2013;8(5):e57678. doi: 10.1371/journal.pone.0057678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soter S, Barta I, Antus B. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation. 2013;36(5):1178–1185. doi: 10.1007/s10753-013-9653-8. [DOI] [PubMed] [Google Scholar]
- 11.Brightling CE. Biomarkers that predict and guide therapy for exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(suppl):S214–S219. doi: 10.1513/AnnalsATS.201302-023AW. [DOI] [PubMed] [Google Scholar]
- 12.Eltboli O, Mistry V, Barker B, Brightling CE. Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology. 2015;20(4):667–670. doi: 10.1111/resp.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9:1101–1110. doi: 10.2147/COPD.S54475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alaithan AM, Memon JI, Rehmani RS, Qureshi AA, Salam A. Chronic obstructive pulmonary disease: hospital and intensive care unit outcomes in the Kingdom of Saudi Arabia. Int J Chron Obstruct Pulmon Dis. 2012;7:819–823. doi: 10.2147/COPD.S37611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khilnani GC, Banga A, Sharma SK. Predictors of mortality of patients with acute respiratory failure secondary to chronic obstructive pulmonary disease admitted to an intensive care unit: a one year study. BMC Pulm Med. 2004;4:12. doi: 10.1186/1471-2466-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Sanz MT, Pol-Balado C, Abellas C, Canive-Gomez JC, Anton-Sanmartin D, Gonzalez-Barcala FJ. Factors associated with hospital admission in patients reaching the emergency department with COPD exacerbation. Multidiscip Respir Med. 2012;7(1):6. doi: 10.1186/2049-6958-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C, Zhu H, Shen N, et al. Bacterial infection, airway and systemic inflammation and clinical outcomes before and after treatment of AECOPD, a longitudinal and cross-sectional study. COPD. 2015;12(1):19–30. doi: 10.3109/15412555.2014.898043. [DOI] [PubMed] [Google Scholar]
- 18.Weis N, Almdal T. C-reactive protein – can it be used as a marker of infection in patients with exacerbation of chronic obstructive pulmonary disease? Eur J Intern Med. 2006;17(2):88–91. doi: 10.1016/j.ejim.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Crisafulli E, Torres A, Huerta A, et al. Predicting in-hospital treatment failure (≤7 days) in patients with COPD exacerbation using antibiotics and systemic steroids. COPD. 2016;13(1):82–92. doi: 10.3109/15412555.2015.1057276. [DOI] [PubMed] [Google Scholar]
- 20.Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150(6 pt 1):1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 21.Zanini A, Cherubino F, Zampogna E, Croce S, Pignatti P, Spanevello A. Bronchial hyperresponsiveness, airway inflammation, and reversibility in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1155–1161. doi: 10.2147/COPD.S80992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47. doi: 10.2147/copd.2006.1.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzichini MM, Popov TA, Efthimiadis A, et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154(4 pt 1):866–869. doi: 10.1164/ajrccm.154.4.8887576. [DOI] [PubMed] [Google Scholar]
- 24.Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108(1):10–15. doi: 10.1378/chest.108.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Hospers JJ, Schouten JP, Weiss ST, Rijcken B, Postma DS. Asthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sample. Am J Respir Crit Care Med. 1999;160(6):1869–1874. doi: 10.1164/ajrccm.160.6.9811041. [DOI] [PubMed] [Google Scholar]
- 26.Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB, Respiratory Effectiveness Group Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015;10:2439–2450. doi: 10.2147/COPD.S94259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–2478. doi: 10.2147/COPD.S90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salturk C, Karakurt Z, Adiguzel N, et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis. 2015;10:1837–1846. doi: 10.2147/COPD.S88058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456–460. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 30.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224–1238. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 31.McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest. 2001;119(4):1190–1209. doi: 10.1378/chest.119.4.1190. [DOI] [PubMed] [Google Scholar]
- 32.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 33.Wilson R, Anzueto A, Miravitlles M, et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J. 2012;40(1):17–27. doi: 10.1183/09031936.00090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bafadhel M, Greening NJ, Harvey-Dunstan TC, et al. Blood eosinophils and outcomes in severe hospitalised exacerbations of COPD. Chest. 2016 Feb 3; doi: 10.1016/j.chest.2016.01.026. Epub. [DOI] [PubMed] [Google Scholar]
- 35.Serafino-Agrusa L, Scichilone N, Spatafora M, Battaglia S. Blood eosinophils and treatment response in hospitalized exacerbations of chronic obstructive pulmonary disease: a case-control study. Pulm Pharmacol Ther. 2016;37:89–94. doi: 10.1016/j.pupt.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Altman LC, Hill JS, Hairfield WM, Mullarkey MF. Effects of corticosteroids on eosinophil chemotaxis and adherence. J Clin Invest. 1981;67(1):28–36. doi: 10.1172/JCI110024. [DOI] [PMC free article] [PubMed] [Google Scholar]