Abstract
Background
Much research has focused on the deleterious neurobiological effects of childhood adversity that may underlie internalizing disorders. While most youth show emotional adaptation following adversity, the corresponding neural mechanisms remain poorly understood.
Methods
In this longitudinal community study, we examined the associations among childhood family adversity, adolescent internalizing symptoms, and their interaction on regional brain activation and amygdala/hippocampus functional connectivity during emotion processing in 132 adolescents.
Results
Consistent with prior work, childhood adversity predicted heightened amygdala reactivity to negative, but not positive, images in adolescence. However, amygdala reactivity was not related to internalizing symptoms. Furthermore, childhood adversity predicted increased fronto-amygdala connectivity to negative, but not positive, images, yet only in lower internalizing adolescents. Childhood adversity also predicted increased fronto-hippocampal connectivity to negative images, but was not moderated by internalizing. These findings were unrelated to adolescence adversity or externalizing symptoms, suggesting specificity to childhood adversity and adolescent internalizing.
Conclusions
Together, these findings suggest that adaptation to childhood adversity is associated with augmentation of fronto-subcortical circuits specifically for negative emotional stimuli. Conversely, insufficient enhancement of fronto-amygdala connectivity, with increasing amygdala reactivity, may represent a neural signature of vulnerability for internalizing by late adolescence. These findings implicate early childhood as a critical period in determining the brain’s adaptation to adversity, and suggest that even normative adverse experiences can have significant impact on neurodevelopment and functioning. These results offer potential neural mechanisms of adaptation and vulnerability which could be used in the prediction of risk for psychopathology following childhood adversity.
Keywords: childhood adversity, adolescence, anxiety, depression, neuroimaging, stress adaptation
Introduction
Childhood adversity, such as parental mental illness and household dysfunction, is common, affecting nearly two-thirds of youth by age 18 (1). Much research has focused on childhood adversity as a risk factor for developing mood and anxiety disorders (2). However, many youth show emotional adaptation even in the face of severe childhood adversity and do not develop mental illness (3; 4). However, the neurobiological mechanisms conferring adaptation to childhood adversity remain poorly understood. Such knowledge is vital for predicting individual outcomes following childhood adversity, determining which youth should receive early intervention, and developing biologically informed treatments for symptomatic youth.
Many neuroimaging studies have documented neural abnormalities during emotion processing in relation to childhood adversity. What is less clear, however, is which of these abnormalities may be adaptive, versus those which directly contribute to psychopathology. For example, amygdala hyperactivation has been reported across many types of childhood adversity (e.g. poverty, caregiver deprivation, interpersonal violence, maltreatment, stressful life events) (5–16), appears to be specific to negative emotional stimuli (6; 9; 12; 14) [though see (13)], and is generally independent of symptom levels (5–13). Together, these studies suggest that amygdala hyperactivation to negative stimuli may be an adaptive response to early life adversity, perhaps allowing enhanced threat detection. In contrast, prefrontal findings during emotion processing have been more variable and include mixed findings (increased and decreased activation) in the medial prefrontal cortex (mPFC) (5; 17), dorsolateral (dl)PFC (5; 7; 9; 18) and ventrolateral (vl)PFC (5–7; 17) in relation to interpersonal violence/maltreatment, caregiver deprivation, and poverty. Notably, abnormal prefrontal activation following early life adversity may also be specific to negative stimuli (6; 9). Furthermore, adversity-related increases in dorsal/lateral prefrontal activation may serve a compensatory role in emotion regulation (7; 9; 18).
Relative to brain activation studies, even less is known about emotion-related functional connectivity patterns which may confer adaptation versus vulnerability following childhood adversity. Gee and colleagues found that more “mature” mPFC-amygdala connectivity to negative stimuli following caregiver deprivation may be partially adaptive, in that it was associated with some reduction in anxiety symptoms (6). Relatedly, work from our group has shown that trauma-exposed youth with PTSD show reduced mPFC-amygdala connectivity to negative stimuli, which in turn was inversely related to PTSD severity (19). An intriguing possibility then, is that while amygdala hyperactivity to emotional stimuli may be a typical response to childhood adversity, augmentation of coupling between the amygdala and prefrontal regulatory regions may be a crucial determinant of adaptive emotion regulatory responses. Consistent with this notion, prefrontal-amygdala connectivity is associated with emotion regulation success and lower anxiety in healthy adults (20; 21).
A major limitation of prior emotion-related imaging studies of childhood adversity is that they have not incorporated measures of childhood adversity and emotional adaptation in the same individual brain model. This risks conflating adaptive and maladaptive sequelae of adversity, given that they may have opposing effects in the same circuits. In addition, prior studies have focused on severe adversity (e.g., maltreatment, caregiver deprivation), leaving it unclear whether similar neural sequelae occur with more normative types of adversity. Prior work in the present community sample of adolescents revealed decreased intrinsic mPFC-amygdala connectivity in relation to normative levels of family adversity and experiences of maltreatment, which mediated some risk for adolescent internalizing symptoms (22; 23). However, it remains unclear how normative experiences of childhood adversity may impact prefrontal-amygdala function and connectivity during emotion processing, and which patterns may serve an adaptive role. Finally, to our knowledge, no studies have examined the effects of childhood adversity on hippocampal functional connectivity during emotion processing. The hippocampus plays an important role in the contextual gating of fear and anxiety (24), and we have previously reported reduced intrinsic mPFC-hippocampus functional connectivity in relation to maltreatment experiences (23).
To address these knowledge gaps, we explored the neural substrates of adversity adaptation during emotion processing in a prospective, longitudinal community sample of adolescents. To index childhood adversity, we focused on family adversity levels during childhood (infancy to age 11), given our prior work showing that childhood, but not adolescent, adversity predicts weaker intrinsic fronto-amygdala and –hippocampus connectivity (22; 23). We defined emotional adaptation as the relative absence of internalizing (anxiety and depressive) symptoms (25) in adolescence, spanning ages 15–18. At age 18, adolescents completed functional MRI while performing an emotion processing task in which they rated negative, positive, and neutral images (26). Group-level analyses examined the effects of childhood adversity, adolescent internalizing, and their interaction on activation and functional connectivity in prefrontal-amygdala and -hippocampal pathways. We hypothesized that childhood adversity would be associated with increased amygdala reactivity to negative, but not positive, emotional content. However, emotional adaptation would be associated with adversity-related augmentation of prefrontal-amygdala and –hippocampus connectivity to negative emotional content. Attenuated recruitment of these pathways following childhood adversity would, in turn, be associated with greater internalizing symptoms in adolescence (i.e. childhood adversity by internalizing interaction). Within these analyses, we explored the specificity of neural findings to adolescent adversity, externalizing symptoms, and potential sex differences.
Methods
Participants
Recruitment for the Wisconsin Study of Families and Work (originally Wisconsin Maternity Leave and Health Project) (27) began in 1990 and was designed to gather information on parental leave and health outcomes from a community sample in and around two cities in southern Wisconsin. A total of 570 women and their partners were initially recruited from clinics and hospitals while attending routine prenatal visits. Mothers had to be over 18 years old, in their second trimester of pregnancy, and living with the baby’s biological father. Selection for the present study was based on proximity to the laboratory and MRI exclusionary criteria. A total of 138 participants completed MRI. Of these, 6 participants were missing data on either childhood adversity or adolescent internalizing, resulting in a final sample of 132 adolescents (69 female; Mage = 18.63). See Table 1 for participant/family characteristics. Note that our prior intrinsic functional connectivity studies (22; 23) represent a subsample of the current set of adolescents. Informed consent (and parental permission in childhood) was obtained for all assessments. University of Wisconsin-Madison Institutional Review Boards approved all procedures.
Table 1.
Participant and family characteristics.
| N | 132 |
| Age, m (range, SD) | 18.63 years (18.15–19.48, ±0.26) |
| Sex (female), n (%) | 69 (52%) |
| Race/ethnicity, n (%) | Caucasian, 119 (90%) Native American/Alaskan, 7 (5%) African American, 5 (4%) Asian, 1 (1%) |
| Family income at child’s age 4.5 yrs, m (range, SD) | $68,296 ($20,000–$300,000, ±$40,676) |
| Parental education at child’s age 3.5 yrs, m (range, SD) | Mother 15.2 years (10–20, ±2.0) Father 14.9 years (10–20, ±2.2) |
| Lifetime internalizing diagnoses, n (%) | Any diagnosis, 38 (29%) Major depressive disorder, 19 (14%) Social anxiety disorder, 16 (12%) Specific phobia, 8 (6%) Generalized anxiety disorder, 3 (2%) Panic disorder, 2 (2%) Depressive disorder NOS, 1 (1%) PTSD, 1 (1%) |
| Lifetime externalizing diagnoses, n (%) | Attention deficit hyperactivity disorder (5.2%) Oppositional defiant disorder (2.6%) |
Behavioral measures
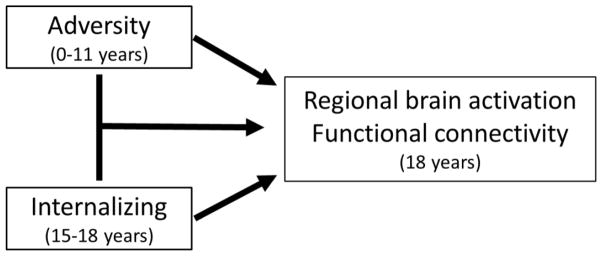
See Figure 1 for a schematic of behavioral measures and their use in the fMRI model. Childhood adversity was based on a composite of maternal reports of normative types of family adversity including maternal depression, negative parenting, parental conflict/family anger, maternal role overload, and financial stress (27). We focused on family adversity because it encompasses a broad array of common family stressors, was available prospectively, and would be less likely to introduce bias when included with adolescent internalizing in the same brain model. The adversity composite was created at each time point using principle components analysis (PCA), then averaged across seven assessments spanning the child’s infancy to age 11. Adolescent internalizing symptoms were assessed four times annually, from ages 15–18, with the adolescent version of the MacArthur Health and Behavior Questionnaire (HBQ) (25). At each time point, PCA was used to create a composite score across reporters - mother, teacher (age 15 only), and adolescent. Composite scores were then averaged across time points. Internalizing was comprised of HBQ subscales measuring symptoms of generalized anxiety, social anxiety, and depression. See Supplemental Methods for description of additional measures for adolescent adversity, externalizing, and clinical diagnoses.
Figure 1.
Summary of behavioral measures and use in analysis for fMRI. The statistical model for fMRI analysis included effects of childhood adversity (infancy to age 11), adolescent internalizing symptoms (ages 15–18), and their interaction on regional brain activation and functional connectivity of the amygdala and hippocampus during emotion processing. This model allows for testing of the effects of childhood adversity on neural patterns as moderated by internalizing status in adolescence.
fMRI Experimental Task
During fMRI, participants completed an emotion processing task in an event-related design as previously described (19; 26). In this task version, participants viewed 180 images from the International Affective Picture System (IAPS) (28), evenly split among negative, neutral, and positive images. The task also included presentation of neutral male faces after image offset in two-thirds of trials, with the intent of examining the effect of emotional content on subsequent face processing. Each image was presented for 4 s, and each face for 500 ms. The current analyses focused only on IAPS responses (negative-neutral, positive-neutral), though all stimuli were modeled at the individual level. Participants were not explicitly instructed to regulate their emotional responses, but were instructed to rate picture valence via button press. The task consisted of five runs approximately eight minutes each. Additional task details can be found in Supplemental Methods.
Image Acquisition and Processing
Structural and functional images were collected on a 3T MRI scanner (Discovery MR750, GE, Milwaukee, WI, USA) with an 8-channel RF head coil array. T1-weighted structural images (1mm3 voxels) were acquired axially with an isotropic MPRAGE sequence (TE=3.18ms, TR=8.13ms, TI=450ms, FA=12°). Functional scans were acquired using a gradient echoplanar sequence (64 x 64 in-plane resolution, 240 mm FOV, TE=25ms, TR=2s, FA=60°, 30 x 5mm interleaved sagittal slices, 265 volumes per run).
Anatomical images were segmented and transformed to MNI space using linear (12 parameter affine) and nonlinear (DARTEL) (29) warps with Statistical Parametric Mapping (SPM)8 (Wellcome Trust Centre for Neuroimaging, UK). For functional data, the first 4 volumes of each time-series were removed due to T1-equilibrium effects. Functional data were concatenated across runs, slice-time-corrected, realigned to the last volume of each run, and coregistered with the anatomical image using Analysis of Functional NeuroImages (AFNI) (30). Images were resampled to 2 x 2 x 2 mm3 voxels and smoothed with an 8 mm Gaussian (FWHM) kernel.
Individual Level Analysis
Each participant’s functional data were entered into a general linear model (GLM) in AFNI (3dDeconvolve), including regressors for each stimulus condition (positive, negative, or neutral IAPS; face presentation) convolved with a sine basis function. Each trial was modeled in two epochs to examine brain activation during reactivity (2–6s post image onset) and recovery (6–14s post image onset) periods (26). Six motion parameters were included as nuisance regressors. Motion parameters were unrelated to childhood adversity or adolescent internalizing (all p ≥ 0.2). Additionally, time-points were censored if the motion of a point 87 mm from the center of rotation was greater than 2 mm/degrees. At the run level, a run was excluded if more than 25% of time points were censored. This resulted in the exclusion of 1 run from 4 subjects. Finally, linear and quadratic trends were modeled to control for correlated drift. For the current study, contrasts of interest included negative-neutral and positive-neutral IAPS. Results from the first level GLM were then transformed to MNI space with the anatomical warp parameters using SPM.
Group Level Analysis
Individual level regression coefficients were submitted to random effects, group level analyses in AFNI (3dttest++). A single group test was used for negative-neutral and positive-neutral contrasts with covariates including childhood adversity, adolescent internalizing, and their interaction. All covariates were mean centered, with the interaction term generated from the mean-centered variables. A priori regions of interest included the PFC and bilateral amygdala/hippocampus using masks generated in AFNI. Multiple comparison correction was applied at the cluster level following Monte Carlo simulations in AFNI (3dClustSim). Multiple comparison correction was performed separately for the amygdala/hippocampus complex to avoid type II error given the small volume of this region (31). Additional results outside of a priori regions surviving whole brain correction are reported in Supplemental Information. Using a voxelwise p = 0.01, the cluster forming threshold for corrected α ≤ 0.05 was 295 voxels for the PFC, 39 voxels for amygdala/hippocampus, and 471 voxels for whole brain.
Functional Connectivity Analyses
A psychophysiological interaction (PPI) analysis was conducted within AFNI to examine task-dependent connectivity with the amygdala and hippocampus using the full hemodynamic response time course. As in our previous studies (22; 23), binary masks of the left and right amygdala and hippocampus were defined by placing 4 mm radius spheres at locations of the amygdala and mid-hippocampus according to the Talairach Daemon (32). A GLM was carried out for each participant as above, with additional regressors for each seed region time series, and the interaction of task and time series. Individual-level PPI coefficients were then entered into a random-effects, group level analysis as for the activation analysis, with multiple comparison correction as above.
Secondary analyses
Secondary analyses were conducted in SPSS (v. 21) on extracted cluster averages obtained in the voxel-wise analyses. Cluster averages were examined for outliers, and any outliers were winsorized to the next nearest non-outlier value. Next, a GLM of the primary model was repeated in SPSS, which confirmed the primary imaging results (see Supplemental Information). Subsequent analyses examined potential sex differences, and specificity of effects to adolescent adversity and externalizing symptoms.
Results
Childhood adversity and adolescent internalizing characteristics
Childhood adversity was very consistent across time points (intraclass correlation [ICC] = 0.88, F116,348 = 8.63, p < 0.001). For a descriptive summary of adversity experiences, see Supplemental Results. Adolescent internalizing symptoms were also very consistent across time points (ICC = 0.90, F113,339 = 10.46, p < 0.001). Nearly one-third (n = 38) of youth had a lifetime diagnosis of any internalizing disorder (Table 1). Lifetime internalizing diagnoses increased with adolescent internalizing levels, ranging from 11% to 46% for internalizing Z scores <−0.5 and >0.5, respectively.
Childhood adversity, adolescent internalizing, and regional brain activation during emotion processing
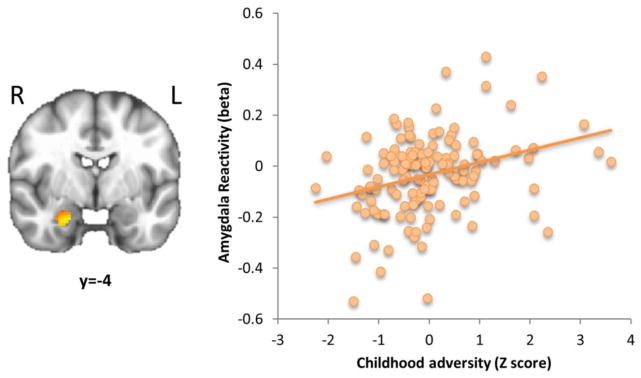
Analysis of a priori regions for the negative-neutral image contrast revealed that right amygdala reactivity was positively correlated with childhood adversity (k = 141 voxels, peak t = 3.07, xyz = 20, −4, −24; Figure 2), but showed no relationship with adolescent internalizing or an adversity by internalizing interaction. No significant effects were found in the negative-neutral contrast for PFC or hippocampal activation. In the positive-neutral contrast, no significant effects were observed with amygdala or PFC activation. However, right hippocampus reactivity was negatively correlated with internalizing (k = 36 voxels, peak t = −3.95, xyz = 36, −20, −18). No significant findings were observed for either contrast in the recovery period when split by face and no face trials. See Supplemental Table 1 for results outside of a priori regions.
Figure 2.
Childhood adversity predicts greater amygdala reactivity to negative vs. neutral images in adolescence. Reactivity was defined as activation 2–6 seconds after image onset. A scatterplot of amygdala reactivity vs. childhood adversity (Z-scored) is displayed on the right. N=132. k=141 voxels, p<0.05 corrected for the amygdala/hippocampus search region.
Childhood adversity, adolescent internalizing, and functional connectivity of the amygdala and hippocampus during emotion processing
Complete results for a priori search regions can be found in Table 2. See Supplemental Table 2 for results outside of a priori regions.
Table 2.
Summary of results from the psychophysiological interaction (PPI) analysis in a priori regions using a seed-based approach with the amygdala or hippocampus. Clusters shown survived correction (α ≤ 0.05) within a priori search regions of the prefrontal cortex or amygdala/hippocampus complex. Peak coordinates (x, y, z) are based on the MNI atlas in LPI orientation. The statistical model included childhood adversity (A), adolescent internalizing (I), and their interaction term (A X I). BA = Brodmann area, amyg = amygdala, hipp = hippocampus, dm/dlPFC = dorsomedial/dorsolateral prefrontal cortex.
| Contrast | Seed | Effect | Cluster | BA | k | t | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Negative-neutral | L amygdala | I | R amygdala | -- | 39 | −3.40 | 26 | −6 | −26 |
| R amygdala | A | B mPFC | 9,10 | 699 | 3.29 | −2 | 58 | 2 | |
| R amygdala | A X I | B dmPFC | 9,10 | 333 | −2.92 | 0 | 52 | 18 | |
| L hippocampus | A | B dm/dlPFC | 8,9 | 480 | 2.82 | 2 | 42 | 50 | |
| R hippocampus | A | L dm/dlPFC | 8,9,32 | 1054 | 2.66 | 0 | 38 | 58 | |
| R hippocampus | A | R dm/dlPFC | 8,9 | 939 | 3.76 | 20 | 46 | 42 | |
| Positive-neutral | No significant effects | ||||||||
Amygdala functional connectivity
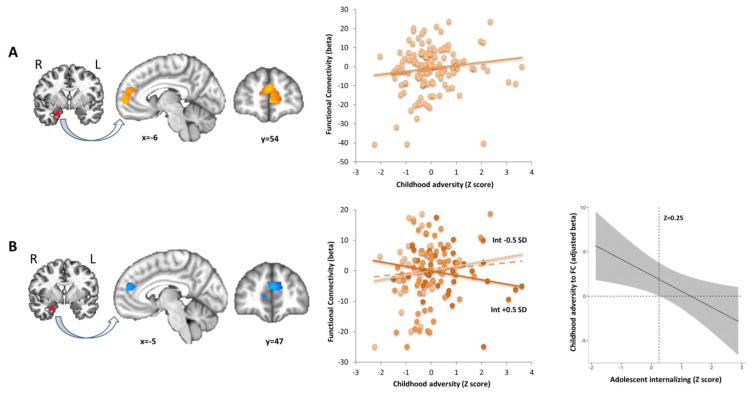
Analysis of the negative-neutral contrast revealed that childhood adversity positively predicted functional connectivity of the right amygdala to bilateral medial prefrontal cortex (mPFC, Brodmann areas [BA] 9, 10; Figure 3A). Furthermore, an adversity by internalizing interaction was present in an overlapping cluster in the dorsomedial (dm)PFC. Here, childhood adversity effects on amygdala-dmPFC functional connectivity were moderated by internalizing levels, such that adversity-related increases in connectivity were attenuated in higher internalizing adolescents (Figure 3B). To further explore this interaction, a conditional effects plot examined the effect of childhood adversity on amygdala-dmPFC connectivity across the full range of internalizing symptoms. Childhood adversity predicted significantly greater amygdala-dmPFC connectivity only in adolescents with internalizing Z-scores < 0.25, while no significant association between adversity and connectivity was observed at higher internalizing levels (Figure 3B). Finally, no relationships were observed among childhood adversity, adolescent internalizing, or their interaction with fronto-amygdala connectivity in the positive-neutral contrast.
Figure 3.
Childhood adversity predicts greater amygdala-mPFC functional connectivity in adolescence but is moderated by internalizing symptoms. Functional connectivity estimates were derived from the negative vs. neutral image contrast, using a seed based approach. The seed region and connectivity results are shown on the left, with scatterplots of childhood adversity (Z-scored) vs. functional connectivity cluster averages in the middle panel. (A) Main effect of childhood adversity on amygdala-mPFC connectivity (k=699 voxels, p<0.05 corrected). (B) A childhood adversity by adolescent internalizing interaction in an overlapping cluster revealed that childhood adversity predicts greater amygdala-dmPFC connectivity in lower but not higher internalizing adolescents (k=333 voxels, p<0.05 corrected). The middle panel shows a scatterplot depicting the interaction, with trend lines shown for childhood adversity vs. functional connectivity at adolescent internalizing Z-scores less than −0.5, or greater than 0.5 (i.e. ± 0.5 SD). Points/lines are color coded for internalizing levels. The dashed line represents the average effect across all participants. The right panel shows a conditional effects plot demonstrating the effect of childhood adversity on amygdala-mPFC connectivity across the full range of internalizing levels. Childhood adversity predicted significantly greater amygdala-mPFC connectivity only in adolescents with internalizing Z-scores < 0.25 (vertical dashed line). N=132. mPFC = medial prefrontal cortex, Int = internalizing, SD = standard deviation.
Hippocampus functional connectivity
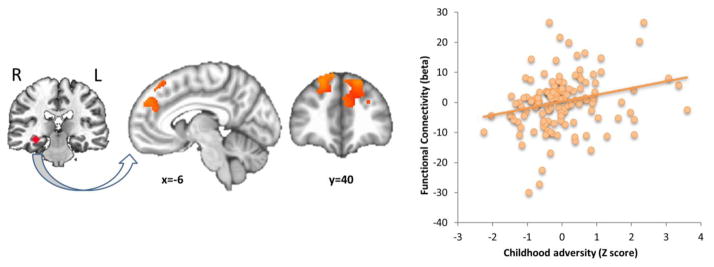
Analyses of the negative-neutral contrast revealed that childhood adversity positively predicted functional connectivity of the left and right hippocampus to bilateral dm/dlPFC (BA 8, 9, 32; Figure 4), but no adversity by internalizing interaction. No relationships were observed among childhood adversity, adolescent internalizing, or their interaction with fronto-hippocampal connectivity in the positive-neutral contrast.
Figure 4.
Childhood adversity predicts greater hippocampus-dm/dlPFC functional connectivity in adolescence. Functional connectivity estimates were derived from the negative vs. neutral image contrast, using a seed based approach. The seed region and connectivity results are shown on the left, with a scatterplot of childhood adversity (Z-scored) vs. functional connectivity cluster averages on the right. Shown in the scatterplot are results for hippocampus-left dm/dlPFC connectivity. A similar pattern was observed for hippocampus-right dm/dlPFC connectivity. N=132. k=1054 voxels (left dm/dlPFC) and 941 voxels (right dm/dlPFC), p<0.05 corrected for PFC and whole brain search regions. dm/dlPFC = dorsomedial/dorsolateral prefrontal cortex.
Potential sex differences in the effects of childhood adversity and adolescent internalizing on brain activation and functional connectivity
Given our prior work demonstrating a greater impact of childhood adversity on intrinsic amygdala-prefrontal connectivity in females compared to males (22; 23), we explored possible sex differences in our primary findings. Within this sample, there was a main effect of sex for adolescent internalizing symptoms as expected (Z-scored averages of 0.19 and −0.21 for females and males, respectively, F1,128 = 6.38, p = 0.01). Using extracted clusters from the negative-neutral contrast, we conducted a GLM including sex, interactions of sex with childhood adversity and adolescent internalizing, and their 3-way interaction. We found no significant main effects of sex or interactions of sex explaining the findings above.
Developmental timing of adversity effects on brain activation and functional connectivity
Next, we asked whether the neural effects of adversity were specific to exposure in childhood versus adolescence. Using extracted clusters from the negative-neutral contrast, we repeated our original GLM (childhood adversity, adolescent internalizing, and their interaction) while including recent adolescent adversity (past 6 months negative events), and the interaction of adolescent adversity with internalizing. In each case, the original effects of childhood adversity and childhood adversity by internalizing interactions remained, with no significant effects of adolescent adversity or adolescent adversity by internalizing. Furthermore, substituting adolescent adversity for childhood adversity entirely revealed no significant effects of adolescent adversity nor adolescent adversity by internalizing interactions, suggesting specificity of neural effects to adversity in childhood.
Symptom specificity of brain activation and connectivity findings: internalizing versus externalizing
Finally, we asked whether adolescent externalizing, versus internalizing, showed any relationship to our primary brain findings. Externalizing and internalizing symptoms were modestly correlated (r = 0.40, p < 0.001). Using clusters from the negative-neutral contrast, we repeated our original GLM while including adolescent externalizing, and the interaction of childhood adversity with externalizing. In each case, the original effects of childhood adversity and childhood adversity by internalizing interactions remained, with no significant effects of externalizing or childhood adversity by externalizing. Furthermore, substituting externalizing for internalizing entirely revealed no significant effects of externalizing nor childhood adversity by externalizing interactions, suggesting specificity of reported effects to adolescent internalizing.
Discussion
Our study offers novel insights on how normative experiences of childhood adversity may alter the brain’s emotion circuitry by adolescence, and how adaptive neural patterns may become compromised in vulnerable adolescents. Consistent with prior studies of more severe adversity, childhood adversity was associated with greater amygdala reactivity in adolescence. At the same time, childhood adversity predicted greater functional connectivity between the amygdala and hippocampus to dorsal prefrontal regions important in the regulation of fear and anxiety. However, adversity-related augmentation of prefrontal-amygdala connectivity was attenuated in higher internalizing adolescents, despite increasing amygdala reactivity. Importantly, these findings were specific to adversity during childhood, to symptoms of internalizing in adolescence, and to negative emotional content. Together, these findings suggest that even normative experiences of childhood adversity bias the amygdala towards reactivity to negative content, yet also adaptively augment prefrontal regulatory pathways, which in turn are compromised in more vulnerable youth. These results implicate childhood as a critical developmental period in the brain’s response to adversity, potentially tipping emotion regulatory circuits towards adaptation or vulnerability by late adolescence.
The present findings revealed that childhood adversity is associated with augmentation of prefrontal-amygdala coupling in lower internalizing adolescents, suggesting a potential neural mechanism of adaptation to adversity. It is worth noting, however, that higher internalizing adolescents also tended to have higher levels of childhood adversity. Furthermore, maternal depressive symptoms in childhood, part of our adversity composite, could also partially reflect heritable vulnerability factors transmitted to the child. On the other hand, maternal depression is, in and of itself, a significant form of childhood/family adversity (33). Thus, attenuated augmentation of prefrontal-amygdala coupling in higher internalizing adolescents could reflect a combination of both greater childhood adversity and heritable vulnerability, reflecting the complex interplay between genetic predisposition and early-life environment (3; 34).
Interestingly, amygdala reactivity was not itself associated with internalizing symptoms, consistent with prior studies of childhood adversity (5–13), suggesting it may be a more general response to childhood adversity allowing improved detection of potential threat. While amygdala hyperactivation is commonly reported in studies of internalizing disorders (35; 36), our findings suggest that augmentation of dorsal prefrontal-amygdala coupling may be a crucial determinant in emotional adaptation following childhood adversity, by counteracting increased amygdala reactivity. The dorsal prefrontal-amygdala pathway is notable for its role in effortful emotion regulation (37), and connectivity between dorsal/lateral PFC and the amygdala has been associated with emotion regulation success and lower anxiety levels in healthy adults (20; 21). Consistent with this regulatory hypothesis, work from our group using the current task in youth with PTSD revealed decreased amygdala-dmPFC coupling, which further related to illness severity (19). Furthermore, recent studies have demonstrated that, controlling for symptom severity, childhood maltreatment is associated with increased dorsolateral prefrontal activation in emotion tasks requiring cognitive control (9; 18), which may counteract re-experiencing symptoms (18) and amygdala hyperactivity (9).
Within this framework, one possible interpretation is that vulnerable youth show impaired augmentation of dorsal prefrontal-amygdala coupling following adversity, which then leads to deficient emotion regulation and internalizing symptoms. Alternatively, the development of internalizing symptoms in adolescence may cause a “degradation” of prefrontal-amygdala coupling, in effect negating adversity augmentation of this pathway. In either case, reduced dorsal prefrontal-amygdala coupling to negative stimuli appears to be a key neural marker of vulnerability for internalizing symptoms following childhood adversity. Future longitudinal neuroimaging studies in adolescence (before and after development of internalizing) would be warranted to fully explore the developmental course of these effects.
Similar to the amygdala, we found that childhood adversity predicted enhanced coupling between the hippocampus and dm/dlPFC to negative emotional content. The hippocampus is notable for its role in providing contextual information to the prefrontal cortex in gating fear and emotional responses (24). Rodent studies suggest that the hippocampus is capable of both promoting and extinguishing conditioned fear responses based on context (38). The current findings suggest that adaptive neural responses to childhood adversity involve increased coupling between the hippocampus and dm/dlPFC, which may allow for more flexible engagement of regulatory circuits based on environmental context. Consistent with this notion, enhanced mPFC-hippocampus coupling in trauma-exposed adults appears to mitigate the development of PTSD symptoms. Furthermore, lower gray matter volume in both hippocampus and dmPFC has been shown to mediate the relationship between childhood adversity and adult anxiety symptoms (39). Thus, impaired adversity-related augmentation of this circuit could contribute to impaired contextual modulation of fear and anxiety in adolescence, as has been observed in anxiety disorders and PTSD (24).
Limitations
While this study has a number of strengths including the large sample size, longitudinal design, and examination of normative types of adversity, there are limitations. First, differences in brain function could represent a predisposing trait to experience childhood adversity. Second, temporal overlap in brain and internalizing measures precludes direct inference on brain differences contributing to internalizing, or vice versa, and will require future studies employing longitudinal neuroimaging. Third, we do not have an independent measure of emotion regulation, such as corrugator activity (21), to directly demonstrate the benefit of augmented fronto-amygdala connectivity. Relatedly, PPI analyses cannot determine directionality, i.e. top-down vs. bottom-up changes in connectivity. Future studies employing causal modeling approaches would be warranted to explore these effects. Fourth, while our findings suggest that childhood adversity is particularly important in the neural differences reported here, we cannot exclude the possibility that different types of adversity captured by our childhood and adolescent measures account for apparent specificity to childhood. Additionally, it is possible that other forms of adversity outside the home, such as exposure to violence or bullying, may influence the brain’s adaptive capacity. Finally, we recognize that our definition of emotional adaptation was restricted to the relative absence of adolescent internalizing/externalizing symptoms, and doesn’t necessarily generalize to other indicators such as well-being which would merit exploration in future studies.
In summary, the current data suggest neural signatures of adaptation to childhood adversity involving augmentation of fronto-amygdala and –hippocampal pathways important in the regulation of fear and anxiety. Furthermore, adversity-related augmentation of fronto-amygdala connectivity was attenuated in higher internalizing adolescents, suggesting that either vulnerable youth fail to benefit from this adaptation, or that it becomes degraded with the development of internalizing. Finally, our results suggest that childhood, but not late adolescence, is a particularly important developmental period in determining neural adaptation to adversity. These findings have great relevance for understanding the development of adversity-related psychopathology such as depression, anxiety-disorders, and PTSD, the majority of which emerge by late adolescence (40). These findings offer neural markers of vulnerability which could be used in the prediction of risk for psychopathology following childhood adversity, the institution of timely interventions in at-risk youth, and as treatment targets in those suffering from internalizing disorders and PTSD.
Supplementary Material
Acknowledgments
This work was supported by US National Institutes of Health grants P50 MH084051 and P50 MH052354 (R.J.D., M.J.E.), R01 MH044340 (M.J.E.); the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development; and the HealthEmotions Research Institute, Department of Psychiatry, University of Wisconsin School of Medicine and Public Health. Partial support for R.J.H. was provided by the National Institute of Mental Health (K08 MH100267), the American Academy of Child & Adolescent Psychiatry, and the Brain and Behavior Research Foundation. We thank J. Armstrong for general management of the WSFW, M. Anderle, R. Fisher, L. Angelos, A. Dyer, C. Hermes, A. Koppenhaver and C. Boldt for assistance with data collection and recruitment, and J. Ollinger, G. Kirk, N. Vack, J. Koger and I. Dolski for general, technical and administrative assistance.
Footnotes
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuMont KA, Widom CS, Czaja SJ. Predictors of resilience in abused and neglected children grown-up: the role of individual and neighborhood characteristics. Child Abuse Negl. 2007;31:255–274. doi: 10.1016/j.chiabu.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29:449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21:R947–948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child Maltreatment and Neural Systems Underlying Emotion Regulation. J Am Acad Child Adolesc Psychiatry. 2015;54:753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am J Psychiatry. 2015;172:276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry. 2014;53:800–813.e10. doi: 10.1016/j.jaac.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci USA. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crozier JC, Wang L, Huettel SA, De Bellis MD. Neural correlates of cognitive and affective processing in maltreated youth with posttraumatic stress symptoms: does gender matter? Dev Psychopathol. 2014;26:491–513. doi: 10.1017/S095457941400008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonzo GA, Huemer J, Etkin A. History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. Journal of Psychiatric Research. 2016;74:45–54. doi: 10.1016/j.jpsychires.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf RC, Herringa RJ. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62:1575–1581. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: developing the Macarthur health and Behavior Questionnaire. J Am Acad Child Adolesc Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Schuyler BS, Kral TRA, Jacquart J, Burghy CA, Weng HY, Perlman DM, et al. Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc Cogn Affect Neurosci. 2014;9:176–181. doi: 10.1093/scan/nss131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- 29.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 31.Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, et al. Response to learned threat: An FMRI study in adolescent and adult anxiety. Am J Psychiatry. 2013;170:1195–1204. doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, et al. Exploring risk factors for the emergence of children’s mental health problems. Arch Gen Psychiatry. 2006;63:1246–1256. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- 34.Rutter M. Resilience as a dynamic concept. Dev Psychopathol. 2012;24:335–344. doi: 10.1017/S0954579412000028. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorka AX, Hanson JL, Radtke SR, Hariri AR. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol Mood Anxiety Disord. 2014;4:12. doi: 10.1186/2045-5380-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.