Abstract
This paper reviews basic methods and recent applications of length-based fiber bundle analysis of cerebral white matter using diffusion magnetic resonance imaging (dMRI). Diffusion weighted imaging (DWI) is a dMRI technique that uses the random motion of water to probe tissue microstructure in the brain. Diffusion tensor imaging (DTI) is an extension of DWI that measures the magnitude and direction of water diffusion in cerebral white matter, using either voxel-based scalar metrics or tractography-based analyses. More recently, quantitative tractography based on diffusion tensor imaging (qtDTI) technology has been developed to help quantify aggregate structural anatomical properties of white matter fiber bundles, including both scalar metrics of bundle diffusion and more complex morphometric properties, such as fiber bundle length (FBL). Unlike traditional scalar diffusion metrics, FBL reflects the direction and curvature of white matter pathways coursing through the brain and is sensitive to changes within the entire tractography model. In this paper, we discuss applications of this approach to date that have provided new insights into brain organization and function. We also discuss opportunities for improving the methodology through more complex anatomical models and potential areas of new application for qtDTI.
Keywords: Diffusion tensor imaging, White matter, Quantitative tractography, Aging
INTRODUCTION
Advances in diffusion weighted imaging (DWI) technology have allowed researchers to characterize the structural integrity of white matter tissue. Diffusion tensor imaging (DTI) is an extension of DWI utilized to non-invasively examine neuronal tracts to quantitatively measure white matter integrity (1,22,34,37,46). Highly advanced DTI methods have been developed in recent years and have significantly improved the utility of diffusion tensor measurements to detect subtle white matter changes in both healthy and diseased populations (13–14,17,39–41). One example includes the integration of quantitative tractography based on diffusion tensor imaging (qtDTI) technology that has enhanced our ability to examine specific detail about the direction and curvature of white matter pathways using in vivo imaging (17). This method is highly sensitive to white matter changes within entire tracts and, therefore, may be more advantageous than methods that involve placing regions of interest on two-dimensional scalar DTI parameter maps (17). In this review, we describe the fundamentals of the diffusion tensor model and qtDTI technology. We then review the existing literature on length-based metrics using qtDTI, followed by a discussion of the strengths and limitations of qtDTI. Finally, a brief review of future applications is provided.
DIFFUSION MR TECHNIQUES
DTI Physical Basis
DTI is a noninvasive magnetic resonance imaging (MRI) technology that measures water diffusion at each voxel in the brain. Water molecules diffuse differently along tissues depending on tissue microstructure and the presence of anatomical barriers. One simple and useful way to characterize diffusion at a location in the brain is along a spectrum between isotropic and anisotropic. Diffusion that is highly similar in all directions (i.e., isotropic diffusion) is typically observed in grey matter and cerebrospinal fluid. By contrast, directionally dependent diffusion (i.e., anisotropic diffusion) is observed in white matter due to the linear organization of the fiber tracts. Water within these tracts preferentially diffuses in one direction because physical barriers such as axonal walls and myelin restrict water movement in other directions (5,24,47,48). Neuropathological mechanisms associated with multiple conditions, including subcortical ischemia, neurodegeneration, and traumatic brain injury, cause reductions in the linear organization of white matter pathways with corresponding reductions in linear anisotropy (5,19,48,52). DTI is sensitive to these changes in linear anisotropy even when white matter integrity appears healthy based on structural neuroimaging methods (referenced as normal appearing white matter) (4,30), making DTI a powerful in vivo imaging method for the examination of the microstructural integrity of white matter.
DTI Scalar Metrics
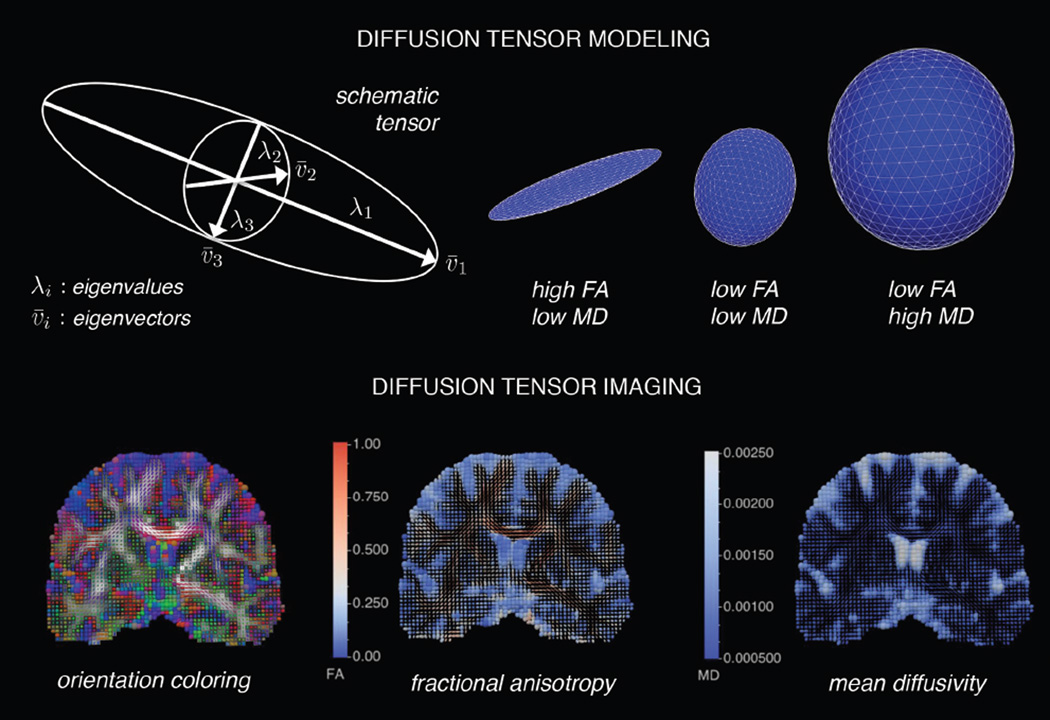
A symmetric 3×3 diffusion tensor characterizes water diffusion in brain tissues. This model represents the diffusion pattern with a second-order tensor that can be decomposed into three non-negative eigenvalues and three eigenvectors that describe the magnitude and orientation of water diffusion in each voxel (Figure 1). Eigenvalues describe the shape and size of the tensor, independent of orientation, while eigenvectors describe the orientation of the tensor, independent of shape and size. The tensor model parameterizes the diffusion in each voxel with an ellipsoid whose diameter in any direction estimates the diffusivity in that direction and whose major principle axis is oriented in the direction of maximum diffusivity The major axis of the ellipsoid (v1) points in the direction of the maximum diffusivity (λ1) of a voxel. The direction of the maximum diffusion is oriented in the direction of the major fiber tract in the voxel. The directions perpendicular to the main fiber orientation along the medium (v2) and minor axes (v3) of the diffusion ellipsoid are also computed (λ2, λ3) in the tensor analysis. DTI scalar metrics are functions of three diffusion eigenvalues (λ1, λ2, λ3). Axial diffusivity (AD = λ1) is the maximum diffusivity in the voxel and decreases with greater axonal injury (15,29). Radial diffusivity
is the average of the diffusivity perpendicular to the major axis and increases with abnormal myelination (1). Mean diffusivity
is the average of the diffusivity values of the three axes of the diffusion ellipsoid and is sensitive to cellularity, edema, and necrosis (46) (Figure 2). Lastly, fractional anisotropy
parameterizes the degree to which the diffusion ellipsoid deviates from spherical. FA is a normalized measure ranging from zero to one that decreases with axonal degeneration, abnormal myelination, and fiber orientation dispersion (27, 35–36, 47) (Figure 2). These scalar metrics describe microstructural properties of white matter; however, inclusion of the full structure of the tensor model assists in determining subtle changes related to tract directionality (17,25).
Figure 1.
Visual depiction of tensor-based modeling and diffusion tensor images: an illustration of tensor-based modeling and diffusion tensor imaging. The top panel shows a single tensor model, which can be decomposed into eigenvectors and eigenvalues. The eigenvectors (v1, v2, v3) represent the major, medium, and minor principle axes of the ellipsoid, and the eigenvalues (λ1, λ2, λ3) represent the diffusivities in these three directions, respectively. The eigenvalues can be used to describe the shape with fractional anisotropy (FA) and mean diffusivity (MD). The bottom panel shows diffusion tensor images, which are composed of glyphs representing the tensor models in each image voxel. The glyphs are ellipsoid shaped and can be colored based on fiber orientation, FA, MD, etc
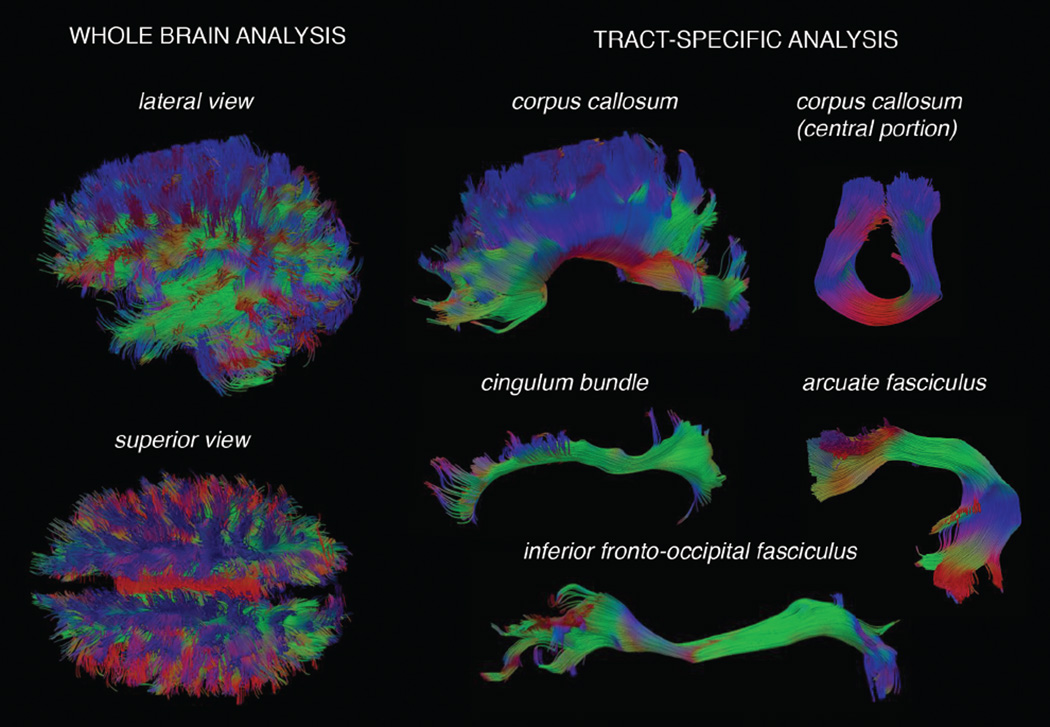
Figure 2.
Visual depiction of whole brain and tract-specific fiber bundles in cerebral white matter: an illustration of tractography modeling of white matter. The left panel shows whole brain tractography models, which consist of a complex pattern of connections. The right panel shows specific bundles extracted from whole brain tractography, allowing anatomically specific metrics to be computed.
DTI Tractography
DTI tractography is a technique for creating geometric models that reflect the large-scale structure of fiber bundles. These models are created based on voxel-wise estimates of local fiber orientation using the primary tensor eigenvector, which is indicative of the direction of the dominant fiber bundle. The primary advantage of tractography compared with regional analysis of scalar metrics is the integration of data across an entire white matter tract (17). This process can be repeated to provide both a curve representing the three-dimensional (3D) path as well as diffusivity properties sampled along the fiber bundle. The trajectories are then graphically depicted using 3D rendering of lines, tubes, or surfaces (31,54). Tractography can be performed using both deterministic and probabilistic approaches. In deterministic tractography, white matter tracts are reconstructed by selecting a seed region and performing streamline integration based on the preferred direction of the diffusion ellipsoids until one of several stopping criteria is reached. Stopping criteria include decreased anisotropy, sharp curvature, and reaching tissue boundaries (33). However, deterministic tractography is limited by the accumulation of errors during tracking and sensitivity to seeding conditions (26). Probabilistic tractography is an alternative approach that more completely samples the space of possible tracks and accounts for uncertainty during tracking (7). This technique estimates the most likely fiber orientations at each voxel along with the probability distribution that a fiber would run along those directions (46). These probability distributions are then used to sample from a large population of probable paths based on complex diffusion models (7). While probabilistic tracking technology helps to overcome complex anatomy and uncertainty, it is limited in the morphometric properties that can be measured, such as fiber bundle length (FBL).
Quantitative Tractography Based on Diffusion Tensor Imaging (qtDTI)
qtDTI technology combines scalar metrics with tractography to estimate bundle-specific properties that characterize the structural properties of fiber bundles, including both simple statistical summaries of bundle properties—for example, average FBL, total length, and average scalar metrics (AD, RD, MD, and FA). These simpler metrics can also be combined to form more complex composite metrics, such as intracranial volume (ICV)-normalized length and anisotropy-weighted FBL (17). Alterations in microstructure result in lowered anisotropy or sharp changes in fiber orientation, which lead to fiber termination during tractography (36). These outcomes are potentially useful for detecting white matter injuries, such as those associated with inflammation in multiple sclerosis, which can cause such microstructural changes and associated changes in bundle metrics (42). Compared with traditional DTI scalar metrics such as FA, qtDTI technology is effective in detecting tract specific alterations that may be distributed anywhere along the tractography model (17). This novel approach has high potential to advance our current understanding of white matter structure and function in healthy and diseased populations.
WHITE MATTER FIBER LENGTHS DECREASE WITH AGE
Postmortem Evidence of Reduced White Matter Fiber Length
Postmortem studies of human tissue have revealed significantly shorter total neuronal fiber lengths among older adults compared with younger adults (32,38,49). Specifically, Marner and colleagues (32) quantified total nerve fiber lengths in adults between the ages of 18 to 93 by using stereologic methods in myelinated nerve fibers. Results revealed a 10% decrease in myelinated nerve fibers per decade of life, with a total decrease of 45% from age 20 to 80 (32). Tang and colleagues (49) also used stereologic methods to examine potential age-related shortening of white matter fiber lengths and demonstrated that the total length of the cerebral fibers was significantly longer in younger individuals (118,000 km) compared with older individuals (86,000 km) (49). This loss of total nerve fiber length with age was accompanied by a decline in the number of small-diameter myelinated fibers.
Application of qtDTI in the Context of Age-Related White Matter Atrophy in Healthy Older Adults
The postmortem studies described above provide evidence that the total length of white matter fibers represents a biomarker of age-related white matter degradation (31,49), yet this could not be confirmed in vivo prior to the development of qtDTI. Our group recently reported a significant negative relationship between FBL and age, specific to the anterior thalamic radiation (3) and uncinate fasciculus (44). The anterior thalamic radiation is formed by fibers interconnecting the anterior and medial thalamic nuclei and the frontal lobe via the anterior limb of the internal capsule. The uncinate fasciculus connects the hippocampus and amygdala in the temporal lobe with the frontal lobe. Additional publications reported significant correlations between shortened FBL in the temporal and frontal lobes and increased age (6,9,43). These results suggest that volumetric reductions in white matter among older adults are likely due to shortened FBL in major white matter tracts (10).
The Relationship between qtDTI In Vivo and Cognition in Healthy Older Adults
Associations between fiber length and age-related cognitive decline could not be examined prior to the development of in vivo qtDTI technology. Our group recently reported that performance on tests of executive functioning was associated with shorter FBL in the frontal, occipital, and parietal lobes in older adults (6). Additionally, lower performance on tests of processing speed was associated with shorter FBL across all four lobes of the brain. The findings of shorter mean lobar FBL as a correlate of poorer cognitive performance provides a functional outcome related to reduced FBL and cognitive aging. Results suggest a possible role for qtDTI in identifying older adults at risk for clinically relevant cognitive dysfunction, including prodromal dementia.
Utilizing qtDTI In Vivo to Examine Risk Factors for Reduced White Matter Integrity
Our group has extended this research to determine the capacity of qtDTI to identify risk variables for suboptimal brain health in a healthy older adult population (9,43–44). The epsilon 4 (e4) isoform of apolipoprotein E (ApoE) and the angiotensin (AGT) M268T polymorphism (rs699; historically referred to as M235T) are two genetic risk factors for reduced brain health in older adults, with the ApoE e4 (ApoE4) allele also representing a known risk factor for developing Alzheimer’s disease (AD) (16,45). Members of our group utilized qtDTI to investigate differences in FBL among individuals with an e4 allele compared with those with e3 alleles (43) and with the MetMet (MM) genotype of the AGT M268T polymorphism compared with the ThrThr (TT) genotype (44). Both studies revealed an effect of genotype group on FBL in specific white matter tracts. FBL in the left UF were significantly shorter in e4 carriers compared to non-carriers (43). Similarly, healthy older adults with the TT genotype exhibited shorter FBL in the left superior longitudinal fasciculus and cingulate gyrus segment of the cingulum compared with their MM counterparts (44). Our group has also revealed that higher body mass index (BMI) was independently and significantly associated with shorter white matter FBL in the temporal lobe (9).
While age may represent a salient factor in white matter integrity of a healthy older adult population, additional factors such as BMI and genetic polymorphisms can contribute to subtle, yet identifiable, alterations to white matter fiber bundles that are detectable with qtDTI. Collectively, these three studies lend support to the utility of qtDTI in the assessment of cerebral white matter changes associated with common risk factors that often lead to suboptimal brain health in older adults.
APPLICATION OF qtDTI TECHNOLOGY TO DETERMINE WHITE MATTER INTEGRITY IN A CLINICAL POPULATION
qtDTI technology has also been used to examine white matter fiber lengths in individuals with subcortical ischemic vascular disease (SIVD) compared with healthy controls to determine the sensitivity of qtDTI to detect white matter changes in a clinical population. SIVD is a condition that is associated with significant white matter microstructural damage in the brain (12,20–21,28). Prior studies have demonstrated the sensitivity of traditional DTI indices to the white matter alterations associated with SIVD in specifically defined regions of interest, such as the corpus callosum and the corona radiate, in addition to the presence of white matter hyperintensities (WMH) that are characteristic of the disease (20–21). Members of our group investigated the utility of qtDTI metrics, particularly those associated with length (e.g., average fiber length, FA-weighted fiber length, normalized fiber length), to examine white matter tract integrity and its relation to cognitive performance in a group of individuals with SIVD (17). Average FA was included to investigate the relative performance of traditional DTI compared to qtDTI in identifying white matter alterations. Both average FA and qtDTI metrics, particularly those associated with fiber length, revealed poorer white matter integrity of transcallosal fibers in individuals with SIVD compared with healthy controls. Reduced fiber length was additionally associated with worse performance on tests of executive functioning and processing speed in the SIVD group. Effect sizes were consistently smaller for average FA values compared with qtDTI metrics, suggesting that qtDTI may be a more robust indicator of white matter tract damage in SIVD compared with traditional DTI scalar metrics.
STRENGTHS AND LIMITATIONS OF qtDTI TECHNOLOGY
The first major strength is that qtDTI provides multi-faceted measurements of fiber bundles that provide complementary measures, such as FBL, volume, FA, and MD, that can detect and gauge the magnitude of various aspects of white matter anatomy. As discussed earlier, FBL can provide a valuable signal that goes beyond diffusivity measurements alone. Furthermore, composite measures such as anisotropy-weighted FBL are potentially more sensitive to pathology than any one alone, as they can reflect both fiber termination and overall changes in anisotropy (17). The second major strength is the anatomical specificity of qtDTI, which is valuable for localizing pathological effects that would be undetectable when simpler whole-brain analysis is performed. Together, these aspects make qtDTI a unique and powerful tool for understanding structural aspects of fiber bundles.
Several limitations of qtDTI technology warrant discussion. First, qtDTI is sensitive to imaging artifacts such as partial-volume averaging of fiber bundle populations with varying degrees of myelination, fiber orientation, and/or axon caliber. Partial-volume confounds can be somewhat managed by decreasing the voxel size and increasing the gradient strengths and number of directions. However, these adjustments reduce signal-to-noise ratios and increase scan time and post-processing complexity. Additional artifacts include subject motion, magnetic susceptibility, and echo planar imaging distortion. While these artifacts are often difficult to avoid, they can be readily detected by inspection, and negative effects on bundle reconstruction can often be avoided by quality control (51).
FUTURE APPLICATIONS AND NEW INNOVATIONS OF qtDTI TECHNOLOGY
As a novel method, qtDTI remains in the early stages of development, and research is needed to examine reproducibility and reliability. Studies including histological techniques and known markers of white matter pathology (e.g., WMH) will support the validity of the approach. Furthermore, qtDTI technology has the potential to broaden our understanding of the associations between white matter integrity and cognitive development in children. It is also possible that these metrics (i.e., FBL) can be used to improve sensitivity to injury and abnormal development. However, studies in newborns and children are necessary to determine the applicability of qtDTI metrics in this population, particularly in relation to work on myelin water fraction mapping (18). There is also much knowledge to gain by going beyond the relatively simple single tensor model to explore more complex diffusion models. Such methods can potentially improve the reconstruction of crossing bundles and enable the quantitation of features such as fiber dispersion and free water contamination. Current efforts to address these limitations include tractography methods that utilize more complex models (11), such as constrained spherical convolution (50), ball-and-sticks diffusion model (8), and neurite orientation dispersion and density imaging (53). Finally, there is a great deal to learn about the relationship between qtDTI and other imaging modalities. In particular, the combination of qtDTI and functional MRI has the potential to provide a much more complete model of brain integrity, as it would provide a parallel view of both structural and functional brain integrity.
SUMMARY
qtDTI is a relatively novel imaging approach that exhibits high potential to advance our current understanding of the organization and function of the human brain. Although traditional DTI metrics provide important information about white matter integrity within a single voxel, qtDTI technology has facilitated the examination of specific detail about the direction and curvature of white matter pathways in vivo. While this method is currently in the early stages of technological advancement, research to date has provided novel insights into cerebral white matter integrity in adult populations. Overall, qtDTI represents a potentially useful tool in future investigations of white matter fiber bundles in healthy and clinical populations.
Acknowledgments
Study Funding. Supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant number R01 NS052470 and R01 NS039538 and National Institutes of Health/National Institute of Mental Health grant R21 MH090494. Recruitment database searches were supported in part by National Institutes of Health/National Center for Research Resources grant UL1 TR000448.
Footnotes
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol. Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Baker LM, Laidlaw DH, Conturo TE, Hogan J, Zhao Y, Luo X, Correia S, Cabeen R, Lane EM, Heaps JM, Bolzenius J, Salminen LE, Akbudak E, McMichael AR, Usher C, Behrman A, Paul RH. White matter changes with age utilizing quantitative diffusion MRI. Neurology. 2014;83(3):247–252. doi: 10.1212/WNL.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu C. The basis of anisotropic water diffusion in the nervous system-a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 6.Behrman-Lay AM, Usher C, Conturo TE, Correia S, Laidlaw DEL, Lane EM, Bolenzius J, Heaps JM, Salminen LE, Baker LM, Cabeen R, Akbudak E, Luo X, Yan P, Paul RH. Fiber bundle length and cognition: a length-based tractography MRI study. Brain Imaging Behav. 2015;9(4):765–775. doi: 10.1007/s11682-014-9334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RC, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 8.Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolzenius JD, Laidlaw DH, Cabeen RR, Conturo TE, McMichael AR, Lane EM, Heaps JM, Salminen LE, Baker LM, Gunstad J, Paul RH. Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging Behav. 2013;7(3):300–306. doi: 10.1007/s11682-013-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DE, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biol. Psychiat. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Cabeen RP, Bastin ME, Laidlaw DH. Kernel regression estimation of fiber orientation mixtures in diffusion MRI. Neuroimage. 2016;127:158–172. doi: 10.1016/j.neuroimage.2015.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chui HC. Subcortical ischemic vascular dementia. Neurol. Clin. 2007;25(3):717–740. doi: 10.1016/j.ncl.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccarelli O, Parker GJM, Toosy AT, Wheeler-Kingshott CAM, Barker GJ, Boulby PA, Miller DH, Thompson AJ. From diffusion tractography to quantitative white matter tract measures: a reproducibility study. Neuroimage. 2003;18(2):348–359. doi: 10.1016/s1053-8119(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 14.Ciccarelli O, Toosy AT, Parker GJM, Wheeler-Kingshott CAM, Barker GJ, Miller DH, Thompson AJ. Diffusion tractography based group mapping of major white-matter pathways in the human brain. Neuroimage. 2003;19(4):1545–1555. doi: 10.1016/s1053-8119(03)00190-3. [DOI] [PubMed] [Google Scholar]
- 15.Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32(3):1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- 16.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 17.Correia S, Lee SY, Voorn T, Tate DE, Paul RH, Zhang S, Salloway SP, Malloy RE, Laidlaw DH. Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI. Neuroimage. 2008;42(2):568–581. doi: 10.1016/j.neuroimage.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deoni SC, Dean DC, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edlow BL, Copen WA, Izzy S, Bakhadirov K, van der Kouwe A, Glenn MB, Greenberg SM, Greer DM, Wu O. Diffusion tensor imaging in acute-to-subacute traumatic brain injury: a longitudinal analysis. BMC Neurol. 2016;16(1):1. doi: 10.1186/s12883-015-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erkinjuntti T. Subcortical ischemic vascular disease and dementia. Int. Pscyhogeriatr. 2003;15:23–26. doi: 10.1017/S1041610203008925. [DOI] [PubMed] [Google Scholar]
- 21.Fu JL, Zhang T, Chang C, Zhang YZ, Li WB. The value of diffusion tensor imaging in the differential diagnosis of subcortical ischemic vascular dementia and Alzheimer’s disease in patients with only mild white matter alterations on T2-weighted images. Acta Radiol. 2012;53(3):312–317. doi: 10.1258/ar.2011.110272. [DOI] [PubMed] [Google Scholar]
- 22.Gunning-Dixon EM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psych. 2009;24(2):109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttman CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- 24.Hagman P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl. 1):S205–S223. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- 25.Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. Am. J. Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S, Zhang P, Han T, Liu W, Liu M. Tri-linear interpolation-based cerebral white matter fiber imaging. Neural Regener. Res. 2013;8(23):2155–2164. doi: 10.3969/j.issn.1673-5374.2013.23.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Lin L, Xue Y, Duan Q, Sun B, Lin H, Chen X, Wei X, Zhang Z. Micro structural white matter abnormalities and cognitive dysfunction in subcortical ischemic vascular disease: an atlas-based diffusion tensor analysis study. J. Mol. Neurosci. 2015;56(2):363–370. doi: 10.1007/s12031-015-0550-5. [DOI] [PubMed] [Google Scholar]
- 29.Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. BBA-Mol. Basis Dis. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clin. Neuropsychol. 2007;21(1):73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- 31.Margulies DS, Bottger J, Watanabe A, Gorgolewski KJ. Visualizing the human connectome. Neuroimage. 2013;80:445–461. doi: 10.1016/j.neuroimage.2013.04.111. [DOI] [PubMed] [Google Scholar]
- 32.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003;462(2):144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- 33.Mori S, Crain BJ, Chacko VP, Van Zijl P. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. Am. J. Neuroradiol. 2008;29(4):632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007;245(2):367–384. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- 37.O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SCR, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57(12):2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- 38.Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJG, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp. Gerontol. 2003;38(1):95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 39.Parker GJM. Tracing fiber tracts using fast marching. P. Int. Soc. Magn. Reson. 2000;85 [Google Scholar]
- 40.Parker GJ, Stephan KE, Barker GJ, Rowe JB, MacManus DG, Wheeler-Kingshott CA, Ciccarelli O, Passingham RE, Sprinks RL, Lemon RN, Turner R. Initial demonstration of in vivo tracing of axonal projections in the macaque brain and comparison with the human brain using diffusion tensor imaging and fast marching tractography. Neuroimage. 2002;15(4):797–809. doi: 10.1006/nimg.2001.0994. [DOI] [PubMed] [Google Scholar]
- 41.Parker GJ, Wheeler-Kingshott CA, Barker GJ. Estimating distributed anatomical connectivity using fast marching methods and diffusion tensor imaging. IEEE Trans. Med. Imaging. 2002;21(5):505–512. doi: 10.1109/TMI.2002.1009386. [DOI] [PubMed] [Google Scholar]
- 42.Roosendaal SD, Geurts JJG, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44(4):1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Salminen LE, Schofield PR, Lane EM, Heaps JM, Pierce KD, Cabeen R, Laidlaw DH, Akbudak E, Conturo TE, Correia S, Paul RH. Neuronal fiber bundle lengths in healthy adult carriers of the ApoE4 allele: a quantitative tractography DTI study. Brain Imaging Behav. 2013;7(3):274–281. doi: 10.1007/s11682-013-9225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salminen LE, Schofield PR, Pierce KD, Zhao Y, Luo X, Wang Y, Laidlaw DH, Cabeen RP, Conturo TE, Tate DE, Akbudak E, Lane EM, Heaps JM, Bolzenius JD, Baker LM, Cagle LM, Paul RH. Neuromarkers of the common angiotensinogen polymorphism in healthy older adults: a comprehensive assessment of white matter integrity and cognition. Behav. Brain Res. 2016;296:85–93. doi: 10.1016/j.bbr.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt R, Schmidt H, Fazekas E, Launer LJ, Niederkorn K, Kapeller P, Lechner A, Kostner GM. Angiotensinogen polymorphism M235T, carotid atherosclerosis, and small-vessel disease-related cerebral abnormalities. Hypertension. 2001;38(1):110–115. doi: 10.1161/01.hyp.38.1.110. [DOI] [PubMed] [Google Scholar]
- 46.Sherbondy AJ, Dougherty RE, Ben-Shachar M, Napel S, Wan dell BA. ConTrack: finding the most likely pathways between brain regions using diffusion tractography. J. Vis. 2008;8(9):15. doi: 10.1167/8.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013;7(31):1–14. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun SW, Neil JJ, Liang HE, He YY, Schmidt RE, Hsu CY, Song SK. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magn. Reson. Med. 2005;53(6):1447–1451. doi: 10.1002/mrm.20488. [DOI] [PubMed] [Google Scholar]
- 49.Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJG. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol. Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- 50.Tournier JD, Calamante E, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35(4):1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Vilanova A, Zhang S, Kindlmann G, Laidlaw D. An introduction to visualization of diffusion tensor imaging and its applications. In: Weickert J, Hagen H, editors. Visualization and processing of tensor fields. The Netherlands: Springer Berlin Heidelberg; 2006. pp. 121–153. [Google Scholar]
- 52.Zhang S, Demiralp C, Laidlaw DH. Visualizing diffusion tensor MR images using streamtubes and streamsurfaces. IEEE Trans. Vis. Comput. Graphics. 2003;9(4):454–462. [Google Scholar]
- 53.Wu XP, Gao YJ, Yang JL, Xu M, Sun DH. Quantitative measurement to evaluate morphological changes of the corpus callosum in patients with subcortical ischemic vascular dementia. Acta Radiol. 2015;56(2):214–218. doi: 10.1177/0284185114520863. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DG. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]